Highlights
- BCG was associated with lower odds on stunting when given early in life and with higher odds when given later in infancy.
- Similar associations were found for DTP1 and measles vaccination and with hemoglobin concentration as outcome.
- Time-dependency of non-specific effects provides a new perspective in the field although confirmation is required.
Vaccines possess effects on health beyond their target disease, so called “non-specific effects”. Our results suggest that timing of vaccination may be important for these effects. In African children, BCG vaccination was associated with lower odds on stunting when given in the first month of life and higher odds on stunting when given later in infancy. Comparable time patterns were found for DTP1 and measles vaccination and with hemoglobin concentration as outcome. Findings were made possible by a Big Data design, using data on 368,450 African children that varied considerably in vaccination times and background characteristics. These results, if confirmed in further research, may provide a fundamental new perspective on the non-specific effects of vaccines.
Abstract
Background
Bacillus Calmette-Guérin (BCG) vaccination possesses effects on health beyond its target disease, the so called “non-specific effects”. We evaluate these effects, as well as the effect of timing of BCG and other vaccinations, on stunting in Sub-Saharan African (SSA) children under five.
Methods
We use a Big Data design, including cross-sectional data for 368,450 children from 33 SSA countries. Logistic regression analysis is used with control factors at child, mother, household and context level.
Results
Overall, BCG vaccination did not affect stunting in SSA children (OR 1.00 [0.98–1.03]). Timing of BCG vaccination was of importance (βtime = 0.067 [0.061–0.073]): compared to unvaccinated children, BCG was associated with lower odds on stunting for children vaccinated early in life (OR 0.92 [0.89–0.94]) and higher odds for children vaccinated later in infancy (OR 1.64 [1.53–1.76]). Similar findings were done for diphtheria-tetanus-pertussis (DTP)1 and measles vaccination, and when hemoglobin concentration was used as outcome variable.
Conclusions
We found a general time-dependent pattern of non-specific effects of vaccination, with positive associations for vaccinations given early in life and negative associations for vaccinations given later in infancy. If confirmed in further research, our findings may provide a new perspective on the non-specific effects of vaccination.
Keywords
Non-specific effects ; Vaccination ; Stunting ; Time-dependency ; Policy
1. Introduction
Live attenuated vaccines, such as Bacillus Calmette-Guérin (BCG), possess beneficial non-specific effects outside the scope of their target disease that have been associated with lower mortality rates (WHO, 2014a , Nankabirwa et al., 2015 , Garly et al., 2003 and Aaby et al., 2011 ). Vaccination with BCG increases immune responses and protects against unrelated pathogens (Kleinnijenhuis et al., 2012 , Kleinnijenhuis et al., 2014 , Jensen et al., 2015 and Clark et al., 1976 ), possibly through a combination of trained immunity, induced by epigenetic reprogramming of innate immune cells (Kleinnijenhuis et al., 2012 ), and heterologous T-helper (Th)1/Th17 immunity (Kleinnijenhuis et al., 2014 ). These general immune modulatory and antimicrobial effects of BCG may also affect stunting. Stunting reflects failure to reach linear growth potential in the early years of life and has a highly multifactorial etiology that includes nutritional factors, infectious diseases, and socio-economic factors (reviewed in Prendergast and Humphrey, 2014 ). Associations between several infectious diseases and stunting have been established, whereby the most profound effect is seen for diarrheal infections (Checkley et al., 2008 ). In addition to wasting of nutrients, infection-related inflammation could lead to stunting through down-regulation of insulin-like growth factor 1 (IGF-1) expression (Prendergast et al., 2014 ).
Stunting affects approximately 165 million children under five, many of whom live in Africa (36.5%) (Black et al., 2013 ) where stunting rates in many areas are over 40% (Global Data Lab, 2016 ). Besides being associated with an increased mortality, stunting has negative long-term effects on cognitive and psychosocial development (Crookston et al., 2011 ), school performance (Martorell et al., 2010 ), and economic productivity (Hoddinott et al., 2008 ). It is therefore an important outcome to establish the total impact of non-specific effects of vaccination on health status. Recently, the World Health Organization (WHO) has made a 40% reduction of stunting in children under five before 2025 a global target, underlining its importance (WHO, 2014b ).
The beneficial non-specific effects of BCG suggest that this vaccine may be used to reduce stunting in low- and middle-income countries. If BCG vaccination indeed reduces stunting, there would be substantial potential for improvement, as coverage of BCG vaccination has stalled at 77–85% in Africa since 2009 (WHO, 2014c ). Apart from vaccination coverage, timing of vaccination may be important, as the childs immune function changes with age (Kollmann et al., 2012 ) and early immunization had stronger consequences related to non-specific effects in one study (Aaby et al., 2011 ). This is important knowing that timing of vaccinations still differs widely between and within low- and middle-income countries (Clark and Sanderson, 2009 ).
We aim to study the overall effect of BCG and other vaccinations on stunting, as well as the role of timing of vaccination, on a newly developed database including data on 368,450 children under five living in 33 Sub-Saharan African countries. Given the significant variation in coverage and timing of vaccination in the daily setting in Sub-Saharan Africa, our data make it possible to study the overall effect, as well as the effect of the timing of vaccination in a much broader setting than in earlier research. To gain insight into the scope of our findings, we repeated our analyses for other vaccinations (diphtheria-tetanus-pertussis [DTP] and measles vaccine [MV]) and for another outcome (hemoglobin concentration).
2. Methods
2.1. Study Population
This study used retrospective and cross-sectional data from the Demographic and Health Surveys (DHS). DHS household surveys have been conducted in many low-income countries since the 1980s, collecting demographic, health, and nutritional indicators (www.dhsprogram.org ). Each DHS consists of a household survey and separate womens and mens surveys. In the womens survey, all usual resident women aged 15 to 49 are invited for an oral interview which includes a complete birth history with detailed questions, including retrospective vaccination and stunting information, on the children born in the last five years. The team executing the DHS ensures protection of human subjects in agreement with local and international laws.
A combined dataset was derived from the Database Developing World (DDW, www.globaldatalab.org ) that included all available DHS (n = 76) for Sub-Saharan African countries (n = 33) between 1998 and 2014 that included BCG vaccination and height-for-age measurement. Included were all children aged 1–60 months for whom valid information was available on stunting and BCG vaccination before age 12 months. Children aged one month or less were excluded, because of the difficulty of differentiating between stunting and fetal growth failure and of interpreting the effect of vaccination. The cut-off at 12 months for BCG vaccination was based on the importance of children being vaccinated before this point in life. There were 419,167 children aged 1–60 months in the data, of whom 50,717 were excluded because no information on stunting or BCG vaccination was available. The final study population, therefore, consisted of 368,450 children. To obtain national representative samples, the data were adjusted according to weights provided by DHS. These weights were recoded to a mean of one for all analyses performed, so that their application did not increase the sample size.
2.2. Core Measures
BCG vaccination was retrieved from vaccination cards upon visit. This was done for 183,476 of the 368,450 children (49.8%). For the 184,974 children (50.2%) of whom no card was available or vaccination was not recorded, the mother was asked whether the child was vaccinated. Timing of vaccination was calculated by subtracting the date of birth from the date of vaccination. The analysis of timing of vaccination was restricted to 171,075 children (46.4%) with valid dates on a vaccination card.
To obtain information on stunting, length or height was measured in centimeters to a precision of 1 decimal with a Shorr measuring board following a standardized DHS protocol (ICF/DHS, 2012 ). The measurement was voluntary and was not performed when the parent refused or the child was too sick. The assignment of anthropometric z-scores was performed by the DHS program (Rutstein and Rojas, 2006 ) based on the WHO Child Growth Standards (WHO. Multicentre Growth Reference Study Group, 2006 ). Children with scores ≤−2 were considered stunted.
We performed additional analyses using hemoglobin concentration as outcome measure. This was measured in 47 of the 76 DHS surveys, which resulted in a study population of 149,868 children aged 6–60 months. This age range differs from that for stunting, since fetal hemoglobin is usually replaced by adult hemoglobin at the age of 6 months. To measure hemoglobin concentration, the blood of the children was obtained through a finger or heel prick. Consent to draw a droplet of blood was asked after reading a consent statement to the parent or responsible adult. Obtained blood was collected in a micro-cuvette and analyzed with a battery operated portable Hemocue analyzer. Hemoglobin concentration was measured in g/dL with a precision of 1 decimal (ICF/DHS, 2012 ). To exclude outliers and input errors, the lowest and highest 0.1% of the hemoglobin concentrations were excluded from the analyses.
2.3. Important Covariates
We included covariates at the level of the child, the mother, the household, and the context. Inclusion of covariates was based on literature, expert opinion and availability in DHS data. Child characteristics included age (in months), sex, other vaccinations, birth order, preceding birth interval, twin status (singleton or multiple birth), size at birth (very small, smaller than average, average, larger than average, very large), and vitamin A supplementation (Benn et al., 2010 and Varela-Silva et al., 2009 ). Age of the child was supplemented with age squared to control for non-linearity in the relationship between age and stunting. Characteristics of the mother were age (in years), height z-scores, body mass index (BMI, in kg/m2 ), breastfeeding for 24 months, place of delivery (home, public hospital, private hospital, other), education (highest level), and marital status (married or living together, widowed, divorced or not living together) (Corsi et al., 2016 ). At the household level, the place of residence (rural, urban) and household wealth were included. Household wealth was measured by the International Wealth Index, a cross-nationally comparable index, running from 0 to 100, based on the households possession of consumer durables (TV, fridge, car etc.), housing characteristics and access to basic services (Smits and Steendijk, 2015 ). Geographical information regarding sub-national district level was used to control for differences in context in which the households were living. Within the 33 countries, 285 districts were distinguished. To address missing values in the covariates, the dummy variable adjustment procedure was used (Allison, 2001 ). There were no missing values in the core measures.
2.4. Statistical Analysis
To determine the effects of vaccination on stunting, logistic regression analysis was performed. For the analysis with hemoglobin concentrations as dependent variable, linear regression was used. All analyses were controlled for the abovementioned covariates at the level of the child, the mother, and the household. The analysis of timing of vaccination was performed for each vaccination separately, but included controls for vaccination status with regard to the other vaccinations. To control for variation in the context in which the children lived, a fixed effects design (Wooldridge, 2013 ) was used for all regression models, whereby dummy variables for the 285 sub-national regions and for the survey years were included. In this way, all (measured and unmeasured) context factors related to stunting at the regional level are controlled for.
The effect of timing of BCG vaccination was studied with both a continuous and a categorical variable. The categorical time variable consisted of the categories “before 1 month”, “months 1–2.99”, “months 3–4.99”, “months 5–6.99”, “months 7–11.99”, “time unknown”, and “not vaccinated” (reference). Because MV is usually given at 9 months of age, there were few cases of MV in the early time frame. We therefore increased the upper age limit for this analysis to 15 months and chose the categories for MV as “before 6 months”, “months 6–7.99”, “months 8–9.99”, “months 10–11.99”, “months 12–14.99”, “time unknown”, and “not vaccinated” (reference).
The independence of the BCG and DTP1 findings was tested in several sensitivity analyses: by analyzing the effects of BCG for children who had not received DTP1, by analyzing the effects of DTP1 for children who had not received BCG and by testing for interaction between the effects of early BCG and DTP1 vaccination.
All analyses were performed in SPSS Statistics V22.0 (IBM, NY, USA) and P = 0.05 was taken as a threshold for significance.
3. Results
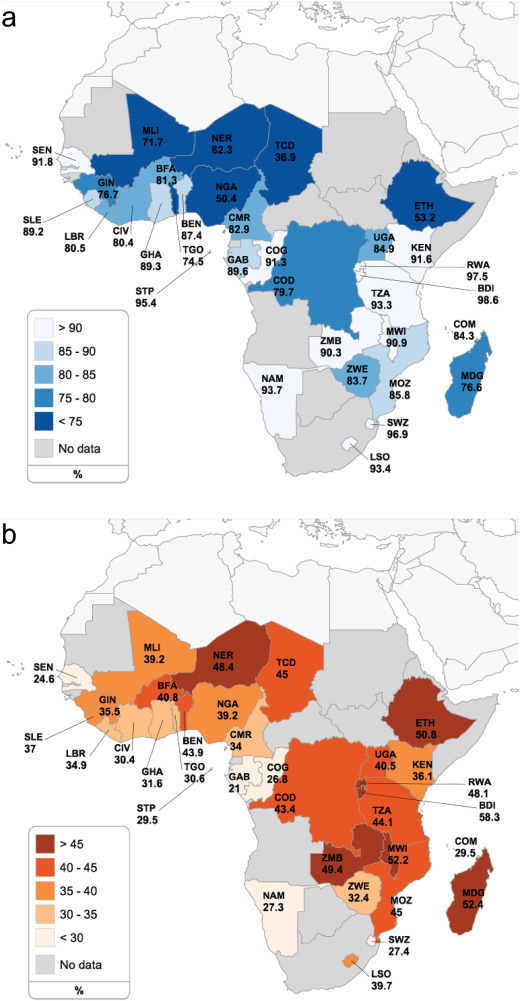
Of the 368,450 children included, 287,552 (78.0%) had received vaccination with BCG and 151,332 children (41.1%) had a length- or height-for-age z-score (LAZ/HAZ) of two standard deviations below the median and were classified as being stunted. Both BCG coverage and stunting levels differed widely across the 33 Sub-Saharan countries (Fig. 1 a, b and Supplementary Table 1). Baseline characteristics of children and mothers are present in Supplementary Tables 2 and 3.
|
|
|
Fig. 1. Prevalence of BCG vaccination and stunting in 33 Sub-Saharan African countries. Geographical distribution for BCG vaccination coverage (a) and stunting (b) among children aged 1–60 months in Sub-Saharan Africa. BDI: Burundi, BEN: Benin, BFA: Burkina Faso, CIV: Cote d'Ivoire, CMR: Cameroon, COD: Democratic Republic of Congo, COG: Congo, COM: Comoros, ETH: Ethiopia, GAB: Gabon, GHA: Ghana, GIN: Guinea, KEN: Kenya, LBR: Liberia, LSO: Lesotho, MDG: Madagascar, MLI: Mali, MOZ: Mozambique, MWI: Malawi, NAM: Namibia, NER: Niger, NGA: Nigeria, RWA: Rwanda, SEN: Senegal, SLE: Sierra Leone, STP: Sao Tome and Principe, SWZ: Swaziland, TCD: Chad, TGO: Togo, TZA: Tanzania, UGA: Uganda, ZMB: Zambia, ZWE: Zimbabwe. |
In the group vaccinated with BCG, 113,786 (39.6%) of the children was stunted, compared to 37,548 (46.4%) in the unvaccinated group, resulting in a crude odds ratio (OR) of 0.76 [95% CI: 0.74–0.77] in favor of BCG vaccination. After correction for relevant child, maternal, social-economic, and context factors the association with BCG vaccination disappeared (OR 1.00 [0.98–1.03]) (Supplementary Table 4).
3.1. Timing of Vaccination
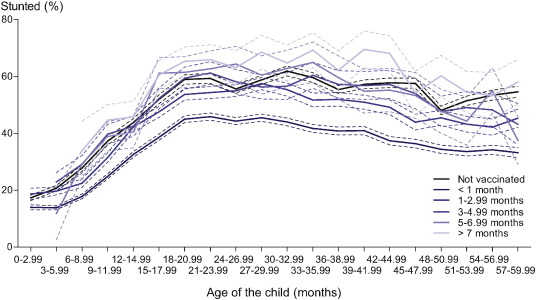
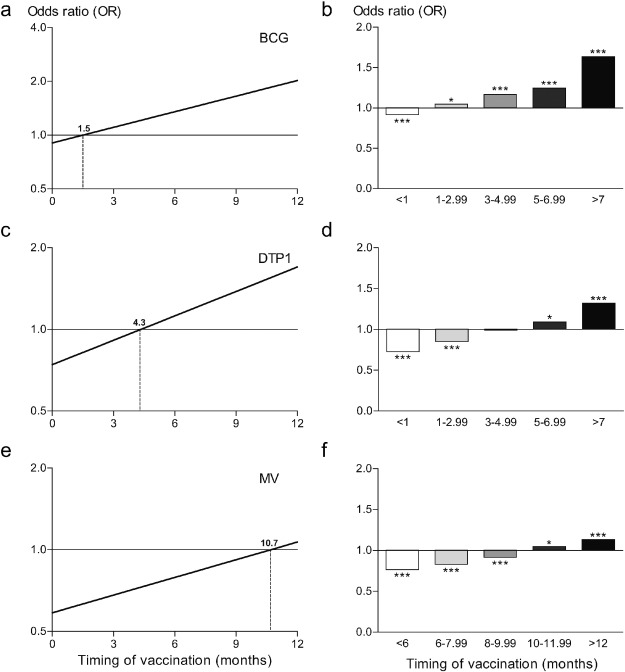
There was a clear time-dependency of the association between BCG vaccination and stunting within the first year of life (βtime = 0.067 [0.061–0.073]). As expected, we found children who received a BCG vaccination early in life to be less stunted than children without BCG vaccination. However, rather unexpected, we found children who received the BCG vaccination later in infancy to be more stunted than children without BCG vaccination (Fig. 2 ). The association shifted from a reduced odds on stunting to an increased odds on stunting at 1.5 months (Fig. 3 a). Broken down into relevant time periods, vaccination with BCG was associated with a lower odds on stunting when given before 1 month of age (OR 0.92 [0.89–0.94]). In contrast, vaccination with BCG was associated with increased odds on stunting when given between 1–2.99, 3–4.99 or 5–6.99 months of age (ORs 1.05 [1.01–1.09], 1.17 [1.11–1.23] and 1.25 [1.16–1.34], respectively) and with the highest odds on stunting when given after 7 months of age (OR 1.64 [1.53–1.76]) (Fig. 3 b). There was no indication of selection with respect to the children without vaccination cards, as this group had a coefficient similar to the group with vaccination cards (ORdifference 0.995 [0.977–1.013]). Repeating the analyses using HAZ as outcome measure instead of the dichotomous stunting variable, resulted in a similar time-dependent association (Supplementary Fig. 1).
|
|
|
Fig. 2. Relation between stunting and age for different categories of timing of BCG vaccination. The non-linearity of the relation between percentage of stunted children and age is shown with a leveling of the percentage of stunted children around month 15. Compared to BCG unvaccinated children, lower percentages of stunted children are seen for early BCG vaccination, while higher percentages of stunted children are seen for late BCG vaccination. |
|
|
|
Fig. 3. Time-dependent effects of vaccination on stunting. Time-dependency of vaccination with BCG (a and b), DTP1 (c and d) and MV (e and f) is shown for odds on stunting in Sub-Saharan African children aged 1–60 months. Timing as continuous variable showed a positive relation to odds on stunting for all vaccines (a, c and e) with shifts from reduced odds ratios to increased odds rations at 1.5 months (BCG), 4.3 months (DTP1) and 10.7 months (MV) compared to unvaccinated children for the specified vaccine as indicated by the dashed vertical lines. Broken down into relevant time periods the same trend is visible (b, d and f). *P < 0.05, ***P < 0.001 compared to unvaccinated children. A 2 log scale is used for the ordinates in a, c and e. |
3.2. Other Vaccinations
To explore whether the findings were restricted to BCG vaccination, or reflect a more general timing effect of vaccination, we examined the effects of other vaccinations in our data.
For DTP1, a similar time-dependent pattern was observed as for BCG (βtime = 0.069 [0.063–0.076]), although the shift from a reduced odds to an increased odds on stunting occurred somewhat later at 4.3 months (Fig. 3 c). This time-dependent association was also seen with HAZ as outcome measure (Supplementary Fig. 1). Broken down into the same time periods as BCG, the association between DTP1 and stunting showed many similarities to the BCG pattern. DTP1 vaccination was associated with the lowest odds on stunting when given before 1 month of age (OR 0.73 [0.68–0.77]). Vaccinations with DTP1 given later in infancy were associated with an increased odds on stunting (ORs of 1.09 [1.03–1.16] and 1.32 [1.24–1.41] between 5–6.99 months and after 7 months of age, respectively) (Fig. 3 d).
For MV vaccination we found a time-dependent association as well (βtime = 0.050 [0.043–0.057]) that shifted from a reduced odds to an increased odds on stunting at 10.7 months (Fig. 3 e). Again, the time-dependent association could be reproduced using HAZ as outcome measure (Supplementary Fig. 1). However, as this vaccine is scheduled later in life, the number of children who received this vaccination in the early months of life was small, making a direct comparison with BCG and DTP1 difficult. Nevertheless, the same tendency was seen with the lowest odds on stunting in the earliest category (< 6 months OR 0.78 [0.71–0.82]) and the highest odds on stunting in the latest category (> 12 months OR 1.13 [1.08–1.19]) (Fig. 3 f).
Since children who receive a BCG vaccination often receive DTP1 vaccination as well, the time-dependent association might be caused by only one of the vaccinations. We therefore analyzed the association between BCG and stunting for children who had not received DTP1 and the association between DTP1 and stunting for children who had not received BCG. These sensitivity analyses displayed the same time-dependency of the association, showing the robustness of the findings (Supplementary Fig. 2 and Supplementary Tables 5 and 6). When studying the timing associations of BCG and DTP1 vaccinations simultaneously, the coefficients were mildly attenuated. However, the time-dependency remained clearly visible (Supplementary Fig. 3 and Supplementary Tables 7 and 8). Moreover, we performed an interaction analysis to find out whether combining early vaccination with BCG and DTP1 would either strengthen or attenuate their separate associations. This interaction analysis revealed a similar association as the additive model without an interaction term (β − 0.268 vs. − 0.268) (Supplementary Tables 7 and 9).
3.3. Hemoglobin Concentration
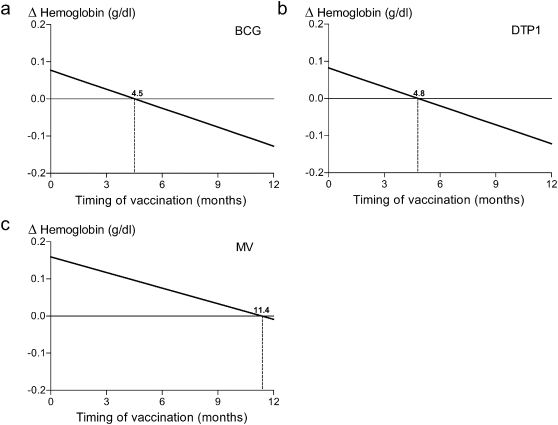
Subsequently, we tested the time associations on the childs hemoglobin concentration, another marker that mirrors the overall wellbeing of the child and is also influenced by infections and inflammation (Weiss and Goodnough, 2005 ). Knowing that this marker is more reversible than stunting, it provides the opportunity to study prolonged effects of vaccination. Consistently, a similar association and time pattern of BCG vaccination as for stunting was found for hemoglobin concentration (βtime = − 0.017 [− 0.024–0.011]) (Fig. 4 a). Association with a higher hemoglobin concentration was seen for vaccination before 1 month of age (β = 0.079 [0.045–0.112]), while vaccination given more than 7 months after birth tended to be associated with a lower hemoglobin concentration (β = − 0.074 [− 0.151–0.004]). DTP1 as well as MV showed similar results for hemoglobin concentration, with decreasing concentrations upon delayed vaccination (DTP1 βtime = − 0.017 [− 0.024–0.009]; MV βtime = − 0.014 [− 0.021–0.007]) (Fig. 4 b, c).
|
|
|
Fig. 4. Time-dependent effects of vaccination on hemoglobin concentrations. Time-dependency of vaccination with BCG (a), DTP1 (b) and MV (c) is shown for alterations in hemoglobin concentrations in Sub-Saharan African children aged 6–60 months. Timing as continuous variable showed a negative relation to hemoglobin concentrations for all vaccines (a, b and c) with shifts from improved concentrations to decreased concentrations at 4.5 months (BCG), 4.8 months (DTP1) and 11.4 months (MV) compared to unvaccinated children for the specified vaccine as indicated by the dashed vertical lines. |
4. Discussion
In this study we found evidence that the timing of BCG vaccination within the first year of life might be important in determining the size and direction of its non-specific effects on stunting and hemoglobin concentration. Early vaccination reduced the odds on stunting (OR 0.92 [0.89–0.94]), while vaccination later in infancy increased the odds on stunting (OR 1.64 [1.53–1.76]), compared to unvaccinated children. The positive associations of early vaccination, as well as the negative associations of vaccination later in infancy were also seen for hemoglobin concentration. Similar patterns were observed for vaccination with DTP1 and MV.
Our results are in line with prior studies that examined the role of vaccines on child morbidity and mortality. Vaccination with BCG, which is given early in the EPI schedule (WHO, 2015 ), is associated with reduced child mortality (WHO, 2014a , Nankabirwa et al., 2015 , Garly et al., 2003 and Aaby et al., 2011 ). DTP vaccinations are generally not associated with such an effect and may even be deleterious (WHO, 2014a ). Our study indicates that this may be related to the timing of vaccination. This finding is supported by studies that show detrimental effects of DTP vaccination when given after or in combination with MV (late vaccination) (Aaby et al., 2003 and Aaby et al., 2007 ), while beneficial effects are reported for early DTP vaccination (Vaugelade et al., 2004 and Lehmann et al., 2005 ). Similar to our findings, combining BCG and DTP1 vaccination was reported to be the most beneficial in several studies (Aaby et al., 2015a and Hirve et al., 2012 ). In case of MV, vaccination at 4–8 months was also associated with lower child mortality than vaccination at 9–11 months (Aaby et al., 2015b ). Regarding child morbidity, BCG vaccination influenced acute lower respiratory tract infections (ALRI) in a time-dependent manner, in a study by Hollm-Delgado et al. (2014) . However, while these authors ascribed this effect to concurrent DTP administration, our present data suggest that the timing of BCG vaccination itself is crucial.
An underlying mechanism for the time-dependent association of both BCG and DTP1 vaccination could lie in the different functional status of the neonatal and child immune system. The neonatal immune system has an anti-inflammatory profile mirroring that of the mother during pregnancy, while the child immune system shifts to a more pro-inflammatory profile, increasingly reflecting that of adults (reviewed in Kollmann et al., 2012 ). It may be hypothesized that when vaccines are given early in life, induction of both specific and non-specific effects results in a more balanced immune reactivity, while when vaccines are given later they trigger a stronger pro-inflammatory response upon encountering a pathogen. On the other hand, unvaccinated neonates may be less able to cope with repeated infections after environmental exposure, leading to prolonged inflammation. As inflammation is associated with down regulation of the expression of growth hormone (GH)/IGF-1, prolonged or increased inflammation negatively influences linear growth, as well as erythropoiesis (Prendergast et al., 2014 and Boyer et al., 1992 ). This would explain the benefits seen for stunting and hemoglobin concentration in children vaccinated early, but not late, in life compared to unvaccinated children, although this hypothesis requires experimental and clinical confirmation in future studies.
We used a Big Data design, including information on 368,450 African children showing much more variation in timing of vaccination and living situation than children in high-income countries. In this way we were able to study effects of timing of vaccination in a much broader perspective than has been possible in earlier research. The smaller observational studies that are usually done, lack the power and variation in timing necessary to detect these effects, while advancing or postponing vaccination in clinical trials might be deemed unethical.
Limitations of the study lie within its observational character. Although we controlled for confounding on several levels, it cannot be excluded that residual confounding is present. For example, it cannot be precluded that part of our results are due to variation in maternal competency. Mothers that have their children vaccinated tend to be better mothers and caretakers for their children. This is indeed visible in the factors we controlled for during analysis (Supplementary Table 3). Proxy variables regarding the childs health show that early vaccinated children received more other vaccinations and vitamin A, were more often delivered in a hospital and had higher educated mothers than unvaccinated children. However, this does not hold for all proxy variables, since unvaccinated children were most often breastfed, an important nutritional factor. Moreover, maternal competency does not explain why children who are vaccinated later in infancy do worse than unvaccinated children, as also the late vaccinated children outperformed unvaccinated children on the abovementioned factors. The worse situation for children vaccinated later in infancy can neither be explained by more distant proxies. BMI of the mother and the position of the child in the family are better for late vaccinated children. This makes maternal competency as an explanation less likely. Given that the factors mentioned in Supplementary Table 3 were all used as control factors in our models, it does not seem likely that our results are influenced by the differences described here. However, it cannot be ruled out completely that our results have been influenced by other aspects of maternal competency which were not available in our data.
A second limitation is the incompleteness of survey data. The time-dependency of the non-specific effect of BCG vaccination is based on vaccination dates of only 46.4% of the children. Our results do not suggest differences between the groups with and without vaccination dates (OR 0.995 [0.977–1.013]). However, non-random missing of vaccination dates among the different timing of vaccination groups could potentially influence the results. For example, we observed that the childs median age increased over the timing of vaccination groups and that vaccination data for older children were less likely to have originated from a card. These observations indicate that groups with a later timing of vaccination have relatively more missing dates of BCG vaccination. However, the households of older children where a vaccination card was available are probably the better organized households. Furthermore, the data covers only living children, which given the high child mortality rates in Sub-Saharan Africa means that the ones who received their vaccinations at a later time are on average a stronger population. In both cases, the impact of non-random missing would be expected to have led to an attenuation rather than an aggravation of the timing effect.
Other possible disturbing factors include differences in acquisition of the outcome measures and inter-regional variations. Differences in acquisition have been minimized by the use of objective ascertainment of vaccination dates (vaccination cards) and height measurements and the fact that during collection of the data, collectors were not aware of any study that was going to be conducted with the outcomes. Inter-regional variation was addressed by including fixed-effects dummies at the level of 285 sub-national areas, which effectively control for all stunting-related risk factors that vary among these areas.
Although definitive causal relationships cannot be drawn from our cross-sectional data, the uniformity of the findings across different vaccines and two outcome measures, the plausibility of the underlying hypothesis, as well as the consistency with results of previous studies suggests that the timing of vaccinations is important for their overall health effects.
If confirmed in further research, our findings may provide an interesting new perspective on the non-specific effects of vaccination and aid global efforts to reduce stunting and related negative health outcomes. Firstly, with regard to stunting, it seems that full and early coverage of vaccinations could substantially reduce stunting in low- and middle-income countries. A simulation analysis (Supplementary Table 10 and accompanying text) indicates that if all children in this study would have received BCG and DTP1 vaccination before 1 month of age, a decrease in stunting of approximately 10% could be achieved. Secondly, improvement of timing of vaccinations may be reached with relatively small and achievable adaptations to the existing vaccination practice. Presently, multi-dose BCG vaccine vials are provided together with the instructions that a certain minimal percentage of the dosages should be administered, which limits health care workers in vaccinating small numbers of children (Hutchins et al., 1993 ). Using smaller multi-dose or single dose vials could overcome this problem and in certain situations is even the most cost-effective option (Dhamodharan and Proano, 2012 ). Lastly, the effects need not be limited to Africa, since stunting is a major health problem in several other world regions with suboptimal coverage and timing of vaccination, such as Latin America and South Asia (Black et al., 2013 and Clark and Sanderson, 2009 ). Extending the interventions to these regions could further contribute to the Global Target set by the WHO to reduce stunting with 40% by 2025.
5. Conclusion
In conclusion, our data indicate that timing of vaccinations plays a role in determining the strength and direction of their non-specific effects on two important health indicators: linear growth and hemoglobin concentration. If confirmed in further epidemiological and immunological research, these findings may shed a new light on the non-specific effects of vaccinations and may have consequences for vaccination programs and schedules worldwide.
Author Contributions
The study was designed by MLTB, JS, MGN and AvdV. MLTB performed the database preparation. Data analysis was performed by MLTB and JS. All authors contributed in preparing the manuscript.
Conflict of Interest
None.
Funding
This work was not supported by any funding agency. MGN is supported by a Vici grant of the Netherlands Organization for Scientific Research and an ERC Consolidator Grant (nr. 310372). These organizations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Reporting
The data were obtained from the DHS program (www.dhsprogram.com ) under the condition that the authors do not pass the data to other researchers. However, other researchers can apply directly to the DHS program to obtain the data.
Ethical Approval
The team executing the DHS ensures protection of human subjects in agreement with local and international laws.
Acknowledgements
The manuscript represents valid work and neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publication elsewhere. The content of the work presented here is solely the responsibility of the authors and does not necessarily represent the official views of any health organizations. The authors thank the Demographic and Health Surveys program (www.dhsprogram.org ) for generously providing the data for this study.
Appendix A. Supplementary data
Supplementary figures and tables.
References
- Aaby et al., 2003 P. Aaby, H. Jensen, B. Samb, et al.; Differences in female–male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria–tetanus–pertussis and inactivated poliovirus: reanalysis of West African studies; Lancet, 361 (9376) (2003), pp. 2183–2188
- Aaby et al., 2007 P. Aaby, S. Biai, J.E. Veirum, et al.; DTP with or after measles vaccination is associated with increased in-hospital mortality in Guinea-Bissau; Vaccine, 25 (7) (2007), pp. 1265–1269
- Aaby et al., 2011 P. Aaby, A. Roth, H. Ravn, et al.; Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period?; J. Infect. Dis., 204 (2) (2011), pp. 245–252
- Aaby et al., 2015a P. Aaby, J. Nielsen, C.S. Benn, J.F. Trape; Sex-differential and non-specific effects of routine vaccinations in a rural area with low vaccination coverage: an observational study from Senegal; Trans. R. Soc. Trop. Med. Hyg., 109 (1) (2015), pp. 77–84
- Aaby et al., 2015b P. Aaby, C.L. Martins, H. Ravn, A. Rodrigues, H.C. Whittle, C.S. Benn; Is early measles vaccination better than later measles vaccination?; Trans. R. Soc. Trop. Med. Hyg., 109 (1) (2015), pp. 16–28
- Allison, 2001 P. Allison; Missing Data; Sage Publications Ltd., London (2001)
- Benn et al., 2010 C.S. Benn, A.B. Fisker, B.M. Napirna, et al.; Vitamin A supplementation and BCG vaccination at birth in low birth weight neonates: two by two factorial randomised controlled trial; BMJ, 340 (2010), p. c1101
- Black et al., 2013 R.E. Black, C.G. Victoria, S.P. Walker, et al.; Maternal and child undernutrition and overweight in low-income and middle-income countries; Lancet, 382 (9890) (2013), pp. 427–451
- Boyer et al., 1992 S.H. Boyer, T.R. Bishop, O.C. Rogers, A.N. Noyes, L.P. Frelin, S. Hobbs; Roles of erythropoietin, insulin-like growth factor 1, and unidentified serum factors in promoting maturation of purified murine erythroid colony-forming units; Blood, 80 (10) (1992), pp. 2503–2512
- Checkley et al., 2008 W. Checkley, G. Buckley, R.H. Gilman, et al.; Multi-country analysis of the effects of diarrhoea on childhood stunting; Int. J. Epidemiol., 37 (4) (2008), pp. 816–830
- Clark and Sanderson, 2009 A. Clark, C. Sanderson; Timing of childrens vaccinations in 45 low-income and middle-income countries: an analysis of survey data; Lancet, 373 (9674) (2009), pp. 1543–1549
- Clark et al., 1976 I.A. Clark, A.C. Allison, F.E. Cox; Protection of mice against Babesia and Plasmodium with BCG ; Nature, 259 (5541) (1976), pp. 309–311
- Corsi et al., 2016 D.J. Corsi, I. Mejía-Guevara, S.V. Subramanian; Risk factors for chronic undernutrition among children in India: estimating relative importance, population attributable risk and fractions; Soc. Sci. Med., 157 (2016), pp. 165–185 (Nov 14. pii: S0277-9536(15)30222-7)
- Crookston et al., 2011 B.T. Crookston, K.A. Dearden, S.C. Alder, et al.; Impact of early and concurrent stunting on cognition; Matern. Child Nutr., 7 (4) (2011), pp. 397–409
- Dhamodharan and Proano, 2012 A. Dhamodharan, R.A. Proano; Determining the optimal vaccine vial size in developing countries: a Monte Carlo simulation approach; Health Care Manag. Sci., 15 (3) (2012), pp. 188–196
- Garly et al., 2003 M.L. Garly, C.L. Martins, C. Balé, et al.; BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG?; Vaccine, 21 (21 − 22) (2003), pp. 2782–2790
- Global Data Lab, 2016 Global Data Lab; Sub-national area database: stunting figures; (2016) Available from: www.globaldatalab.org/areadata (Website accessed April 10, 2016)
- Hirve et al., 2012 S. Hirve, A. Bavdekar, S. Juvekar, C.S. Benn, J. Nielsen, P. Aaby; Non-specific and sex-differential effects of vaccinations on child survival in rural western India; Vaccine, 30 (50) (2012), pp. 7300–7308
- Hoddinott et al., 2008 J. Hoddinott, J.A. Maluccio, J.R. Behrman, R. Flores, R. Martorell; Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults; Lancet, 371 (9610) (2008), pp. 411–416
- Hollm-Delgado et al., 2014 M.G. Hollm-Delgado, E.A. Stuart, R.E. Black; Acute lower respiratory infection among Bacille Calmette-Guerin (BCG)-vaccinated children; Pediatrics, 133 (1) (2014), pp. e73–e81
- Hutchins et al., 1993 S.S. Hutchins, H.A. Jansen, S.E. Robertson, P. Evans, R.J. Kim-Farley; Studies of missed opportunities for immunization in developing and industrialized countries; Bull. World Health Organ., 71 (5) (1993), pp. 549–560
- ICF/DHS, 2012 ICF/DHS; Biomarker Field Manual; ICF International/Demographic and Health Surveys, Calverton, Maryland (2012)
- Jensen et al., 2015 K.J. Jensen, N. Larsen, S. Biering-Sorensen, et al.; Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial; J. Infect. Dis., 211 (6) (2015), pp. 956–967
- Kleinnijenhuis et al., 2012 J. Kleinnijenhuis, J. Quintin, F. Preijers, et al.; Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes; Proc. Natl. Acad. Sci., 109 (43) (2012), pp. 17537–17542
- Kleinnijenhuis et al., 2014 J. Kleinnijenhuis, J. Quintin, F. Preijers, et al.; Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity; J. Innate Immun., 6 (2) (2014), pp. 152–158
- Kollmann et al., 2012 T.R. Kollmann, O. Levy, R.R. Montgomery, S. Goriely; Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly; Immunity, 37 (5) (2012), pp. 771–783
- Lehmann et al., 2005 D. Lehmann, J. Vail, M.J. Firth, N.H. de Klerk, M.P. Alpers; Benefits of routine immunizations on childhood survival in Tari, Southern Highlands Province, Papua New Guinea; Int. J. Epidemiol., 34 (1) (2005), pp. 138–148
- Martorell et al., 2010 R. Martorell, B.L. Horta, L.S. Adair, et al.; Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low- and middle-income countries; J. Nutr., 140 (2) (2010), pp. 348–354
- Nankabirwa et al., 2015 V. Nankabirwa, J.K. Tumwine, P.M. Mugaba, T. Tylleskär, H. Sommerfelt, PROMISE-EBF Study Group; Child survival and BCG vaccination: a community based prospective cohort study in Uganda; BMC Public Health, 15 (2015), p. 175
- Prendergast and Humphrey, 2014 A.J. Prendergast, J.H. Humphrey; The stunting syndrome in developing countries; Paediatr. Int. Child Health, 34 (4) (2014), pp. 250–265
- Prendergast et al., 2014 A.J. Prendergast, S. Rukobo, B. Chasekwa, et al.; Stunting is characterized by chronic inflammation in Zimbabwean infants; PLoS One, 9 (2) (2014), Article e86928
- Rutstein and Rojas, 2006 S.O. Rutstein, G. Rojas; Guide to DHS Statistics; ORC Macro, Calverton, Maryland (2006)
- Smits and Steendijk, 2015 J. Smits, R. Steendijk; The international wealth index (IWI); Soc. Indic. Res., 122 (1) (2015), pp. 65–85
- Varela-Silva et al., 2009 M.I. Varela-Silva, H. Azcorra, F. Dickinson, B. Bogin, A.R. Frisancho; Influence of maternal stature, pregnancy age, and infant birth weight on growth during childhood in Yucatan, Mexico: a test of the intergenerational effects hypothesis; Am. J. Hum. Biol., 21 (5) (2009), pp. 657–663
- Vaugelade et al., 2004 J. Vaugelade, S. Pinchinat, G. Guiella, E. Elguero, F. Simondon; Non-specific effects of vaccination on child survival: prospective cohort study in Burkina Faso; BMJ, 329 (7478) (2004), p. 1309
- Weiss and Goodnough, 2005 G. Weiss, L.T. Goodnough; Anemia of chronic disease; N. Engl. J. Med., 352 (10) (2005), pp. 1011–1023
- WHO, 2014a WHO; Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. Meeting of the strategic advisory group of experts on immunization; (2014) Available from: http://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf (Website accessed March 5, 2015)
- WHO, 2014b WHO; Global Nutrition Targets 2025: Stunting Policy Brief (WHO/NMH/NHD/14.3); World Health Organization, Geneva (2014)
- WHO, 2014c WHO; Global and Regional Immunization Profile: African Region; World Health Organization, Geneva (2014) (Available from: www.who.int/immunization/monitoring_surveillance/data/gs_afrprofile.pdf?ua=1 . Website accessed April 17, 2015)
- WHO, 2015 WHO; Summary of WHO Position Papers — Recommended Routine Immunizations for Children; World Health Organization, Geneva (2015) (Available from: www.who.int/immunization/policy/Immunization_routine_table2.pdf?ua=1 . Website accessed April 17, 2015)
- WHO. Multicentre Growth Reference Study Group, 2006 WHO. Multicentre Growth Reference Study Group; WHO child growth standards based on length/height, weight and age; Acta Paediatr., 450 (2006), pp. 76–85
- Wooldridge, 2013 J.M. Wooldridge; “Fixed Effects Estimation”. Introductory Econometrics: A Modern Approach. Fifth International Ed; Mason, OH, South-Western (2013)
Document information
Published on 06/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?