Summary
Breast cancer is one of the most common origins of metastatic lesions in the central nervous system. Many patients with a breast cancer and concurrent brain tumor(s) were diagnosed to have a metastatic lesion or lesions in the brain, based exclusively on their image findings without further pathologic verification, and received radiotherapy alone thereafter. It is, however, possible that a different pathology such as primary brain malignancy, which actually warrants a specific treatment modality, may occur in such patients with an already known malignancy. We, herein, reported a 61-year-old female patient who suffered from an anaplastic oligodendroglioma 1 year after her diagnosis of breast cancer. Demographic data, characteristic imaging findings, treatment, and outcome of the patient were discussed.
Keywords
anaplastic oligodendroglioma;breast cancer;high-grade glioma;metastatic brain lesions
1. Introduction
Breast cancer is one of the most common origins of the metastatic lesions in the central nervous system (CNS). As estimated, approximately 10–15% of breast cancer patients suffer from the CNS metastatic lesion(s) throughout the course of treatment.1 Many patients with a breast cancer and concurrent brain tumor(s), however, do not receive a surgical extirpation or biopsy for the CNS lesions, because characteristic radiological findings such as a ring-like enhancing lesion or multiple lesions with peritumoral edema in the computed tomography (CT) and magnetic resonance imaging (MRI) often have made a putative diagnosis of malignant metastatic lesion or lesions. These patients are often treated with whole-brain radiotherapy (WBRT) to the brain plus systemic chemotherapy for local tumoral control, because most patients with metastatic lesions, especially those with multiple small lesions (less than 2 × 2 × 2 cm3) that originated from breast cancer, response well to the radiation treatment.1 ; 2 It is, however, possible that primary brain malignant gliomas, although relatively rare in incidence, may develop in patients with a systemic malignancy, and such patients actually need a treatment modality already developed and tailored for primary CNS malignancy.3 We reported a female patient who suffered from an anaplastic oligodendroglioma 1 year apart after her diagnosis of breast cancer. Demographic data, characteristic imaging findings, tailored treatment, and final outcome of the patient were discussed.
2. Case report
A 61-year-old woman was diagnosed to have a right-sided invasive ductal carcinoma (T2N2M0, stage IIIA) and left-sided ductal carcinoma in situ 1 year prior to her neurologic evaluation. She had no family history of breast cancer, and had undergone right-side modified radical mastectomy and left-side total mastectomy thereafter. The tumor was confirmed to be an adenocarcinoma that was strongly positive for estrogen and progesterone receptors but was negatively stained for human epidermal growth factor receptor-2 (HER2). The patient was further treated with local radiotherapy (50 Gy for right chest wall and 50 Gy for right supraclavicular lymph nodes) and chemotherapy with cyclophosphamide, doxorubicin, and paclitaxel, without notable adverse effects.
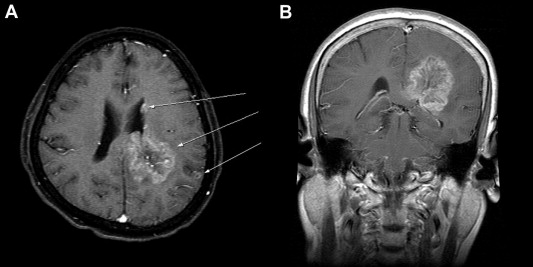
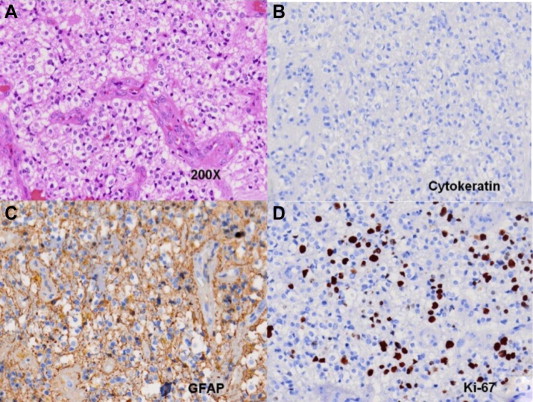
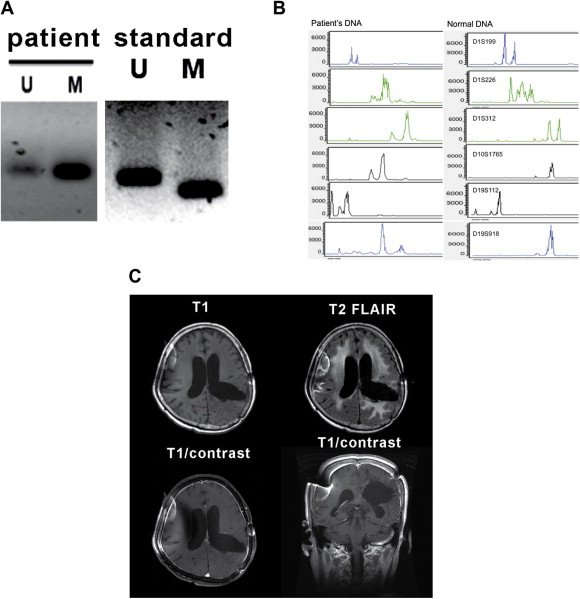
The patient experienced progressive recent and remote memory impairment and cognitive dysfunction 1 year after her diagnosis of breast cancer. She was also found to have mild weakness (motor grade = 4+/5) of the right upper and lower limbs. Cranial functions and other neurologic examinations were unremarkable. Further CT and MRI examinations of the brain demonstrated that the patient had multiple brain lesions, including a large (4 × 4 × 5 cm3 in size) brain tumor located at left parieto-occipital subcortical regions extending into the ipsilateral splenium of the corpus callosum, and two other small, but apart, lesions in the lateral ventricle wall and the parietal cortex of the ipsilateral brain (Fig. 1). The patient was diagnosed to have a multifoci primary brain malignancy rather than secondary metastases, because the largest brain lesion was relatively infiltrative and deeply invasive to the corpus callosum. She subsequently underwent a craniotomy for gross total resection of the largest brain lesion. Pathologic examination demonstrated the tumor to be an anaplastic oligodendroglioma with plenty of cells having hyperchromatic, pleomorphic nuclei and abundant pale eosinophilic cytoplasm, and with vascular proliferation (Fig. 2A). Further immunohistochemical examinations showed that the tumor was strongly positive for glial fibrillary acidic protein (GFAP) but was negative for cytokeratin staining (Fig. 2B and 2C). Moreover, the Ki-67 labeling index was found to be approximately 40% (Fig. 2D), indicating a relatively high mitotic activity. Furthermore, the methylation-sensitive polymerase chain reaction (PCR) of the tumoral tissues demonstrated the O6-methylguanine-DNA methyltransferase (MGMT) promoter of the tumor to be a methylated one (Fig. 3A). Capillary electropherograms of PCR products resulting from amplification of microsatellite loci on chromosomes 1p and 19q revealed that the tumor samples contained 1p/19q codeletion (Fig. 3B). The patient then received external beam radiotherapy up to 60 Gy with concurrent daily temozolomide treatment (75 mg/kg), followed by standard temozolomide monotherapy (200 mg/kg) for the subsequent 12 months. The patient tolerated the treatment well and made an uneventful recovery. Her Karnofsky performance scale was improved from a score of 60 to that of 70 after the neurosurgical treatment. In addition, she remained progression free and did not have notable intracranial recurrence up to 24 months after her brain surgery (Fig. 3C).
|
|
|
Figure 1. Magnetic resonance imaging of the patients brain. (A) Axial T1-weighted image with gadolinium enhancement shows a large left-sided parieto-occipital lesion, located at the subcortical region but extending into the ipsilateral corpus callosum, and two concurrent small lesions in the ipsilateral lateral ventricle wall and parietal cortex (arrows). (B) Coronal section of the largest lesion shows compression of the left lateral ventricle and extension into the corpus callosum. |
|
|
|
Figure 2. (A) Hematoxylin and eosin staining shows plenty of tumor cells with hyperchromatic, pleomorphic nuclei and abundant pale eosinophilic cytoplasm, and vascular proliferation. Immunohistochemical examinations showed that the tumor was (B) negative for cytokeratin staining but (C) strongly positive for glial fibrillary acidic protein. (D) The Ki-67 labeling index is about 40%. |
|
|
|
Figure 3. (A) Examinations for MGMT promoter of the tumor proved the tumor sample to be a methylated one. (B) Amplification of microsatellite loci on chromosomes 1p and 19q revealed that the tumor samples contained 1p/19q codeletion. Follow-up MRI examinations of the brain were taken at 24 months postoperatively. (C) Axial and coronal T1-weighted images with gadolinium enhancement as well as T2-FLAIR revealed no notable recurrence of the tumors. M = methylated MGMT promoters; MGMT = O6-methylguanine-DNA methyltransferase; U = unmethylated MGMT promoters. |
3. Discussion
Primary brain tumors may occur in patients who have systemic malignancies, especially in the era with improved surgical techniques for tumoral extirpation, followed by well-developed radiotherapy and chemotherapy, or even target therapy.5 ; 6 In a retrospective study, Maluf et al6 have identified 21 patients with a histologic diagnosis of concurrent high-grade glioma after a prior diagnosis of a solid or hematological malignancy. In particular, breast cancer is the most common cancer in women. It has been estimated that approximately 10–15% of breast cancer patients will suffer from the CNS metastatic lesion(s) throughout the course of the treatment.1 In contrast, an annual incidence of concurrent primary brain malignancy in patients with other systemic cancers can be expected to be much less than 1 per million in the general population, solely based on the annual incidence rates of 10–15 cases per 100,000 who develop primary brain tumors.7 Thus, it is evident that in patients with a known systemic malignancy such as breast cancer, the incidence of metastatic brain lesion(s) is far more prevalent than that of the concurrent primary brain malignancy.
It is noteworthy that in most breast cancer patients, a diagnosis of CNS metastases is often determined by either contrast-enhanced CT or MRI of the brain alone. Arslan et al1 have retrospectively collected 259 breast cancer patients with CNS metastases and have found that only 32 patients (13%) underwent surgery for pathological verification of their CNS metastases. Kim et al2 also reported a retrospective study including 400 breast cancer patients with CNS metastases and have noted that only five patients (1.3%) had underwent surgical intervention for pathological confirmation. However, it is clinically essential to differentiate between primary and metastatic intracerebral lesions in patients with systemic malignancies, thus offering appropriate treatment protocols. Different surgical planning and therapeutic modalities between the two disease entities may affect the final outcome of patients greatly, when an inappropriate diagnosis is made solely based on the imaging diagnosis.3 In the study of Maluf et al, it has been observed that four patients were misdiagnosed as having brain metastases. These patients had received WBRT alone and, thus, the surgical intervention and appropriate chemotherapy tailored for primary CNS malignant gliomas were much delayed.3
Distinguishing the high-grade glioma from brain metastases solely on the basis of contrast-enhanced CT or MRI is virtually difficult, because many cases of these two disease entities may have similar imaging characteristics.4 Server et al4 have reported the use of 3 T MRI with vascular permeability and vascular perfusion (relative cerebral blood volume and blood flow) imaging methods to distinguish high-grade gliomas from metastases. They, however, have noted that the specificity and accuracy were actually inconsistent for most cases. However, some MRI findings might provide clues to differentiate between a primary brain malignancy and a brain metastasis. It has been demonstrated that an infiltrative lesion involving the corpus callosum, lesions involving deep white matter structures, and extensive infiltration involving both gray and white matter may provide more possibility to suggest a glioma rather than a metastatic lesion.5 In our case, the infiltrative tumor involving the corpus callosum raised the suspicion of a primary brain tumor, which was subsequently confirmed after surgical resection and histopathologic examinations. Thus, this patient could receive a treatment modality tailed for high-grade glioma, and has made a favorable disease control up to 24 months postoperatively. In the patient, the nature of the anaplastic oligodendroglioma with a combination of methylated MGMT promoter and 1p/19q codeletion has certainly made the tumor more amenable to chemotherapy, with a complete response following surgical extirpation.8 ; 9
In this case, we could not exclude completely the possibility that the other two small nodules observed in the ipsilateral contiguous cortical and ventricular regions, as seen in Fig. 1, were actually metastatic lesions originated from her breast cancer. In a follow-up period of 2 years, the patient, however, did not have notable metastatic lesions from her breast cancer. A high mitotic activity of the anaplastic oligodendroglioma, as evidenced by 40% of the Ki-67 labeling index, also suggested a relatively high risk for developing early intracranial seeding of the anaplastic oligodendroglioma along the white matter and CSF pathways. Alternatively, it was also likely that this multifocal tumor had phenotypes derived from different progenitor cells of tumors (polyclonal origin). A favorable outcome observed with the patient, however, support that the presence of intracranial tumor dissemination on initial diagnosis does not, in itself, preclude aggressive treatment for high-grade glioma if a patient is otherwise well.
In summary, in patients with systemic malignancies and concurrent infiltrative brain lesion(s), primary brain malignancy should be included in the differential diagnosis, in addition to secondary metastases. We emphasize that surgical intervention for histopathologic examinations is needed for such patients, especially when there are characteristic MRI findings suggestive of gliomas rather than metastases.
References
- 1 U.Y. Arslan, B. Oksuzoglu, S. Aksoy, et al.; Breast cancer subtypes and outcomes of central nervous system metastases; Breast, 20 (2011), pp. 562–567
- 2 H.J. Kim, S.A. Im, B. Keam, et al.; Clinical outcome of central nervous system metastases from breast cancer: differences in survival depending on systemic treatment; J Neurooncol, 106 (2011), pp. 303–313
- 3 R. Stupp, W.P. Mason, M.J. van den Bent, et al.; Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma; N Engl J Med, 352 (2005), pp. 987–996
- 4 A. Server, T.E.D. Orheim, B.A. Graff, et al.; Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis; Neuroradiology, 53 (2010), pp. 319–330
- 5 M. Piccirilli, M. Salvati, S. Bistazzoni, et al.; Glioblastoma multiforme and breast cancer: report on 11 cases and clinico-pathological remarks; Tumori, 91 (2005), pp. 256–260
- 6 F.C. Maluf, L.M. DeAngelis, J.J. Raizer, et al.; High-grade gliomas in patients with prior systemic malignancies; Cancer, 94 (2002), pp. 3219–3224
- 7 K.J. Zülch; Epidemiology of brain tumor: general statistical and biological data; P. Bailey (Ed.), Brain Tumors: Their Biology and Pathology, Springer, New York (1986), pp. 85–114
- 8 J.J. Zhu, T. Santarius, X. Wu, et al.; Screening for loss of heterozygosity and microsatellite instability in oligodendrogliomas; Genes Chromosomes Cancer, 21 (1998), pp. 207–216
- 9 M. Esteller, J. Garcia-Foncillas, E. Andion, et al.; Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents; N Engl J Med, 343 (2000), pp. 1350–1354
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?