Keywords
Sensorineural hearing loss ; Inner ear ; Hearing ; Omega 3 ; Endothelial dysfunction
Hearing impairment is the most prevalent sensory deficit [1] .
Sensorineural hearing loss (SNHL) is the most common type of permanent hearing loss and it occurs when there is damage to the inner ear (cochlea), or to the nerve pathways from the inner ear to the brain. Most of the time, SNHL cannot be medically or surgically corrected.
SNHL can result from genetic, environmental, or combined etiologies that prevent normal function of hearing, but, despite detailed investigation, the main cause remains usually unknown. Clinical and experimental studies have shown that ischemia contributes to several SNHL [2] , such as sudden sensoneural hearing loss, presbyacusis and noise-induced hearing loss. All of these SNHL can be related to alteration in blood flow [3] .
The aim of the study is finding a relationship between idiopathic SNHL and endothelial dysfunction.
52 patients affected by SNHL from March to April 2015 at the Unit of Otolaryngology of Campus Bio-Medico University of Rome were enrolled. The exclusion criteria were:
- Fluctuating cochlea-vestibular dysfunction suggestive of endolymphatic hydrops (history of vertigo with either fluctuating hearing loss, aural pressure or episodic tinnitus preceding the ISSHL episode);
- Cerebello-pontine angle pathology at MRI;
- History of otologic surgery;
- Head and/or neck trauma or barotrauma in the 10 weeks prior to audiological evaluation
- otitis media in the last 10 weeks;
- neurologic disorders predisposing to deafness;
- recent use of ototoxic medications;
- other major comorbidities (heart failure, severe pulmonary, hepatic or renal dysfunction).
All included patients underwent to audiological evaluation and to cardiovascular assessment with brachial artery FMD evaluation. The study was approved by the institutional ethics committee.
All of the patients underwent a standard pure tone audiometry and the hearing threshold at each frequency was calculated. The air conduction pure tone average was obtained by the air conduction at 250, 500, 1000, 2000, 4000, and 8000 Hz.
Noninvasive endothelial function assessment was performed with ultrasound detection of brachial artery reactivity to flow as previously reported [4] by a single operator. Patients were asked to abstain from smoking, drinking coffee or tea and from physical activity for 60 min, and to discontinue medications for 12 h before endothelial function assessment. Two-dimensional brachial artery imaging was obtained on the dominant forearm with the patient supine using a 10-MHz multifrequency linear array probe attached to a high-resolution ultrasound machine. Straight segments (8 to 10 mm in length) of the artery were identified above the antecubital fossa, perpendicular to the ultrasound beam and along its long axis. Flow-mediated dilation (FMD) due to shear-induced endothelial nitric oxide release was evaluated after occlusion of the forearm circulation. Briefly, a longitudinal image was selected to calculate brachial artery diameter (first baseline value), then a blood pressure cuff was inflated on the upper arm to 50 mm Hg above the systolic pressure for 5 min and then deflated. 1 min after cuff deflation, a second longitudinal scan was obtained, and brachial artery diameter was measured (post-occlusion value). FMD was expressed as percent diameter variation. Brachial artery diameters were measured from near-to-far blood-wall interface (intima-media interfaces) at end-diastole in the cardiac cycle (onset of the R-wave at ECG-gating during image acquisition). Five cardiac cycles were analyzed and averaged for each scan. Intraobserver variations were 1.25 ± 0.8% in 10 randomly selected patients with two repeated measurements. FMD values < 11% were considered as indicative of endothelial dysfunction [5] and [6] .
All data was taken from medical records of our institution and analyzed. All the authors were also treating physicians of the included patients. Statistical analysis was carried out using STATA/IC 12 (STATA Corp., College Station, Texas) and significance was defined as a p value < 0.05.
At the end of our selection process, 34 patients were prospectively included and retrospectively analyzed. Patients showed a median age of 48; (19–65 years).
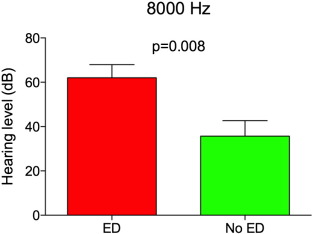
The patients with altered FMD showed a worst hearing level threshold for all evaluated frequencies, reaching statistical significance at 8000 Hz.
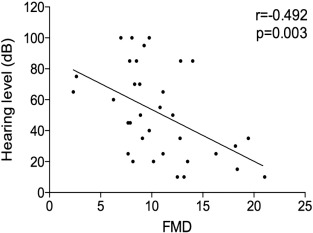
The degree of hearing loss, calculated at 8000 Hz, was significant correlated with flow mediated dilatation (FMD)(r = − 0.492 and p = 0.003)(Fig. 1 ). That is, patients with higher degrees of hearing loss had worst FMD (p < 0.05) (Fig. 2 ).
|
|
|
Fig. 1. Correlation between pure tone average and flow mediated dilatation (FMD). From this inferred a high statistical significance (r = + 0.492 and p = 0.003) |
|
|
|
Fig. 2. Hearing threshold in patients with (ED) and without (nED) endothelial dysfunction at 8000 Hz: it shows how those ones, who were positive in the FMD, demonstrating an endothelial disease, were also have positive for 8000 Hz hearing loss. Here there were also a significant correlation (p = 0.008). |
At multivariate analysis, FMD values were independently associated with pure tone average at 8000 Hz, even after adjustment for age, gender, BMI and cardiovascular risk factors (beta = − 3.138, p = 0.036.
Sensorineural hearing loss is a common ill-defined clinical condition. Several hypotheses have attempted to explain its pathogenesis. Cochlear blood flow impairment has been considered as one of the pathophysiological mechanism of SNHL.
The anterior inferior cerebellar artery (AICA), with its labyrinthine artery, is the supplier of the inner ear. It develops arterioles branch that radiate running centrifugally over the scala vestibule and across the spiral lamina. Eventually a centripetal system of radiating venules collects blood.
In idiophatic SNHL the basis of cochlea is typically involved, due to its tonotopic organization, known that the hair cell of basal turn of the cochlea are more sensitive to noxa pathogena then the apex [7] .
Basal outer hair cells are also more susceptible to free radical damage, because of the lower level of glutathione [8] .
Our preliminary data show a relationship between SNHL and endothelial dysfunction. Similar studies also confirm the role of endothelial dysfunction on other types of hearing loss [9] . This suggests a therapeutic opportunity for patients affected by progressive or early SNHL; in particular, the ameliorating of microcirculation could slow or stop the progression of the disease including omega-3 (omega-3) polyunsaturated fatty acids (PUFAs). It is known that omega-3 PUFAs improve both small and large arteries vasodilatation and compliance reducing the formation of angiotensin II and enhancing nitric oxide (NO) generation. An additional favorable effect on the microcirculation can be exerted by anti-inflammatory mediators, ameliorating the vascularization of small ear vessels that usually cause inner ear injury. If the correlation between SNHL and endothelial dysfunction is confirmed in studies on larger scale, SNHL without any evident hearing loss risk factors, especially for high frequencies, could be considered a marker of endothelial dysfunction and consequently identify subjects with a major cardiovascular risk.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] K.P. Steel; New interventions in hearing impairment; BMJ, 320 (2000), pp. 622–625
- [2] P.R. Thorne, A.L. Nuttall; Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig; Hear. Res., 27 (1987), pp. 1–10
- [3] M.D. Seidman, W.S. Quirk, N.A. Shirwany; Mechanisms of alterations in the microcirculation of the cochlea; Ann. N. Y. Acad. Sci., 884 (1999), pp. 226–232
- [4] G. Patti, V. Pasceri, R. Melfi, C. Goffredo, M. Chello, et al.; Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation; Circulation, 111 (2005), pp. 70–75
- [5] K.E. Pyke, M.E. Tschakovsky; The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function; J. Physiol., 568 (2005), pp. 357–369
- [6] M.C. Corretti, T.J. Anderson, E.J. Benjamin, D. Celermajer, F. Charbonneau, et al.; Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force; J. Am. Coll. Cardiol., 39 (2002), pp. 257–265
- [7] T. Nakashima, S. Naganawa, M. Sone, M. Tominaga, H. Hayashi, et al.; Disorders of cochlear blood flow; Brain Res. Brain Res. Rev., 43 (2003), pp. 17–28
- [8] F. Scheibe, H. Haupt, C. Ludwig; Intensity-related changes in cochlear blood flow in the guinea pig during and following acoustic exposure; Eur. Arch. Otorhinolaryngol., 250 (1993), pp. 281–285
- [9] M.M. Ciccone, F. Cortese, M. Pinto, C. Di Teo, F. Fornarelli, et al.; Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss; Atherosclerosis, 225 (2012), pp. 511–516
Document information
Published on 19/07/16
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?