Abstract
In this paper, an eco-friendly single-step process for the synthesis of biolubricant basestock from high free fatty acid (FFA) castor oil (CO) via epoxidation reaction was investigated. Influence of various process parameters on the structural modification of CO and their interaction with the maximum oxirane oxygen content (OOC) was optimized. Central composite design (CCD) as one of the tools in response surface methodology (RSM) was used to evaluate the effects of process variables on maximum OOC. Iodine value (IV) and OOC was used to monitor the progress of epoxidation. From the RSM study, the optimal condition inferred was H2O2, 1.65 mol; catalyst loading, 15.14 wt%; temperature, 52.81°C; and reaction time, 2.81 h. At this optimum condition, OOC was found to be 3.85 mass%. Further, the epoxide product was confirmed by 1H, 13C NMR spectral technique and OOC was determined by the standard HBr method. Finally, the significant physico-chemical properties for the prepared epoxide were determined and compared with the castor oil.
Introduction
Currently, plant seed oil/vegetable oil-derived renewable products have been gaining much importance to replace the conventional source of energy due to their depletion at a faster rate [1, 2]. In addition, the use of fossils has stimulated the search for eco-friendly alternatives to conventional resources. Nevertheless, inexhaustible renewable resources can supply a raw material basis for day-to-day life products, and this can avoid contribution to green house effects due to CO2 discharge minimization [3]. In addition to that, proper utilization of renewable raw materials such as plant seed oils can bridge the gap between the fossil reserves’ demand and consumption in the future. Above all, renewable materials could meet the principles of green chemistry in terms of easy degradation and lower toxicity [4]. Moreover, the plant oils offer a wide number of advantages discussed by various technocrats [1, 2, 4]. In this regard, lubricants are one of the areas, which demand an alternative to the conventional lubricant basestocks due to diverse environmental issues reported by many researchers [5, 6]. Therefore, an effective utilization of the bio-based feedstocks for the lubricant basestock synthesis could bring down the dependency on imported petroleum as well as promote the sustainable agricultural initiative [6].
In general, liquid lubricants are the most common form of lubricants; their composition consists of 70–99% basestock and 30–1% of additives to improve the performance properties. However, the ultimate performance of lubricant depends on the basestock which can be synthesized by using plant seed oils [7]. Replacement of lubricant basestocks with the plant-based resources offers a wide range of advantages [5-8]. Even though the plant seed oils offer many advantages, they restrict their direct use due to the inadequate thermal and oxidative stability, hydrolytic stability, and poor cold flow properties [9]. These negative impacts are due to the presence of bis-allylic protons in plant seed oils structure which are highly susceptible to free radical attack, and thereby it undergoes oxidative degradation to form polar oxy compounds [10-13]. Plant seed oil oxidizes similarly to the hydrocarbon mineral oil by following the same free radical oxidation mechanism, but the oxidation rate of plant seed oils is faster than the hydrocarbon mineral oils [14, 15]. This fast rate of oxidation can be attributed to the presence of unsaturated fatty acids in its composition [16]. On the other hand, thermo-oxidative stability, hydrolytic stability, and low temperature performance of nonedible plant oils are much low and poor [17]. However, chemical modification of triglycerides can eliminate the poly unsaturation (for better thermo-oxidative stability), and an optimal extent of chemical change can ameliorate low temperature behavior [18].
Till date, many studies are available in the literature on structural modification of different plant seed oils and their methyl esters to prepare lubricant basestock by various methods, such as epoxidation (chemical or structural modification) [19], genetic modification [20], blending with additives [21], and hydrogenation of double bonds [22]. Among these methods, one of the most significant methods is hydrogenation of unsaturated double bonds in vegetable oils and its methyl esters [23, 24]. Hydrogenation yields total saturation of double bonds, resulting in miserable cold flow properties [23]. Genetic modification approach is tried either to reduce the saturated fatty acid content of the plant seed oils or to reduce the polyunsaturated fatty acid content, as these constituents have a negative impact on the thermo-oxidative stability [23, 25]. Genetically modified oils consistently exhibited improved oleic acid content ranging from 84 to 88% during multiple environmental conditions [26, 27]. However, among all these methods, structural alteration of unsaturated bonds via epoxidation has gained much attention due to high reactivity of three-member oxirane rings. Chemical modification of plant oils at the double bond sites’ results in improved thermo-oxidative stability of the modified product [28].
The present study is focused on epoxidation of castor oil (nonedible oil) which is abundantly available all over India. Castor plant has the botanical name of Ricinus Communis of the family Eurphorbiacae [29]. Castor plant is primitively a tree or shrub that can grow in most tropical and subtropical countries above 10 m high, reaching an age up to 4 years [30]. Especially, castor plant needed a temperature between 15 and 38°C with lower humidity throughout the growing season in order to receive maximum oil yields [30, 31]. Castor oil is a colorless to pale yellow liquid with mild/no odor or no taste, on an average CO seed contains about 46–55% oil by weight [29]. Oil fraction of castor seeds contains a higher amount of the ricinoleic acid as a hydroxylated fatty acid, and this unique structure has given a unique identity to this biological source for industrial synthesis of the variety of compounds [32]. Around the globe, India is the worlds largest exporter of castor oil, and the other major producers are China and Brazil [30]. The total world production of castor seeds is estimated around one million tons, and the oil extracted is about 500,000 tons with productivity of 470 kg of oil per hectare [30, 33]. Irrespective of castor seed origin, season in which it has grown, its fatty acid composition remains unique [34].
Goud et al. [35] reported the epoxidation of castor oil with acetic acid and formic acid, using Amberlite IR-120 as heterogeneous acid catalysts [36, 37]. Further, Salimon et al. [38] also discussed the synthesis of ricinoleic acid epoxide and its characterization. Likewise, Salih et al. [14] described the synthesis of biolubricant basestocks from chemically modified ricinoleic acid-based tetra-esters via epoxidation, ring opening, and esterification reactions [39]. Recently, Hajar et al. [32] reported the production of biolubricant from castor oil substrate using novozyme 435. But very scanty information is available in the literature on the optimization of high FFA castor oil epoxidation process. Therefore, in the current study, an attempt has been made to examine the behavior of the epoxidation reaction and the physicochemical properties of epoxide prepared from high FFA raw material. However, very scanty information is available in the literature on optimization of high FFA castor oil epoxidation to understand the effect of reaction variables on oxirane oxygen content. Therefore, the present communication is aimed to bridge this gap by conducting the study on the modification of high FFA castor oil structure via epoxidation. The reaction was aimed at higher OOC and the main focus was to understand the effect of process parameters and interaction among them. RSM was adopted to optimize the epoxidation parameters and CCD was applied to understand the effect of process variables, using analysis of variance (ANOVA).
Materials and Methods
Materials and analytical techniques
Castor seeds were collected from Cherukupalli (Andhra Pradesh, India). Hydrogen peroxide (purity = 50% v/v) was purchased from Rankem, ion-exchange resin (Amberlite IR-120, strong acid), glacial acetic acid (purity = 99–100%) was obtained from Merck India Ltd. All other reagents used for analysis were of analytical grade and used as received.
Extraction of CO was carried out according to the standard AOAC method. Similarly, physico-chemical characterization of all the samples was carried out as per the standard method reported earlier in some of our studies [16, 40].
Thin layer chromatography
The silica coated aluminum thin layer chromatography (TLC) plates were used for the analysis. The solvent system used was a mixture of hexane and dichloromethane (CH2Cl2) at 2:7 ratio to which few drops of glacial acetic acid were added, and the sample coated plates were visualized in an iodine chamber.
Epoxidation experimental design
Interaction between the process variables on maximum OOC was judged by RSM. A full factorial CCD technique was used for the optimization of the CO epoxidation process variables. Four reaction variables such as substrate molar ratio, that is C=C bonds to H2O2 mole ratio, catalyst loading (wt%), temperature (°C), and reaction time (h) were chosen to understand the effect of these variables on CO epoxidation reaction, as these variables are highly responsible for maximum OOC. The range of epoxidaton reaction variables involved in this study are shown in Table 1 along with lower (−1), medium (0), and higher (+1) levels of the variables. Value of α (alpha) is fixed at level 2 (α = 24/4). A 24 full factorial CCD for four independent variables was used by giving a total number of 30 (=2n + 2n + 6) experiments, where ‘n’ is the number of independent variables. During the optimization study, eight axial experimentations and 16 factorial runs were carried out with six extra replications at the center of design to estimate the pure error.
| Independent variables | Variable levels | ||||
|---|---|---|---|---|---|
| Symbol | Unit | −1 | 0 | +1 | |
| Time | A | h | 2 | 3 | 4 |
| Temperature | B | °C | 50 | 60 | 70 |
| Catalyst Loading | C | wt% | 10 | 15 | 20 |
| Oil: Hydrogen peroxide (H2O2) | D | mol | 1 | 1.5 | 2 |
Epoxidation reaction procedure
Epoxidation of CO was carried out in 250 mL three necked glass reactor equipped with five blade glass stirrer and condenser; the entire setup was immersed in a heating oil bath. During the experiment CO, hydrogen peroxide and acetic acid were measured in a molar ratio; ion-exchange resin (Amberlite, IR-120) was added in weight% based on the organic phase. Initially, 20 g of CO (0.07 mol) was transferred into the reactor and heated to the desired reaction temperature (60°C). Then glacial acetic acid 2.1 g (0.035 mol) and other reactants, that is, hydrogen peroxide and catalysts were added to the reaction mixture. Processing time and temperature conditions used are mentioned in an experimental design matrix (Table S1). Addition of hydrogen peroxide was carried out drop wise for the first-half hours, when the temperature was 5°C below the reaction temperature to avoid the explosion. During epoxidation 14 g stirring speed was maintained to ensure consistent mixing. After complete addition of hydrogen peroxide, the reaction was continued for the desired time duration as mentioned in Table S1. Upon completion of the reaction and prior to the analysis the samples were washed repeatedly with warm Millipore water (40°C) to make it neutral. The sample was concentrated by rotary evaporator. The OOC was determined for the final epoxide product after each run. All the measurements were carried out in duplicate, and the average values are reported.
Preliminary study of process variables
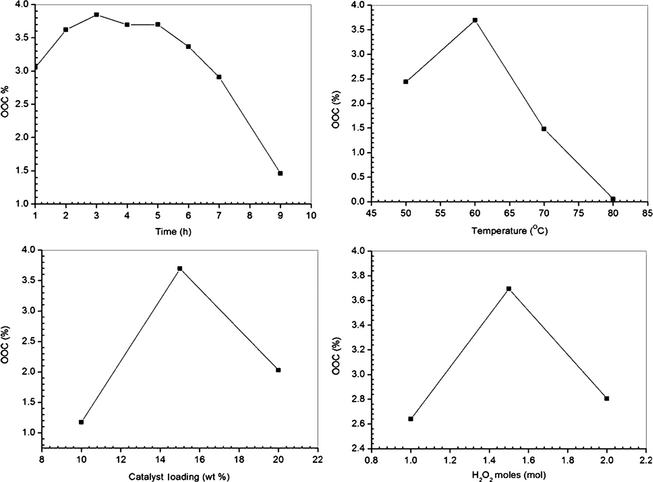
Single parameter optimization process was followed to decide the optimum range of variables. Initially, the reaction time was optimized by continuing the reaction up to 9 h and samples were drawn at regular 1 h intervals to estimate the OOC. Linear increases in the epoxide content were observed up to the reaction time of 3 h, beyond which a gradual decrease was noticed in OOC (Fig. 1). A similar trend was noticed for all other reaction variables. Hence, depending on the preliminary studies, ranges of all the process variables were chosen and coded using the reported expression [41].
|
|
|
Figure 1. Preliminary study data for castor oil epoxidation to find the effects of individual reaction variables (time, catalyst loading, substrate ratio and temperature) on response. |
Statistical analysis
Based on experimental data shown in Table S1, the regression coefficient was determined by design expert software 8.0.7.1 trial version to predict the process response as a function of independent variables and their interactions were used to understand the system behavior. The mathematical relationship between the process variables and response was calculated by the following quadratic polynomial expression:
|
|
(1) |
where Y is the response, that is, the epoxide content, Xiand Xj represent the independent variables, β0 is constant, βi is linear term coefficient, βii is the quadratic term coefficient, βij is cross-term coefficient and ‘n’ is the number of process variables studied and optimized during the study. ANOVA was carried out to estimate the effects of process variables and their possible interaction effects on the maximum OOC in the response surface regression procedure. The goodness and best fit of the model was evaluated by a regression coefficient R2. The response surface and counter plots are obtained using the fitted quadratic polynomial equation generated from regression analysis by keeping two of the independent variables at central value (0) and varying the other two.
Results and Discussion
Model fitting and ANOVA analysis
In order to optimize the epoxidation process variables for maximum epoxide content, a three level, four-factorial CCD was favored. ANOVA as a multivariate technique was studied to determine optimum reaction conditions. All the 30 designed experimental runs (Table S1) were performed and the results were analyzed by multiple regression analysis (Table 2). A quadratic polynomial equation was obtained from the experimental data to predict the epoxide content as shown below in terms of coded variables.
| Source | Sum of squares | Degrees of freedom | Mean square | F value | P-value (prob > F) |
|---|---|---|---|---|---|
| Model | 42.74 | 14 | 3.05 | 2261.27 | <0.0001 |
| A-time | 0.035 | 1 | 0.035 | 25.56 | 0.0001 |
| B-temp | 15.99 | 1 | 15.99 | 11843.23 | <0.0001 |
| C-cat Load | 0.83 | 1 | 0.83 | 611.11 | <0.0001 |
| D-H2O2 | 0.11 | 1 | 0.11 | 81.99 | <0.0001 |
| AB | 2.24 | 1 | 2.24 | 1660.91 | <0.0001 |
| AC | 0.41 | 1 | 0.41 | 305.74 | <0.0001 |
| AD | 7.656E-003 | 1 | 7.656E-003 | 5.67 | 0.0309 |
| BC | 0.35 | 1 | 0.35 | 255.64 | <0.0001 |
| BD | 1.71 | 1 | 1.71 | 1266.18 | <0.0001 |
| CD | 0.39 | 1 | 0.39 | 291.64 | <0.0001 |
| A2 | 1.16 | 1 | 1.16 | 858.43 | <0.0001 |
| B2 | 8.70 | 1 | 8.70 | 6440.64 | <0.0001 |
| C2 | 8.54 | 1 | 8.54 | 6326.76 | <0.0001 |
| D2 | 9.32 | 1 | 9.32 | 6906.31 | <0.0001 |
| Lack-of-fit | 0.018 | 10 | 1.812E-003 | 4.26 | 0.0616 |
| Pure error | 2.128E-003 | 5 | 4.255E-04 |
|
|
(2) |
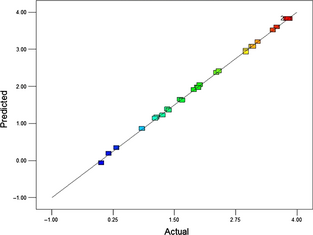
In order to ensure a thorough model fit, to measure the analysis of variance on individual model coefficients test for lack-of-fit need to be estimated. The lack-of-fit is an assessment of failure of a model to represent the data that cannot be reported by random error [42]. Generally, the significant process variables are decided based on the F-value or P-value (also known as a probability of error value or “prob > F” value) [42]. Greater the magnitude of F-value and correspondingly smaller the “prob > F” value, more important is the corresponding coefficient [42]. The results of second-order response surface model in the form of ANOVA for the maximum epoxide content are summarized in Table 2. From the table, it can be seen that F-value of the model is 2261.27, and the corresponding P-value (prob > F) is very small, that is <0.0001 implying that the model is highly significant. The P-values are adopted as a tool to ensure the importance of each co-efficient. In this study, the main linear effects of time (A), temperature (B), catalyst loading (C), and hydrogen peroxide molar ratio (D), cross and quadratic effects of all four process variables (i.e., AB, AC, BC, BD, A2, B2, C2, and D2) are highly significant, as the P-value is very less (<0.0001). The other model term (cross variable) time and hydrogen peroxide molar ratio (AD) are also significant variables, since the P-value is 0.0309 (<0.05). In the present model, the absence of insignificant parameters intending that all the liner, cross, and quadratic terms are highly important for maximum epoxide content. The insignificant lack-of-fit F-value of 4.26 indicates that lack-of-fit is considerably significant relative to the pure error, which signifies that the model is extremely accurate without any noise, and the results are reproducible. Furthermore, the actual values are very much close to the predicted as shown in Figure 2.
|
|
|
Figure 2. Predicted versus actual plot of response (maximum OOC) on castor oil epoxidation. |
The precision of a model is judged by the regression coefficient (R2). The R2 value is always in between 0 and 1, and its order of magnitude suggests the aptness of the model [8]. For a good statistical model, the R2 value should be close to one and the regression value for higher epoxide content is presented as 0.9996, which is close to 1, and it signifies that the 99.96% model behavior can be interpreted for higher epoxide content while only about 0.04% of the full variance cannot be explained by the model. Regression coefficient R2 represents that the accuracy and general ability of the polynomial model is good. The predicted R2 value (0.9982) is in reasonable agreement with the adjusted R2 (0.9993) which recommends prominent corelational statistics between the remarked values and the predicted data. Thus, the regression model provides an excellent explanation of the relationship between the independent process variables and the response variable [42].
Influence of various process variables on maximum OOC
In order to estimate the best reaction condition for maximum epoxide content, the effects of linear, cross and quadratic reaction variable on the epoxidation was studied. The three-dimensional response surface plots and the two-dimensional counter plots, which is the graphical histrionics of the regression equation, are obtained by employing design expert software [26, 43]. These graphical representations of the plots are shown by varying two process variables at a time while keeping the other two variables at a central level (0). During each experiment, samples were withdrawn at regular intervals, washed, neutralized, and analyzed for OOC and α-Glycol content (i.e., oxirane cleavage).
Effect of time and temperature on OOC
Figure S1a and b describes the two-dimensional counter plot and corresponding three-dimensional response surface plot expressing the cross effect of time (A) and temperature (B) on the maximum OOC with respect to other process variables. Figure S1a and b shows that OOC increased with an increase in reaction time from 2 to 4 h, the increasing trend was noticed up to a certain time period of 3 h, beyond which gradual depletion in OOC was noticed (Table S1). Similarly, temperature was varied from 40 to 80°C in order to study the effect of temperature on OOC with respect to time. Results in Figure S1a and b show that OOC content increased with an increase in temperature up to 60°C, further increase in temperature, say 70°C OOC decreases (Table S1). The behavior of 2D and 3D figures revealed that longer reaction time and elevated temperature (beyond 60°C) lowers the OOC which might be due to oxirane cleavage (Fig. S2) [44]. The maximum OOC of 3.85 mass% was attained at the medium temperatures of 60°C and 3 h reaction time. Similar behavior was noticed by Salimon et al. [38] during their study on epoxidation of ricinoleic acid. In the current work, less OOC was obtained, which may be due to higher acid value of castor oil. Okieimen et al. [45] observed similar results during their study on epoxidation of high acid value rubber seed oil. Hence, 60°C temperature was considered as suitable for epoxidation of CO, when molar ratio of unsaturation: acetic acid: hydrogen peroxide was 1:0.5:1.5 and catalysts loading 15 wt%.
Effect of time and catalyst loading on OOC
Keeping time (A) and catalyst loading (C) at central levels, that is, 3 h and 15 wt%, respectively, combined the effect of temperature (B) and hydrogen peroxide (D) was studied aiming at the higher epoxide content. From Figure S3a and b, it can be seen that OOC content increased almost linearly with a certain time period along with catalyst loading, thereafter OOC decreases (Table S1). The obtained results attributed to the hypothesis that availability of enough active surface area of catalyst for longer reaction time during the reaction results in cleavage of oxirane oxygen [44]. Hwang and Erhan [46] reported that presence of higher FFA content led to hydrolysis reaction in acid media thereby decreases the epoxide content due to oxirane cleavage. Hence, within the experimental conditions used in this study, the most favorable catalyst loading and reaction duration appeared to be 15 wt% and 3 h.
Effect of time and substrate molar ratio on OOC
Figure S4a and b shows the effect of varying substrate molar ratio and reaction time on the maximum epoxide content. Increase in the substrate molar ratio from 0.5 to 1.5 mol (Table S1) leads to linear increased in OOC content due to the formation of more peracetic acid and thereby OOC. The maximum OOC was attained with the mole ratio, 1.5 mol and the stability of oxirane observed with this ratio was similar to that observed with 1 molar ratio. However, an increase in the final oxirane value was relatively less when the ratio was further increased to 2.5 mol [47]. The use of a higher substrate molar ratio raised additional problem of agitation and decreases the mass transfer rate thereby decreases the OOC. The maximum substrate ratio and higher reaction time provided an opportunity to react oxirane rings with excess hydrogen peroxide, acetic acid, and by-product water [48]. Therefore, for subsequent experimentation, optimum reaction time, H2O2 moles considered are 3 h and 1.5 mol.
Effect of temperature and catalyst loading on OOC
Effect of reaction temperature (B) and catalyst loading (C) on the maximum OOC was investigated by varying catalyst loading from 5 to 25 wt% (Table S1) and temperature from 40 to 80°C with an interval of 10°C. Increase in the temperature (Fig. S5a and b) showed a favorable effect on epoxidation reaction and maximum OOC was obtained (3.85 mass%) at 60°C and catalyst loading 15 wt%. From Figure S5a and b, it can also be seen that beyond 60°C decrease in OOC was observed. Epoxidation at higher temperatures (>60°C) acts as a medium to oxirane cleavage thereby decreasing the OOC value [49]. Likewise, altering the catalyst loading from 5 to 25% roaring in the epoxide content was observed up to 15 wt%. Beyond which inadequate OOC was noticed due to excess catalyst loading, which leads to oxirane cleavage [39]. However, under a given reaction condition and 15 wt% catalyst loading, it is assumed that reaction is free from mass transfer resistance and the maximum OOC can be obtained at a moderate reaction temperature of 60°C [39]. Hence, 15 wt% catalyst loading and 60°C temperature was considered as the optimum parameters for subsequent experimentation.
Effect of temperature and substrate molar ratio on OOC
The effect of the substrate molar ratio (D) on reaction temperature (B) and their combined interaction during the epoxidation process at constant catalyst loading of 15 wt% and 3 h of reaction time is represented by 3D response surface plot and corresponding 2D contour plot in Figure S6a and b. From the figures, it can be depicted with an increase in both temperature and substrate molar ratio, there was a progressive increase in the OOC which indicates that both the variables have a significant interaction between each other. However, the decrease in OOC was noticed beyond 60°C [39] and substrate molar ratio 1.5. Derawi et al. [50] reported that the rate of epoxidation increased as the concentration of hydrogen peroxide increased in the system, but the stability of oxirane rings was poor at higher substrate molar ratios [51]. On the other hand, Dinda et al. [39] have shown that oxirane ring was quite stable at lower hydrogen peroxide concentrations. Finally from the interaction of these variables, it can be seen that at a medium reaction temperature, the OOC increases with an increase in substrate molar ratio. However, higher reaction temperature and molar ratio showed a negative impact on OOC. Hence, a temperature 60°C and 1.5 substrate molar ratio was considered as optimum condition for further experimentations.
Effect of catalyst loading and substrate molar ratio on OOC
The influence of catalyst loading on varying hydrogen peroxide molar ratio was investigated at various catalyst loadings and various H2O2 molar ratios. Enhanced rate of per acid formation was anticipated with an increase catalyst loading and hydrogen peroxide concentration followed by an increase in the OOC [49, 52]. Figure S7a and b describes the effect of catalyst loading and hydrogen peroxide on the course of epoxide formation. Higher selectivity was achieved at 1.5 moles of hydrogen peroxide per mol of ethylenic unsaturation and catalyst loading 15 wt%. Concentrations of hydrogen peroxide higher than 1.5 mol leads to a higher rate of epoxy ring decomposition as resolved by most of the researchers [53] and also discussed in the previous section. This observation was in agreement with the results shown in Figure S7a and b. Above all, lower epoxide content for high FFA CO was due to the hydrolysis reaction in the aqueous phase and its mechanism is well explained by Blee et al. [54].
Process optimization for epoxidation of high FFA CO
In the current study, this model is aspired to find the epoxidation process for the best fit of variables that gives maximum OOC. The predicted values of epoxidation reaction obtained from the model equation for a maximum amount of epoxide content are substrate ratio 1.65 mol, catalyst loading 15.14 wt%, and 2.81 h of reaction time at 52.8°C reaction temperature (Table S2). These values were chosen from the optimum solutions proposed by RSM optimization tool. The model expects the maximum OOC that can be obtained at these optimum conditions is 4.09 mass%. To confirm the model prediction, the best response variables are tested at the theoretical condition by conducting the laboratory experiment. At the optimized process condition epoxide content was found to be 3.85 mass% which agrees well with the model predicted value suggests that the formulated model was believed to be accurate and reliable (Table S2). In the present study, all the 30 experiments (Table S1) including confirmatory experiments (Table S2) were performed in duplicate at laboratory conditions and the average values are reported.
Physico-chemical characterization of prepared high FFA CO epoxide product
NMR spectroscopy
Epoxidation of high FFA CO was confirmed by 1H NMR spectroscopy; it is one of the significant techniques to confirm the product formation and monitor the progress of reaction. 1H NMR spectrum of the starting material (CO) and final product (CO epoxide) are shown in Figure S8a and b. Significant signals in the 1H NMR spectra of CO shows the presence of unsaturation bands (-CH=CH-) from 5.25 to 5.58 ppm range (Fig. S8a). However, the partial disappearance of unsaturation bands have been noticed (Fig. S8b) after completion of the reaction, which signifies the presence of unsaturation in the end product at optimum condition. Similarly, the peaks (-CH-O-CH-) at 2.8 to 3.1 ppm range indicate the formation of the epoxide product, which are absent in the oil spectra. Furthermore, characterization of the epoxide product by 1H NMR revealed the presence of the ring open product, which was confirmed by the additional peak at 3.41 ppm. Although 1H NMR confirmed the complete conversion of unsaturation, but minimal oxirane cleavage was noticed due to the high FFA content of oil. Padmasiri et al. [47]; Derawi and Salimon [50]; Blee et al. [54] also noticed similar results during their study on mee and rubber oil.
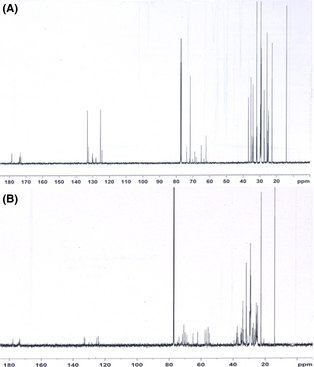
Similarly, Figure 3A and B show 13C NMR spectra of CO and its epoxide. Appearance of peaks at 125–133 ppm represents the olefinic carbons in CO (Fig. 3A). Comparison of Figure 3A and B, revealed the formation of epoxy protons at 53–59 ppm (Fig. 3B) and disappearance of olefinic carbons in the epoxide product at 125–133 ppm (Fig. 3A). The appearance of additional peaks at 53 and 59 ppm in the CO confirms the epoxidation of high FFA CO. Similar results are noticed by Madankar et al. [55] during their study on canola oil.
|
|
|
Figure 3. 13C NMR spectrum of castor oil (A) and its epoxide (B). |
TLC analysis
Thin layer chromatography analysis of CO and its epoxide was carried out in order to confirm the product and mixture of compounds present in the final product (Fig. 4). The TLC spectra of the epoxide product indicate the presence of polar compounds, which was found to be absent in the CO confirms the formation of epoxide. From the CO epoxide TLC spectra, it was clear that some starting material is still present in the end product, which indicates an incomplete reaction. The same can be noticed from IV of the epoxide product (Table 3) and 1H NMR spectrum (Fig. S8a and b) [47].
| Physicochemical properties | CO epoxide | CO |
|---|---|---|
| Acid value (mg KOH/g) | 1.46 | 45.6 |
| Density (kg/m3) | 837.24 | 790.74 |
| Free fatty acid (mg KOH/g) | 0.73 | 22.8 |
| Iodine value (gI2/100 g of oil) | 51.86 | 89.69 |
| Kinematic viscosity (cSt) at 40°C | 249.84 | 193.13 |
| Moisture content (wt %) | 0.21 | 0.9 |
| Pour point (°C) | −15 | −20 |
| Refractive index (at 27.6°C) | 1.479 | 1.477 |
| Viscosity index (VI) | 188.92 | 99.52 |
| Oxirane content (Experimental, mass %) | 3.85 | – |
| Oxirane content (Theoretical, mass %) | 5.35 | – |
| Relative percentage conversion of oxirane (%) | 71.96 | – |
| Glycol content (Theoretical, mol/100 g) | 0.31 | – |
| Glycol content (Experimental, mol/100 g) | 0.18 | – |
| Relative percentage conversion to α-Glycol (mol/100 g) | 40.67 | – |
|
|
|
Figure 4. Thin layer chromatography of castor oil and its epoxide. |
Pour point determination by DSC
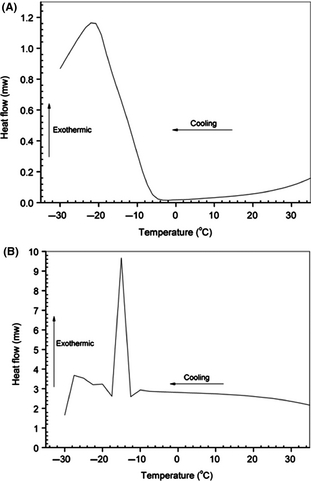
Estimation of pour point is important in order to determine the low-temperature flow behavior of the modified (i.e. epoxide) and unmodified CO. It is a rough indication of the lowest temperature at which the epoxide is promptly pumpable [14]. In the present communication, cold flow properties of CO and its epoxide are determined using the method followed by Borugadda et al. [16]. DSC thermograms of CO and its epoxide are shown in Figure 5A and B. From the thermograms pour point of CO and CO epoxide was found to be −20 and −15 oC, respectively, which revealed that after epoxidation (i.e., the structural modification of CO), the pour point reduced significantly (5°C). This behavior is attributed to the fact that conversion of unsaturation content into epoxide altered the cold flow properties (PP). However, Soriano et al. [56] also observed similar kind of results during PP analysis of oils and esters. Elaborate literature survey on vegetable oils cold flow properties revealed that the cold flow property of plant oils is extremely inadequate, and this restricts their use at lower operating temperatures [10]. Plant oils have a tendency to form macro-crystalline structures at low temperatures through uniform stacking of the triglyceride backbone [10]. Formation of the macro-crystals restricts a free flow of the fluid due to loss of kinetic energy of individual molecules during the self-stacking [10]. In general, the pour point should be low enough to ensure that the epoxide is pumpable at lower temperatures in an application point of view [8]. Finally from this study, it can be seen that the modification in the structure of CO can alter the PP which was regarded as one of the major concerns for the plant oils to be used as an alternative to fossil resources. However, further improvement in the low-temperature properties can be done by additivation.
|
|
|
Figure 5. DSC thermogram of castor oil (A) and its epoxide (B) for pour point determination. |
Thermo-oxidative stability
Thermo-oxidative stability of CO and its epoxide was estimated by onset (under nitrogen atmosphere) and oxidative onset (under oxygen atmosphere) temperatures. TGA thermo grams of (Fig. S9a and b) CO and its epoxide furnish the detailed information related to its degradation behavior in the presence of the inert gas (N2) and reactive gas (O2). Onset temperature is reported as the first perceptible temperature at which the degradation of CO and its epoxides starts. From Figure S9a, it can be seen that CO and its epoxide are stable up to 310 and 308°C (onset temperatures), respectively, in an inert atmosphere. Similarly, the maximum decomposition temperature represents the temperature at which utmost weight loss of CO and epoxide samples occurred, that is, 371.5 and 350.5°C, respectively. Lower thermal stability of epoxide signifies the degradation of epoxide at lower temperature compared to the unmodified CO. This may be due to the presence of high acid media in the mixture which is responsible for the lower thermal stability of CO epoxide [54].
Park et al. [57] reported that oil with saturated fatty acids, and mono unsaturation content has a positive influence and thermally more stable than polyunsaturation. Since the double bonds are converted to epoxide, hence higher thermal stability of epoxide was anticipated compared to unmodified CO, but due to the aforementioned reason, lower thermal stability was noticed. Study on thermal stability of epoxide is of great importance for various applications, and it depends on the chemical composition and structure of the epoxide.
Similarly, oxidative stability was found as a quality indicative parameter under oxygen atmosphere. In the present communication, oxidative stability was specified as the resistance of the epoxide against oxidation in the air atmosphere. Figure S9b describes the TGA plots of CO and its epoxide, from the oxidative onset temperature of the thermogram, it was found to be 320°C for both modified and unmodified CO (Fig. S9b). Similarly, the maximum decomposition temperature was found to be 382 and 354°C, respectively, for CO and its epoxide. In addition to the aforementioned elucidation, this attitude can be justified by the presence of unsaturation in the epoxide sample. Finally, it can be concluded that the CO showed more thermo-oxidative stability than the structurally modified CO epoxide.
Viscosity and viscosity index
In the current study, kinematic viscosities of CO and its epoxide were found to be 193.13 and 249.84 cSt, respectively at 40°C (Table 3). Increase in the epoxide molecular weight can be attributed to the addition of an oxygen molecule at the unsaturation sites thereby forming the oxirane ring [45]. The results of the study depict that CO epoxide viscosity (249.84 cSt) has been improved over the unmodified CO, and this signifies that the synthesized CO epoxide can serve as an acceptable lubricant base stock with enhanced viscosity to reduce friction. The ability of substance to resist free flow is one of the highly significant attributes for many heavy duty and industrial materials such as fuels, lubricants, and surfactants [58]. Therefore, it is desirable that the viscosity must be high enough all the time to keep good oil film between the moving parts to reduce friction [58]. Otherwise, due to loss in the lubricant, there is a tendency to increase the friction thereby resulting in power loss and rapid wear on the machine parts [58]. Viscosity is one of the crucial parameters while selecting a lubricant for a specific application, and failure to use the right lubricant with the required viscosity results in extreme temperature that may result in poor lubrication, equipment failure, and damage [59]. As discussed above, viscosity of acceptable range cannot be covered by conventional vegetable oils, hence in this study, structural modification was attempted to enhance the viscosity and molecular weight. However, viscosity can also be improved by addition of a viscosity enhancer [60].
Similarly, it is also desirable to check the viscosity in terms of the viscosity index (VI) which indicates the lower sensitivity of viscosity variations at higher temperatures. During this study, CO and its epoxide VI was found to be 99.52 and 188.92, respectively. Higher VI of CO epoxide signifies that the addition of an oxygen molecule at the unsaturation sites leads to an overall increase in the molecular weight of the final epoxide product and the viscosity index [14]. The higher VI of epoxide (188.92) almost doubles compared with the viscosity index of castor oil (99.52) indicating that the prepared epoxide can act as a high-temperature lubricant basestock.
Physico-chemical characterization
Significant physico-chemical properties of CO and its epoxide are estimated and given in Table 3. Acid value (AV) of CO and its epoxide was found to be 45.6 and 1.46 mg KOH/g, respectively. Lower acid value of CO epoxide signifies the smooth operation and functioning of the equipment during its usage. FFA is always considered as half of the AV, which signifies the formation of soap when mixed with water. The obtained FFA value was very less (0.73 mg KOH/g) and signifies trouble-free performance of the epoxide. The density of CO was found to be 790.74 kg/m3 and corresponding epoxide density was 837.24 kg/m3. Improved density of CO epoxide was assigned to increase molecular weight of epoxide by addition of an oxygen molecule in the midst of unsaturation sites. Determination of the IV (i.e., quantity of the double bonds) after epoxidation reaction is one of the best ways to confirm and support the completion of the epoxidation reaction. Initial IV of CO was found to be 89.69 (g I2/100 g) whereas after epoxidation the value was 51.86 (g I2/100 g). Higher IV of epoxide signifies incomplete epoxidation reaction and the same has been observed from 1H NMR and TLC spectral analysis (Table 3). The presence of hydroxyl groups was confirmed by α-Glycol content analysis. The theoretical and experimental values of α-glycol content along with the relative percentage conversion of α-glycol is calculated and reported in Table 3.
Another significant property of epoxide is moisture content indicating the presence of water in the epoxide. Presence of moisture in the epoxide supports the bacterial growth which leads to an undesired performance upon usage thereby increasing AV, viscosity, and formation of free radical compounds via oxidation [21]. In this study, the CO epoxide moisture content was found to be 0.21 wt% (Table 3), which indicates safe performance of epoxide during usage. Refractive index for both the samples was found to be almost similar 1.477 and 1.479 (Table 3), which conveys that a very small quantity of heat energy can pass through the CO and epoxide samples, which helps to avoid the thermal degradation of end products during its usage and storage.
Conclusion
This is the first comprehensive report on the synthesis of castor oil epoxide from high FFA castor oil. The optimum condition for CO epoxidation occurred at temperature, 52.81°C; hydrogen peroxide to ethylenic unsaturation molar ratio, 1.65; catalyst loading, 15.14 wt %; and reaction time, 2.81 h. At this optimal condition, maximum epoxide content and relative conversion to oxirane was found to be 3.85mass% and 0.71%. Further, the product was confirmed by 1H-NMR, 13C-NMR, IV, and oxirane analysis. Quadratic polynomial model and ANOVA has well explained the interaction between the process variables. Further the model was examined and validated for the best fit. Comparative evaluation of physico-chemical properties of castor oil and its epoxide revealed surprising results. Among all the evaluated properties, only a few properties showed significant improvement, whereas thermo-oxidative stability and cold flow property results were unsatisfactory which may be due to high FFA content in CO or higher oxirane cleavage. However, the obtained viscosity index of epoxide indicates that the product can be used as high-temperature lubricant basestocks. However, from the present study, it can be concluded that low FFA CO could act as a suitable feedstock for lubricant synthesis with improved physico-chemical properties and anticipated to give improved thermo-oxidative stability and cold flow properties.
Conflict of Interest
None declared.
References
- Kotwal, M., A. Kumar, and S. Darbha. 2013. Three-dimensional, mesoporous titanosilicates as catalysts for producing biodiesel and biolubricants. J. Mol. Catal. A. Chem.377:65–73.
- Borugadda, V. B., and V. V. Goud. 2014a. Synthesis of waste cooking oil epoxide as a bio-lubricant base stock: characterization and optimization study. J. Bioproc. Eng. Bioref.3:57–72.
- Oyedepo, S. O.2012. Energy and sustainable development in Nigeria: the way forward. Energy Sustain. Soc.2:15.
- Eissen, M., J. O. Metzger, E. Schmidt, and U. Schneidewind. 2002. 10 years after Rio - concepts on the contribution of chemistry to a sustainable development. Angew. Chem. Int. Ed. Engl.41:414–436.
- Salimon, J., N. Salih, and E. Yousif. 2011b. Chemically modified biolubricant basestocks from epoxidized oleic acid: Improved low temperature properties and oxidative stability. J. Saudi Chem. Soc.15:195–201.
- Pawan, D. M., G. P. Ravindra, and V. P. Harshal. 2011. Epoxidation of wild safflower (Carthamus oxyacantha) oil with peroxy in of strongly cation exchange resin IR-122 as catalyst. Int. J. Chem. Tech. Res.3:1152–1163.
- Dietrich, H.2002. Recent trends in environmentally friendly lubricants. J. Synthetic Lubrication4:327–347.
- Manivannan, P., and M. Rajasimman. 2011. Optimization of process parameters for the osmotic dehydration of beetroot in sugar solution. J. Food Process Eng34:804–825.
- Aguieiras, E. C. G., C. O. Veloso, J. V. Bevilaqua, D. O. Rosas, M. A. P. Silva, and M. A. P. Langone. 2011. Estolides synthesis catalyzed by immobilized lipases. Enzyme Res.432746:1–7.
- Adhvaryu, A., Z. Liu, and S. Z. Erhan. 2005. Synthesis of novel alkoxylated triacylglycerols and their lubricant base oil properties. Ind. Crops Prod.21:113–119.
- Sharma, B. K., J. M. Perez, and S. Z. Erhan. 2007. Soybean oil-based lubricants: a search for synergistic antioxidants. Energy Fuels21:2408–2414.
- Singh, C. P., and V. K. Chhibber. 2013. Chemical modification in karanja oil for biolubricant industrial applications. J. Drug Deliv. Therapeutics3:117–122.
- Erhan, S. Z., B. K. Sharma, and J. M. Perez. 2006. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crops Prod.24:292–299.
- Salih, N., J. Salimon, E. Yousif, and B. M. Abdullah. 2013. Biolubricant basestocks from chemically modified plant oils: ricinoleic acid based tetra-esters. Chem. Central J.7:128.
- Hamblin, P.1999. Oxidative stabilisation of synthetic fluids and vegetable oils. J. Synthetic Lubrication2:157–181.
- Borugadda, V. B., and V. V. Goud. 2013a. Comparative studies of thermal, oxidative and low temperature properties of waste cooking oil and castor oil. J. Renew. Sustain. Energy5:063104.
- Fox, N. J., B. Tyrer, and G. W. Stachowiak. 2004. Boundary lubrication performance of free fatty acids in sunflower oil. Tribol. Lett.4:275–281.
- Bart, J. C. J., E. Gucciardi, and S. Cavallaro. 2012. Biolubricants: science and technology. Woodhead publishing series in energy No.46, 249. Woodhead Publishing Limited, UK.
- Borugadda, V. B., and V. V. Goud. 2014b. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia54:75–84.
- Adhvaryu, A., and S. Z. Erhan. 2002. Epoxidised soybean oil as a potential source of high temperature lubricants. Ind. Crops Prod.15:247–254.
- Doll, K. M., B. K. Sharma, and S. Z. Erhan. 2007. Synthesis of branched methyl hydroxyl stearates including an ester from bio-based levulinic acid. Ind. Eng. Chem. Res.46:3513–3519.
- Cermak, S. C., K. B. Brandon, and T. A. Isbell. 2006. Synthesis and physical properties of estolides from lesquerella and castor fatty acid esters. Ind. Crops Prod.23:54–64.
- Wadumesthrige, K., S. O. Salley, and K. Y. S. Ng. 2009. Effects of partial hydrogenation, epoxidation, and hydroxylation on the fuel properties of fatty acid methyl esters. Fuel Process. Technol.90:1292–1299.
- Cadenas, F. A. P., F. Kapteijn, M. P. Martijn, A. Jacob, and Z. Moulijn. 2007. Selective hydrogenation of fatty acid methyl esters over palladium on carbon-based monoliths: structural control of activity and selectivity. Catal. Today128:13–17.
- Suliman, E. T., Z. Meng, J. W. Li, J. Jiang, and Y. Liu. 2013. Optimisation of sunflower oil deodorising: balance between oil stability and other quality attributes. Int. J. Food Sci. Technol.48:1822–1827.
- Kinney, A. J., and T. E. Clemente. 2005. Modifying soybean oil for enhanced performance in biodiesel blends. Fuel Process. Technol.86:1137–1147.
- Kinney, A. J.1996. Development of genetically engineered soybean oils for food application. J. Food Lipids3:273–292.
- Holser, R. A.2008. Transesterification of epoxidized soybean oil to prepare epoxy methyl esters. Ind. Crops Prod.1:130–132.
- Castor oil plant. Available at http://en.wikipedia.org/wiki/Castor_oil_plant (accessed 14 November 2014).
- Borugadda, V. B., and V. V. Goud. 2012. Biodiesel production from renewable feedstocks: status and opportunities. Renew. Sustain. Ene. Rev.16:4763–4784.
- Ogunniyi, D. S.2006. Castor oil: a vital industrial raw material. Bioresour. Technol.97:1086–1091.
- Hajar, M., and F. Vahabzadeh. 2014. Artificial neural network modelling of biolubricant production using Novozym 435 and castor oil substrate. Ind. Crops Prod.52:430–438.
- Santana, G. C. S., P. F. Martins, D. L. N. Da Silva, C. B. Batistella, M. R. Filho, and W. M. R. Maciel. 2010. Simulation and cost estimate for biodiesel production using castor oil. Chem. Eng. Res. Design88:626–632.
- Salimon, J., N. Salih, and E. Yousif. 2010. Biolubricants: raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol.5:519–530.
- Goud, V. V., A. V. Patwardhan, and N. C. Pradhan. 2006. Strongly acidic cation exchange resin of sulphonated polystyrene type used as catalyst for epoxidation of castor oil with peracetic acid and performic acid. Solid State Sci. Technol.14:62–68.
- Jankovic, M., O. Borota, and S. S. Fiser. 2012. Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chem. Eng. Process: Process Intensif.62:106–113.
- Leveneur, S., D. Y. Murzin, and T. Salmi. 2009. Application of linear free-energy relationships to perhydrolysis of different carboxylic acids over homogeneous and heterogeneous catalysts. J. Mol. Catal. A: Chem.303:148–155.
- Salimon, J., N. Salih, and E. Yousif. 2011c. Synthetic biolubricant basestocks from epoxidized ricinoleic acid: improved low temperature properties. Chem. Industry60:127–134.
- Dinda, S., A. V. Patwardhan, V. V. Goud, and N. C. Pradhan. 2008. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol.99:3737–3744.
- Borugadda, V. B., and V. V. Goud. 2014c. Thermal, oxidative and low temperature properties of methyl esters prepared from oils of different fatty acids composition: a comparative study. Thermochim. Acta577:33–40.
- Sagiroglu, A., S. S. Isbilir, H. M. Ozcan, H. Paluzar, and N. M. Toprakkiran. 2011. Comparison of biodiesel productivities of different vegetable oils by acidic catalysis. Ind. Eng. Chem. Res.17:53–58.
- Tabrizi, S. A. H., and E. T. Nassaj. 2011. Modeling and optimization of densification of nanocrystalline Al2O3 powder prepared by a sol–gel method using response surface methodology. J. Sol-Gel. Sci. Technol.57:212–220.
- Majumdar, S., and V. K. Lukka. 2011. Pectin based multiparticulate formulation of ketoprofen for bacterial enzyme dependent drug release in colon. Trends Biomater. Artif. Organs25:133–143.
- Chavan, V. P., A. V. Patwardhan, and P. R. Gogate. 2012. Intensification of epoxidation of soybean oil using sonochemical reactors. Chem. Eng. Process: Process. Intensif.54:22–28.
- Okieimen, F. E., C. Pavithran, and I. O. Bakare. 2005. Epoxidation and hydroxlation of rubber seed oil: one-pot multi-step reactions. Eur. J. Lipid Sci. Technol.107:330–336.
- Hwang, H. S., and S. Z. Erhan. 2011. Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J. Am. Oil Chem. Soc.78:1179–1184.
- Padmasiri, K., B. M. O. Gamage, and L. Karunanayake. 2009. Epoxidation of some vegetable oils and their hydrolysed products with peroxyformic acid - optimised to industrial scale. J. Natl Sci. Found Sri Lanka37:229–240.
- Suarez, P. A. Z., M. S. C. Perreira, K. M. Doll, B. K. Sharma, and S. Z. Erhan. 2009. Epoxidation of methyl oleate using heterogeneous catalyst. Ind. Eng. Chem. Res.48:3268–3270.
- Goud, V. V., A. V. Patwardhan, S. Dinda, and N. C. Pradhan. 2007. Kinetics of epoxidation of jatropha oil with peroxyacetic and peroxyformic acid catalysed by acidic ion exchange resin. Chem. Eng. Sci.62:4065–4076.
- Derawi, D., and J. Salimon. 2010. Optimization of epoxidation of palm olein by using performic acid. J. Chem.7:1440–1448.
- Dinda, S., V. V. Goud, A. V. Patwardhan, and N. C. Pradhan. 2011. Selective epoxidation of natural triglycerides using acidic ion exchange resin as catalyst. APJ Chem. Eng.6:870–878.
- Mungroo, R., N. C. Pradhan, V. V. Goud, and A. K. Dalai. 2008. Epoxidation of canola oil with hydrogen peroxide catalyzed by acidic ion exchange resin. J. Am. Oil Chem. Soc.85:887–896.
- Petrovic, Z., A. Zlatanic, C. C. Lava, and S. S. F. Fiser. 2002. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids-kinetics and side reactions. Eur. J. Lipid Sci. Technol.104:293–299.
- Blee, E., S. Summerer, M. Flenet, H. Rogniaux, A. V. Dorsselaer, and F. Schuber. 2005. Soybean epoxide hydrolase. Identification of the catalytic residues and probing of the reaction mechanism with Secondary kinetic isotope effects. J. Biol. Chem.280:6479–6487.
- Madankar, C. S., A. K. Dalai, and S. N. Naik. 2013. Green synthesis of biolubricant base stock from canola oil. Ind. Crops Prod.44:139–144.
- Soriano, N. U., V. P. Migo, and M. Matsumura. 2006. Ozonized vegetable oil as pour point depressant for neat biodiesel. Fuel85:25–31.
- Park, J. Y., D. K. Kim, J. P. Lee, S. C. Park, Y. J. Kim, and J. S. Lee. 2008. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol.99:1196–1203.
- Salimon, J., N. Salih, and E. Yousif. 2011a. Characterization and physicochemical properties of oleic acid ether derivatives as biolubricant basestocks. J. Oleo Sci.60:613–618.
- Lubricant Failure = Bearing Failure. Available at http://www.machinerylubrication.com/Read/1863/lubricant-failure (Accessed 14 November 2014).
- Quinchia, L. A., M. A. Delgado, C. Valencia, J. M. Franco, and C. Gallegos. 2009. Viscosity modification of high oleic sunflower oil with polymeric additive for the design of new biolubricant formulations. Environ. Sci. Technol.43:2060–2065.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?