Abstract
Cisplatin is a chemotherapeutic agent used in the treatment of solid tumors, with clinical use often complicated by kidney toxicity. Nuclear factor (erythroid-derived-2)-like 2 (Nrf2) is a transcription factor involved in kidney protectant effects. The purpose of this study was to determine whether the Nrf2 activators oltipraz, sulforaphane, and oleanolic acid could protect human kidney cells against cisplatin-induced injury and to compare the protective effects between three Nrf2 activators. Human proximal tubule cells (hPTC) and human embryonic kidney 293 cells (HEK293) were exposed to cisplatin doses in the absence and presence of Nrf2 activators. Pre- and delayed-cisplatin and Nrf2 activator exposures were also assessed. Cell viability was enhanced with Nrf2 activator exposures, with differences detected between pre- and delayed-treatments. Both sulforaphane and oltipraz increased the expression of anti-oxidant genes GCLC and NQO1. These findings suggest potential human kidney protective benefits of Nrf2 activators with planned exposures to cisplatin.
Keywords
Nrf2 ; Kidney ; Nephrotoxicity ; Cisplatin ; Sulforaphane ; Oltipraz ; Oleanolic acid
1. Introduction
Cisplatin (cis -diamminedichloroplatinum) is one of the most commonly used chemotherapy drugs for the treatment of solid tumors. Cisplatin and other related platinum-based therapeutics are effective against lung, head and neck, prostate, ovarian, and bladder cancers [1] , [2] and [3] . However, acute kidney injury can develop after a single dose. Existing clinical studies report reductions in estimated glomerular filtration rates, increases in urinary albumin excretion, and elevations in serum creatinine within 10 days following a cisplatin dose in 8–40% of patients [4] , [5] , [6] and [7] . Kidney disease manifestations can also include electrolyte wasting and persistent hypomagnesemia [8] . Nephrotoxicity may limit cisplatins clinical use and resultant treatment efficacy. Current therapies, including fluid administration have variable efficacy in preventing kidney damage. Thus, interventions that can prevent or ameliorate kidney injury in human kidney cells exposed to cisplatin are warranted.
Several mechanisms contribute to the onset and pathogenesis of cisplatin-induced kidney injury, including vascular injury, inflammation, ischemia, oxidative stress, and tubular cell death [3] , [6] , [9] , [10] and [11] . Although cisplatin nephrotoxicity involves many different mechanisms, tubular cell death plays an important role in its progression. Studies using cultured renal tubular cells exposed to cisplatin demonstrated apoptotic and necrotic cell death [12] . These results were confirmed in animals, where both necrosis and apoptosis were induced in renal tubules following cisplatin administration [13] , [14] and [15] . Another study found that cisplatin administration in rats increases oxidative stress resulting in down-regulation of tight junction proteins and potentiation of proximal tubule damage [16] . Reducing exposure of tubule cells to cisplatin is an approach to limit kidney toxicity.
Prior research demonstrated enhanced kidney injury in Nrf2-null mice [17] and [18] suggesting a protective effect from this transcription factor. Under non-stressed conditions, Nrf2 is sequestered in the cytosol by kelch-like ECH associating protein 1 (Keap1) [19] . However, when oxidative stress, electrophilic stress, or the presence of Nrf2 activators becomes prevalent, Nrf2 and Keap1 dissociate resulting in accumulation of free Nrf2 in the cytosol and an increase in Nrf2 translocation into the nucleus [20] . Once in the nucleus, Nrf2 heterodimerizes and binds to antioxidant response elements (ARE) which leads to the transcription of various cytoprotective and antioxidant genes [21] . Activation of transcription factors such as Nrf2 that regulate uptake and efflux transporters localized to kidney proximal tubule is a strategy to modulate cisplatin exposures [6] and [17] . As Nrf2 can regulate proteins involved in the metabolism and excretion of organic chemicals [22] , treatment with Nrf2 activating compounds, including oltipraz, sulforaphane, and oleanolic acid, would be a plausible approach to limit exposure of human kidney cells to cisplatin. For the current study, we aimed to explore the therapeutic potential of known Nrf2 activators to modulate cisplatin-induced human kidney cell injury. Furthermore, we sought to determine optimal exposure regimens for Nrf2 activating compounds for nephrotoxicity prophylaxis and favorable effects on Nrf2 antioxidant genes with exposure to cisplatin.
2. Materials and methods
2.1. Cell culture & reagents
Human embryonic kidney 293 (HEK293, ATCC, Rockville, MD) and human proximal tubule epithelial (hPTC, ScienCell, Carlsbad, CA) cells were used for in vitro studies. HEK293 cells were routinely grown and maintained in Dulbeccos modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin per product information (Life Technologies, Grand Island, NY). Epithelial cell medium (EpiCM, ScienCell) supplemented with 2% fetal bovine serum, 1% epithelial cell growth supplement, and 1% Penicillin/Streptomycin was used to culture hPTC cells. Cells were maintained at 37 °C in a humidified incubator with an atmosphere of 5% CO2 . Cisplatin (Sigma Chemical Co., St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) (Sigma Chemical Co.) to 100 mM. Nrf2 activators (sulforaphane, oltipraz, and oleanolic acid) were purchased (Sigma Chemical Co.) and dissolved in DMSO. Cells were treated with Nrf2 activators either before or after cisplatin exposure. Unless specified otherwise, the doses of Nrf2 activators (sulforaphane, oltipraz, and oleanolic acid) were 5, 12, and 5 μM, respectively. RNA extraction kits were purchased from Life Technologies. PCR reagents and Taqman gene expression assays were obtained from Life Technologies.
2.2. Cell viability assay
HEK293 and hPTCs were plated in a 96-well configuration. Cells were incubated overnight at 37 °C in a humidified incubator with an atmosphere of 5% CO2 prior to treatment. The cell treatments consisted of 1) 0.1% vehicle control (DMSO), 2) cisplatin at 0, 50, 80 μM doses (LC50 23.4–60 μM), 3) Nrf2 activator [sulforaphane (5 μM), oleanolic acid (5 μM), or oltipraz (12 μM)], or 4) cisplatin (at the above doses) combined with a Nrf2 activator at specified doses. Cells were incubated with Nrf2 activators for 12, 24, or 48 h beginning either 3 h prior to cisplatin or 3 h after initiation of cisplatin exposure. After the specified incubation time, MTT reagent (2 mg/mL) (Sigma Aldrich) in DMEM or EpiCM containing no FBS was added and cells were incubated for 4 h at 37 °C. MTT reagent was removed after the 4 h incubation period, MTT solubility solution (2% HCl, 25% H2 O, 73% 2-propanol) was added and cell viability was analyzed at 550 nm by VersaMax microreader plate (Molecular Devices, Sunnyvale, CA).
2.3. Gene expression
Gene expression was only evaluated in hPTCs to enable a closer in vitro approximation to humans. The expression of NFE2L2 /NRF2 and detoxifying enzymes GCLC and NQO1 were evaluated in hPTCs that received Nrf2 activators before and after treatment with cisplatin. For these studies cisplatin doses of low, moderate, and high were chosen. The cell treatments consisted of 1) 0.1% vehicle control (DMSO), 2) cisplatin at 0, 5, 25 and 80 μM doses, 3) Nrf2 activator [sulforaphane (5 μM), oleanolic acid (5 μM), or oltipraz (12 μM)], or 4) cisplatin (5, 25, 80 μM) combined with a Nrf2 activator at the specified doses. Cells were harvested for gene expression studies at 12, 24, or 48 h post treatment. Cells were collected and lysed and total mRNA prepared from cell lysates using Ambion RNA Extraction Kit (Life Technologies). cDNA was generated using Taqman reverse transcription reagents (Applied Biosystems) and an Applied Biosystems 2720 thermal cycler. Commercial gene expression assays: GCLC (Hs00155249), NQO1 (Hs02512143), NFE2L2 (Hs00975961), and housekeeping gene GAPDH (Hs02758991) were used (Applied Biosystems). Real-time PCR was performed using the 7500 Real Time PCR system. Generated data was analyzed by relative quantitation using the comparative CT method (2−ΔΔCT ).
2.4. Statistical analysis
The data were expressed as mean ± SEM. Graphs were created using Graph Pad Prism 4.0 (GraphPad Software, Inc., San Diego, CA). Analysis was performed using GraphPad InStat 3.0. Data were analyzed using the one-way analysis of variance with Student–Newman–Keuls post hoc test for groups of 3 or more. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Cisplatin toxicity: exposure of hPTC and HEK293 cells to cisplatin
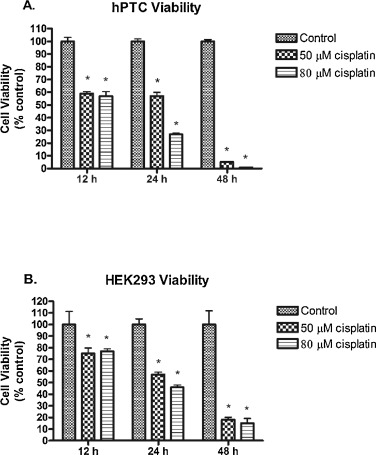
We analyzed the viability of hPTCs and HEK293 cells following exposure to cisplatin (50 and 80 μM) for 12, 24, or 48 h. Cisplatin doses of 50 μM and above resulted in significantly lower hPTC and HEK293 cell viability. hPTCs and HEK293 cells treated with cisplatin alone demonstrated decreased survival over time, with hPTCs demonstrating more sensitivity to cisplatin (Fig. 1 A & B). After 12, 24, and 48 h incubation with cisplatin, only 59%, 57% and 5% of hPTCs treated with 50 μM cisplatin were viable compared to vehicle controls, respectively (Fig. 1 A). HEK293 cells incubated with cisplatin (50 μM) exhibited 75% viability during the first 12 h of exposure (Fig. 1 B). After 24 and 48 h incubation with cisplatin (50 μM), cell viability was 57% and 18%, respectively (Fig. 1 B). Cell viability was further decreased in hPTCs and HEK293 cells treated with 80 μM cisplatin (Fig. 1 A & B).
|
|
|
Fig. 1. Cell viability of hPTCs and HEK293 cells after exposure to 50 or 80 μM cisplatin. (A). hPTC viability after 12, 24 or 48 h incubation with 50 or 80 μM cisplatin compared to vehicle control (DMSO). (B). HEK293 viability after 12, 24 h or 48 h incubation with 50 or 80 μM cisplatin compared to vehicle control (DMSO). Data are means ± SEM. (n = 4-6). *p < 0.05 compared to vehicle control (DMSO). |
3.2. Cisplatin toxicity: pre- and delayed-treatment with NRF2 activators
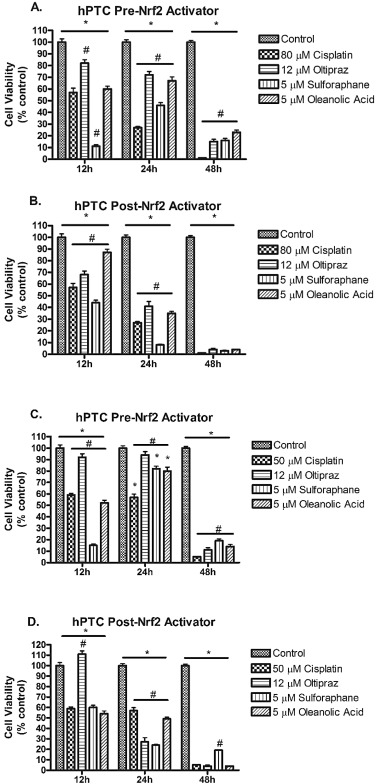
hPTCs cells were treated before (pre-treatment) or after (delayed treatment) cisplatin (50 and 80 μM) with Nrf2 activators for 12, 24, and 48 h, respectively. Preliminary cell viability experiments determined the optimal Nrf2 activator doses for sulforaphane, oleanolic acid, and oltipraz to be 5 μM, 5 μM, and 12 μM, respectively. hPTCs treated with 80 μM cisplatin had decreased cell viability compared to vehicle controls (Fig. 2 A). hPTCs pre-treated with oltipraz or oleanolic acid had greater cell viability relative to cisplatin 80 μM alone treated cells (Fig. 2 A). Sulforaphane demonstrated less impressive results than oltipraz and oleanolic acid when compared to cisplatin 80 μM alone and vehicle control treated cells. However, pre-treatment with sulforaphane displayed greater viability at 24 h and 48 h relative to cisplatin alone treated cells. Delayed-treatment with oltipraz and oleanolic acid at 12 and 24 h demonstrated higher cell viability as compared to cisplatin 80 μM alone treated cells (Fig. 2 B). Delayed-treatment with sulforaphane failed to demonstrate improved cell viability over cisplatin 80 μM alone treated cells. hPTCs treated with 50 μM cisplatin and pre-treatment with Nrf2 activators resulted in comparable cell viability to results observed at 80 μM (Fig. 2 C). Delayed-treatment with oltipraz resulted in significantly enhanced cell viability compared to control and cisplatin 50 μM alone at 12 h (Fig. 2 D).
|
|
|
Fig. 2. Survival of hPTCs treated pre- or delayed-cisplatin (50 or 80 μM) with Nrf2 activators [oltipraz (12 μM), sulforaphane (5 μM), oleanolic acid (5 μM)] incubated for 12, 24, or 48 h. (A) hPTCs treated pre- cisplatin (80 μM) with Nrf2 activators, (B) hPTC treated with delayed- cisplatin (80 μM) with Nrf2 activators, (C) hPTCs treated pre- cisplatin (50 μM) with Nrf2 activators, and (D) hPTCs treated with delayed- cisplatin (50 μM) with Nrf2 activators. Data are means ± SEM. (n = 4–6). *p < 0.05 compared to vehicle controls (DMSO), #p < 0.05 compared to 50 μM or 80 μM cisplatin-treated cells. |
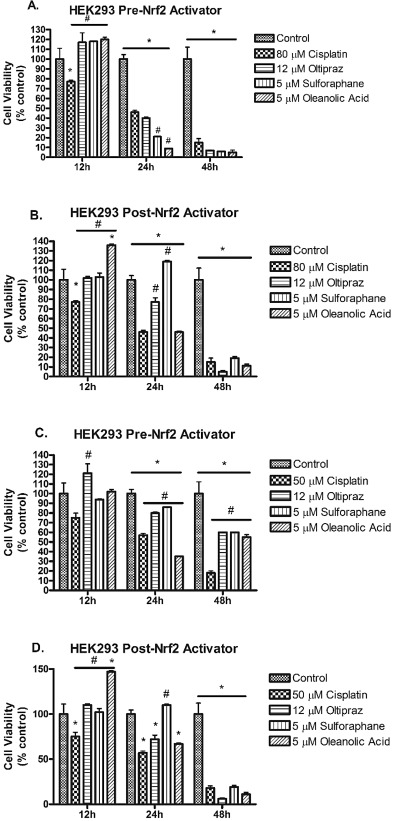
HEK293 cells were also treated before (pre-treatment) or after (delayed treatment) cisplatin (80 μM) with Nrf2 activators for 12, 24, and 48 h. HEK293 cells treated pre- and delayed-cisplatin 80 μM with Nrf2 activators at 12 h resulted in enhanced cell viability in comparison to cisplatin alone (Fig. 3 A). Delayed-treatment with sulforaphane at 24 h resulted in statistically significantly higher cell viability compared to vehicle controls and cisplatin 80 μM alone treatment (Fig. 3 B). Delayed-treatment with oltipraz at 24 h resulted in higher cell viability compared to cisplatin treatment (Fig. 3 B). Pre-treatment with the Nrf2 activators at 24 h and 48 h and delayed-treatment at 48 h resulted in HEK293 cell viability that was less than vehicle controls and cisplatin alone treatments. Pre-treatment with Nrf2 activators resulted in enhanced cell viability compared to cisplatin 50 μM alone treatments at 48 h (Fig. 3 C). HEK293 cells treated with 50 μM cisplatin and delayed treatment with Nrf2 activators displayed comparable cell viability to results observed at 80 μM (Fig. 3 D).
|
|
|
Fig. 3. Survival of HEK293 cells treated pre- or delayed-cisplatin (50 or 80 μM) with Nrf2 activators [oltipraz (12 μM), sulforaphane (5 μM), oleanolic acid (5 μM)] incubated for 12, 24, or 48 h. (A) HEK293 cells treated pre- cisplatin (80 μM) with Nrf2 activators, (B) HEK293 cells treated with delayed-cisplatin (80 μM) with Nrf2 activators, (C) HEK293 cells treated pre-cisplatin (50 μM) with Nrf2 activators, and (D) HEK293 cells treated with delayed-cisplatin (50 μM) with Nrf2 activators. Data are means ± SEM. (n = 4–6). *p < 0.05 compared to vehicle controls (DMSO), #p < 0.05 compared to 50 μM or 80 μM cisplatin-treated cells. |
3.3. Expression of Nrf2, GCLC, and NQO1 in hPTCs
3.3.1. Cisplatin alone
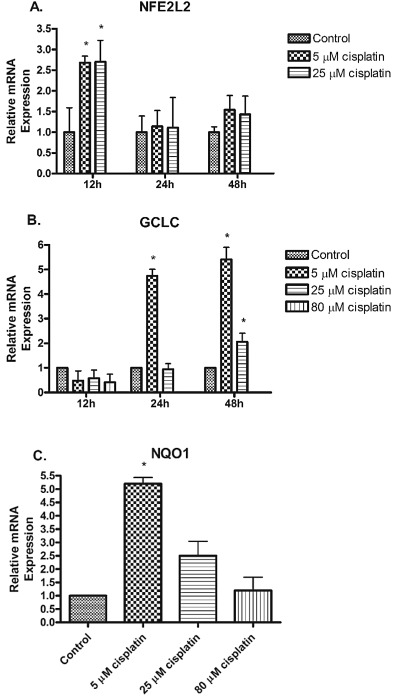
The mRNA expression of antioxidant genes was evaluated in hPTCs exposed to cisplatin (5, 25, and 80 μM). Cisplatin doses were chosen to represent low, moderate, and high exposures in humans. Treatment with 5 μM and 25 μM cisplatin resulted in increased mRNA expression of NFE2L2 at 12 h compared to vehicle controls (Fig. 4 A). There was no observable expression with 80 μM cisplatin dose. Cisplatin treatment at 24 h (5 μM) incubation and at 48 h incubation (5 μM and 25 μM) resulted in increased mRNA expression of the Nrf2 target gene GCLC over vehicle control ( Fig. 4 B). Cisplatin treatment with 5 and 25 μM resulted in increased mRNA expression of NQO1 at 24 h compared to vehicle controls (Fig. 4 C). There was no observable expression at 12 h and 48 h incubation. Treatment with 80 μM cisplatin displayed gene expression that was similar to vehicle control conditions.
|
|
|
Fig. 4. mRNA expression of antioxidant genes NFE2L2, GCLC , and NQO1 in hPTCs. Total mRNA was isolated from hPTCs following treatment with cisplatin 5 μM, 25 μM, or 80 μM after 12, 24, and/or 48 h and probed for (A) NFE2L2 , (B) GCLC , and (C) NQO1 . Gene expression was analyzed by RT-PCR. GAPDH was used as endogenous control. Data are means ± SEM. (n = 3) *p < 0.05 compared to vehicle control (DMSO). |
3.4. Nrf2 activators alone
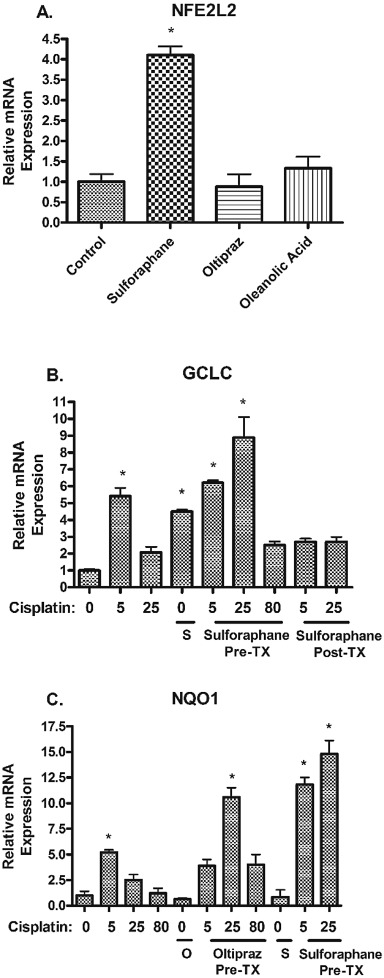
Sulforaphane alone resulted in a 4-fold increase in mRNA expression of NFE2L2 compared to vehicle controls at 24 h (Fig. 5 A). Neither oltipraz nor oleanolic acid led to an increase in NFE2L2 mRNA expression. Treatment with sulforaphane alone resulted in an up-regulation of GCLC expression 4.5-fold, at 48 h compared to vehicle controls (Fig. 5 B).
|
|
|
Fig. 5. mRNA expression of antioxidant genes NFE2L2, GCLC , and NQO1 in hPTCs exposed to Nrf2 activators. (A) NFE2L2 mRNA expression was assessed at 24 h after administration of Nrf2 activators. (B) GCLC mRNA expression was assessed at 48 h after administration of Nrf2 activators and pre- or delayed-cisplatin administration. (C) NQO1 mRNA expression was assessed at 24 h after administration of Nrf2 activators and pre- or delayed-cisplatin administration. Doses of Nrf2 activators were: sulforaphane (5 μM), oltipraz (12 μM), and oleanolic acid (5 μM). S = sulforaphane and O = oltipraz. Gene expression was analyzed by RT-PCR. GAPDH was used as endogenous control. Data are means ± SEM. (n = 3) *p < 0.05 compared to vehicle control (DMSO). |
3.5. Pre- and delayed-treatment with Nrf2 activators
Pre- and delayed-cisplatin treatment with oleanolic acid had no effect on mRNA expression of NFE2L2 or detoxifying genes (GCLC and NQO1 ) (data not shown). Pre- and delayed-cisplatin treatment with sulforaphane resulted in an enhancement of GCLC mRNA expression compared to vehicle controls at 48 h (Fig. 5 B). However, the transcription effects were greater when sulforaphane was administered prior to cisplatin. Pre-treatment with sulforaphane and oltipraz resulted in increased NQO1 mRNA expression at 24 h compared to vehicle controls (Fig. 5 C). Changes to NQO1 mRNA expression were not observed with sulforaphane or oltipraz treatment alone. Pre- and delayed-cisplatin treatment with Nrf2 activators did not enhance mRNA expression of NFE2L2.
4. Discussion
The present study investigated the feasibility of three known Nrf2 activators to mitigate cisplatin toxicity in human kidney cells. HEK293 cells were selected for this study as an immortalized in vitro kidney model. hPTCs were selected for this study to enable a close in vitro approximation to humans. hPTC were used in all gene expressions studies due to the lack of previously published data in primary cells and due to their higher sensitivity to drug-induced toxicity. Exposure to Nrf2 activators produced favorable patterns of viability in both hPTCs and HEK293 cells. hPTCs pre-treated with Nrf2 activators exhibited favorable cell viability compared to cisplatin-only treated cells, with oltipraz and oleanolic acid demonstrating the most favorable cell viability profile. HEK293 cells displayed higher cell viability when treated with pre- and delayed-Nrf2 activators versus cisplatin alone treatment, with all three Nrf2 activators performing similarly. Previous studies demonstrated, in vitro , that pre-treatment with sulforaphane, oleanolic acid, and oltipraz results in higher cell viability upon exposure to cisplatin and other toxicants [23] , [24] and [25] . The previously published data, in addition to our current findings in hPTCs, support cell viability protection of Nrf2 activators with anticipated cisplatin exposures. These data suggest that Nrf2 activators may be promising therapeutics to limit tubular apoptosis and necrosis during AKI and to stimulate subsequent regeneration.
In contrast to previous studies, we investigated a comparison between three known Nrf2 activators. In addition to Nrf2 activators, several studies have evaluated other compounds as potential modulators of cisplatin-induced toxicity and oxidative stress [26] , [27] and [28] . For example, pre-stimulation of the kallikrein system with a high potassium diet was found to reduce tissue markers of oxidative stress in a model of nephrotoxicity induced by cisplatin [26] .
A novel assessment in the current study is the finding of differential effects of the three investigated Nrf2 activators on expression of antioxidant defense genes in human kidney cells and evaluation of pre-versus delayed-exposure regimens. Sulforaphane exhibited the greatest induction of GCLC and NQO1 when administered concomitantly with cisplatin, with the collective results demonstrating greatest antioxidant effects when sulforaphane was administered prior to cisplatin exposure. Since sulforaphane alone resulted in an increase in NFE2L2 mRNA expression, induction of Nrf2 inducible genes such as GCLC prior to cisplatin would contribute to a primed anti-oxidant environment and protect cells against oxidative damage allowing survival and regenerative responses The renoprotective effect of sulforaphane was mediated, at least in part, by activation of the GCLC detoxifying gene.
Oleanolic acid alone resulted in a modest increase in NFE2L2 mRNA expression. These findings are in contrast to previous studies conducted in acetaminophen hepatoxicity models where oleanolic acid increased mRNA expression by 4-fold in livers of wild-type mice [29] . It is important to note that the concentrations of oltipraz and oleanolic acid were lower in our study using human kidney cells versus data previously reported in RAW 264.7 and PC12 cells [24] and [25] , and may contribute to our findings Moreover, previous studies suggest oleanolic acid is unable to activate the Nrf2 pathway due to the lack of functional groups including Michael acceptors [30] and [31] , as well as an inability to induce Nqo1 activity at low doses in vitro in hepa1c1c7 murine hepatoma cells [30] . Doses of Nrf2 activators used in our study were selected based on preliminary cell viability experiments that determined optimal Nrf2 activator doses for sulforaphane, oleanolic acid, and oltipraz.
We examined NFE2L2 expression in hPTCs pre- or delayed-cisplatin with Nrf2 activators, but did not observe any induction. A previous report suggested that mice treated with Nrf2 activators including oltipraz and sulforaphane have enhanced Nrf2-mediated transcription in the liver but not in the kidneys [17] , suggesting a differential tissue-specific Nrf2 activation response. It is currently unknown whether this response is due to differences in tissue disposition related to Nrf2 activator pharmacokinetics [17] . Future studies will be designed to understand how sulforaphane, oltipraz, and oleanolic acid activate Nrf2 in the kidneys. In addition to Nrf2-dependent effects, secondary effects independent of Nrf2 may also contribute to sulforaphane, oltipraz, and oleanolic acids therapeutic benefits. Microarray data suggest sulforaphane-induced gene expression that is independent of Nrf2 in select cell models. [32] and [33] . Sulforaphane was shown to inhibit inflammasomes through a Nrf2-independent mechanism, by modulation of apoptotic pathways [34] . Oleanolic acid can induce metallothionein in both mice and rats. Metallothionein has an ARE sequence in its promoter region which is induced by Nrf1, and is shown to protect against acetaminophen hepatotoxicity and cadmium toxicity [35] and [36] . Oltipraz is not a potent or typical Nrf2 activator and thus its effects may not be solely due to Nrf2 activation. Oltipraz is known to activate aryl hydrocarbon receptor [37] and constitutive androstane receptor, both of which activates NQO1 expression [38] .
In summary, our results demonstrate beneficial effects of Nrf2 activating agents on viability of human kidney cells and expression of antioxidant and efflux transporter genes. Agents such as sulforaphane and oltipraz may be potential therapies to enhance nephro-protectant effects for patients undergoing treatment with known toxicants such as cisplatin. There appears to be compound specific effects of Nrf2 activating agents on antioxidant pathways. While weak activation of Nrf2 was observed with Nrf2 activators, collectively, increased cell viability and increased mRNA expression of Nrf2-target genes provide evidence for the benefits of select Nrf2 activators as potential therapies. Ongoing in vitro and in vivo investigations are evaluating mechanistic pathways involved in kidney cells exposed to toxicants including cisplatin and benefits of Nrf2 activators on nephroprotection.
Transparency document
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK093903 , DK080774 ], the National Institute of Environmental Health Sciences [Grants ES005022 ], and the National Institute of General Medical Sciences [Grants GM107122 ], components of the National Institutes of Health.
References
- [1] R.S. Goldstein, G.H. Mayor; The nephrotoxicity of cisplatin; Life Sci., 32 (7) (1983), pp. 685–690
- [2] A.H. Lau; Apoptosis induced by cisplatin nephrotoxic injury; Kidney Int., 56 (4) (1999), pp. 1295–1298
- [3] D.M. Townsend, M. Deng, L. Zhang, M.G. Lapus, M.H. Hanigan; Metabolism of cisplatin to a nephrotoxin in proximal tubule cells; J. Am. Soc. Nephrol., 14 (1) (2003), pp. 1–10
- [4] N.A.G. dos Santos, M.A.C. Rodrigues, N.M. Martins, A.C. dos Santos; Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update; Arch. Toxicol., 86 (8) (2012), pp. 1233–1250
- [5] C.A. Naughton; Drug-induced nephrotoxicity; Am. Fam. Physician, 78 (6) (2008), pp. 743–750
- [6] N. Pabla, Z. Dong; Cisplatin nephrotoxicity: mechanisms and renoprotective strategies; Kidney Int., 73 (9) (2008), pp. 994–1007
- [7] L.A.B. Peres, A.D.D. Cunha Junior; Acute nephrotoxicity of cisplatin: molecular mechanisms; J. Bras. Nefrol., 35 (4) (2013), pp. 332–340
- [8] J.B. Tarloff, L.H. Lash; Toxicology of the Kidney; (3rd ed.)CRC Press (2005), pp. 779–784
- [9] G. Ramesh, W.B. Reeves; Inflammatory cytokines in acute renal failure; Kidney Int. Suppl (91) (2004), pp. S56–S61
- [10] P. Devarajan; Update on mechanisms of ischemic acute kidney injury; J. Am. Soc. Nephrol., 17 (6) (2006), pp. 1503–1520
- [11] S.F. Ma, M. Nishikawa, K. Hyoudou, R. Takahashi, M. Ikemura, Y. Kobayashi, F. Yamashita, M. Hashida; Combining cisplatin with cationized catalase decreases nephrotoxicity while improving antitumor activity; Kidney Int., 72 (12) (2007), pp. 1474–1482
- [12] W. Lieberthal, V. Triaca, J. Levine; Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs necrosis; Am. J. Physiol., 270 (4 Pt 2) (1996), pp. F700–F708
- [13] J. Megyesi, R.L. Safirstein, P.M. Price; Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure; J. Clin. Invest., 101 (4) (1998), pp. 777–782
- [14] G. Ramesh, W.B. Reeves; TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure; Am. J. Physiol. Ren. Physiol., 285 (4) (2003), pp. F610–F618
- [15] H. Liu, R. Baliga; Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis; Kidney Int., 63 (5) (2003), pp. 1687–1696
- [16] J. Trujillo, E. Molina-Jijon, O.N. Medina-Campos, R. Rodriguez-Munoz, J.L. Reyes, M.L. Loredo, E. Tapia, L.G. Sanchez-Lozada, D. Barrera-Oviedo, J. Pedraza-Chaverri; Renal tight junction proteins are decreased in cisplatin-induced nephrotoxicity in rats; Toxicol. Mech. Methods, 24 (7) (2014), pp. 520–528
- [17] L.M. Aleksunes, M.J. Goedken, C.E. Rockwell, J. Thomale, J.E. Manautou, C.D. Klaassen; Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity; J. Pharmacol. Exp. Ther., 335 (1) (2010), pp. 2–12
- [18] Y. Tanaka, L.M. Aleksunes, M.J. Goedken, C. Chen, S.A. Reisman, J.E. Manautou, C.D. Klaassen; Coordinated induction of Nrf2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity; Toxicol. Appl. Pharmacol., 231 (3) (2008), pp. 364–373
- [19] K. Itoh, N. Wakabayashi, Y. Katoh, T. Ishii, K. Igarashi, J.D. Engel, M. Yamamoto; Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain; Genes Dev., 13 (1) (1999), pp. 76–86
- [20] W. Li, A.N. Kong; Molecular mechanisms of Nrf2-mediated antioxidant response; Mol. Carcinog., 48 (2) (2009), pp. 91–104
- [21] K. Itoh, T. Chiba, S. Takahashi, T. Ishii, K. Igarashi, Y. Katoh, T. Oyake, N. Hayashi, K. Satoh, I. Hatayama, M. Yamamoto, Y. Nabeshima; An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements; Biochem. Biophys. Res. Commun., 236 (2) (1997), pp. 313–322
- [22] L.M. Aleksunes, C.D. Klaassen; Coordinated regulation of hepatic Phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPAR alpha-, and Nrf2-null mice; Drug Metab. Dispos., 40 (7) (2012), pp. 1366–1379
- [23] C.E. Guerrero-Beltran, M. Calderon-Oliver, E. Tapia, O.N. Medina-Campos, D.J. Sanchez-Gonzalez, C.M. Martinez-Martinez, K.M. Ortiz-Vega, M. Franco, J. Pedraza-Chaverri; Sulforaphane protects against cisplatin-induced nephrotoxicity; Toxicol. Lett., 192 (3) (2010), pp. 278–285
- [24] M.E. Killeen, J.A. Englert, D.B. Stolz, M. Song, Y. Han, R.L. Delude, J.A. Kellum, M.P. Fink; The phase 2 enzyme inducers ethacrynic acid dl -sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells ; J. Pharmacol. Exp. Ther., 316 (3) (2006), pp. 1070–1079
- [25] S.J. Tsai, M.C. Yin; Antioxidant and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells; J. Food Sci., 73 (7) (2008), pp. H174–H178
- [26] A. Aburto, A. Barria, A. Cardenas, D. Carpio, C.D. Figueroa, M.E. Burgos, L. Ardiles; Pre-stimulation of the kallikrein system in cisplatin-induced acute renal injury: an approach to renoprotection; Toxicol. Appl. Pharmacol., 280 (2) (2014), pp. 216–223
- [27] R. Hamad, C. Jayakumar, P. Ranganathan, R. Mohamed, M.M. El-Hamamy, A.A. Dessouki, A. Ibrahim, G. Ramesh; Honey feeding protects kidney against cisplatin nephrotoxicity through suppression of inflammation; Clin. Exp. Pharmacol. Physiol., 42 (8) (2015), pp. 843–848
- [28] F. Funk, K. Kruger, C. Henninger, W. Watjen, P. Proksch, J. Thomale, G. Fritz; Spongean alkaloids protect rat kidney cells against cisplatin-induced cytotoxicity; Anticancer Drugs, 25 (8) (2014), pp. 917–929
- [29] S.A. Reisman, L.M. Aleksunes, C.D. Klaassen; Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes; Biochem. Pharmacol., 77 (7) (2009), pp. 1273–1282
- [30] A.T. Dinkova-Kostova, K.T. Liby, K.K. Stephenson, W.D. Holtzclaw, X. Gao, N. Suh, C. Williams, R. Risingsong, T. Honda, G.W. Gribble, M.B. Sporn, P. Talalay; Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress; Proc. Natl. Acad. Sci. U. S. A., 102 (12) (2005), pp. 4584–4589
- [31] M.S. Yates, M. Tauchi, F. Katsuoka, K.C. Flanders, K.T. Liby, T. Honda, G.W. Gribble, D.A. Johnson, J.A. Johnson, N.C. Burton, T.R. Guilarte, M. Yamamoto, M.B. Sporn, T.W. Kensler; Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes; Mol. Cancer Ther., 6 (1) (2007), pp. 154–162
- [32] R. Hu, C. Xu, G. Shen, M.R. Jain, T.O. Khor, A. Gopalkrishnan, W. Lin, B. Reddy, J.Y. Chan, A.N. Kong; Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice; Cancer Lett., 243 (2) (2006), pp. 170–192
- [33] A.D. Kraft, D.A. Johnson, J.A. Johnson; Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult; J. Neurosci., 24 (5) (2004), pp. 1101–1112
- [34] A.J. Greaney, N.K. Maier, S.H. Leppla, M. Moayeri; Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism; J. Leukoc. Biol. (2015) jib-3A0415
- [35] Y. Liu, H. Kreppel, J. Liu, S. Choudhuri, C.D. Klaassen; Oleanolic acid protects against cadmium hepatotoxicity by inducing metallothionein; J. Pharmacol. Exp. Ther., 266 (1) (1993), pp. 400–406
- [36] M. Ohtsuji, F. Katsuoka, A. Kobayashi, H. Aburatani, J.D. Hayes, M. Yamamoto; Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes; J. Biol. Chem., 283 (48) (2008), pp. 33554–33562
- [37] E. Le Ferrec, D. Lagadic-Gossmann, C. Rauch, C. Bardiau, K. Maheo, F. Massiere, M. Le Vee, A. Guillouzo, F. Morel; Transcriptional induction of CYP1A1 by oltipraz in human Caco-2 cells is aryl hydrocarbon receptor- and calcium-dependent; J. Biol. Chem., 277 (27) (2002), pp. 24780–24787
- [38] M.D. Merrell, J.P. Jackson, L.M. Augustine, C.D. Fisher, A.L. Slitt, et al.; The Nrf2 activator oltipraz also activates the constitutive androstane receptor; Drug Metab. Dis., 36 (8) (2008), pp. 1716–1721
Document information
Published on 02/05/17
Accepted on 02/05/17
Submitted on 02/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?