Abstract
Increasingly tighter regulations regarding organic waste, and the demand for renewable chemicals and fuels, are pushing the manufacturing industry toward higher sustainability to improve cost-effectiveness and meet customers’ demand. Food waste valorization is one of the current research areas that has attracted a great deal of attention over the past few years as a potential alternative to the disposal of a wide range of residues in landfill sites. In particular, the development of environmentally sound and innovative strategies to process such waste is an area of increasing importance in our current society. Landfill, incineration and composting are common, mature technologies for waste disposal. However, they are not satisfactory to treating organic waste due to the generation of toxic methane gas and bad odor, high energy consumption and slow reaction kinetics. In fact, research efforts have also been oriented on novel technologies to decompose organic waste. However, no valuable product is generated from the decomposition process. Instead of disposing and decomposing food waste, recent research has focused on its utilization as energy source (e.g., for bioethanol and biodiesel production). Organic waste is also useful to generate useful organic chemicals via biorefinery or white biotechnology (e.g., succinic acid and/or bio-plastics). This article is aimed to summarize recent development of waste valorization strategies for the sustainable production of chemicals, materials, and fuels through the development of green production strategies. It will also provide key insights into recent legislation on management of waste worldwide as well as two relevant case studies (the transformation of corncob residues into functionalized biomass-derived carbonaceous solid acids and their utilization in the production of biodiesel-like biofuels from waste oils in Philippines, as well as the development of a bakery waste based biorefinery for succinic acid and bioplastic production in Hong Kong) to illustrate the enormous potential of biowaste valorization for a more sustainable society. Future research directions and possible sustainable approaches will also be discussed with their respective proofs of concept.
Introduction
Climate change, energy crisis, resource scarcity, and pollution are major issues humankind will be facing in future years. Sustainable development has become a priority for the worlds policy makers since humanitys impact on the environment has been greatly accelerated in the past century with rapidly increasing population and the concomitant sharp decrease of ultimate natural resources. Finding alternatives and more sustainable ways to live, in general, is our duty to pass on to future generations, and one of these important messages relates to waste. Waste from different types (e.g., agricultural, food, industrial) is generated day by day in extensive quantities, generating a significant problem in its management and disposal. A widespread feeling of “environment in danger” has been present everywhere in our society in recent years, which, however, has not yet crystallized in a general concienciation of cutting waste production in our daily lives. Many methods could achieve sustainable development, methods that could not only improve waste management but could also lead to the production of industrially important chemicals, materials, and fuels, in essence, valuable end products from waste.
Waste valorization is the process of converting waste materials into more useful products including chemicals, materials, and fuels. Such concept has already existed for a long time, mostly related to waste management, but it has been brought back to our society with renewed interest due to the fast depletion of natural and primary resources, the increased waste generation and landfilling worldwide and the need for more sustainable and cost-efficient waste management protocols. Various valorization techniques are currently showing promise in meeting industrial demands. One among such promising waste valorization strategies is the application of flow chemical technology to process waste to valuable products. A recent review of Ruiz et al. [1] highlighted various advantages of continuous flow processes particularly for biomass and/or food waste valorization which included reaction control, ease of scale-up, efficient reaction cycles producing more yield, and no required catalyst separation. Although flow chemistry has been known to be used in industries for other processing methodologies, it still remains to be used in biomass/waste valorization – a limitation caused by the large energy needed to degrade highly stable biopolymers and recalcitrant compounds (e.g., lignin). The deconstruction of such biopolymers, most of the time, requires extreme conditions of pressure and temperature – conditions achieved by microwave heating, which is another green valorization technology. These requirements are not simple to satisfy and various techniques (e.g., microwave irradiation) need to be combined to satisfy the prerequisites for a successful transformation of waste. However, the main challenge for this combination is on the scale-up itself. As conceptualized by Glasnov et al. [2] microwave and flow chemistries maybe coupled by attaching back-pressure regulators to flow devices. This approach can revolutionize industrial valorization since it will synthesize products fast (due to microwave heating) on one continuous run (flow process). Although the approach presented is possible, the main challenge of temperature transfer from microwave to flow remains to be solved. A buildup of temperature gradient inside the instrument could lead to various instrument inefficiencies.
Another valorization strategy is related to the use of pyrolysis in the synthesis of fuels. This involves biomass heating at high temperatures in the absence of oxygen to produce decomposed products [3, 4]. Although pyrolysis is a rather old method for char generation, it has been recently utilized to produce usable smaller molecules from very stable biopolymers. This method has been particularly employed in the production of Bio-Oil (a liquid, of relatively low viscosity that is a complex mixture of short-chain aldehydes, ketones, and carboxylic acids). In a study by Heo et al. [5], several conditions for the fast pyrolysis of waste furniture sawdust were studied, and it was found that bio-oil yields do not necessarily increase with temperature. The optimized pyrolysis temperature was set at 450°C (57% bio-oil yield) using a fluidized bed reactor. The reason for the nonlinear dependence bio-oil yield/temperature is the possible decomposition of small molecules into simpler gases. This theory is supported by the increase in the amount of gaseous products found at increasing temperatures. A separate study by Cho et al. [6] employed fast pyrolysis under a fluidized bed reactor to recover BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) from mixed plastics. The highest BTEX yield was obtained at 719°C. The pyrolysis of cotton stalks was also reported to produce second generation biofuels [7]. The study found that at much higher temperatures of pyrolysis the amounts of H2 and CO collected increased, while CO2 levels lowered. The decrease in CO2 production could be due to the degradation of the gas at much higher temperatures producing CO and O2. More recently, synergy between these first proposed technologies (microwave and pyrolysis) has been also reported to constitute a step forward toward more environmentally friendly low temperature pyrolysis protocols for bio-oil and syngas production [8]. Microwave-assisted pyrolysis of a range of waste feedstock can provide a tuneable and highly versatile option to syngas with tuneable H2/CO ratios or bio-oil-derived biofuels via subsequent upgrading of the pyrolysis oil [8].
Aside from energy applications, pyrolysis can also be used to produce advanced materials including carbon nanotubes and graphene-like materials, which have a wide range of applications. These studies along with many others in literature illustrate the potential of pyrolysis to convert waste materials into valuable chemicals.
A third green method of valorization would be on the use of biological microorganisms to degrade complex wastes and produce fuel. The method is used by taking advantage of cellulose (or any biopolymer) degrading enzymes by microorganisms as demonstrated by Wulff et al. [9]. In their work, cellulase Xf818 was isolated from the plant pathogen Xylella fastidiosa (known to cause citrus variegated chlorosis in plants). The gene responsible for the enzyme was also probed and then later on expressed on Escherichia coli. Such enzyme was found to be mostly active in the hydrolysis of carboxymethyl celluloses, oat spelt xylans, and wood xylans.
Bioconversion has been under intensive research for the past years, and one of the most significant advances in the field relates to the possibility of a synthetic control of microorganisms’ metabolic pathways to produce favorable metabolic processes, which will in turn increase the yield of products. A notable example is the use of a bioengineered E. coli to produce higher alcohols including isobutanol, 1-butanol, 2-methyl-1-butanol, 3-methyl-1-butanol and 2-phenylethanol from glucose [10]. The protocol was amenable for the conversion of 2-ketoacid intermediates (from amino acid biosynthesis) into alcohols by amplifying expression of 2-ketoacid decarboxylases and alcohol dehydrogenases.
To model the design for isobutanol, the gene ilvIHCD was over-expressed with the PLlacO1 promoter in a plasmid to amplify 2-ketoisovalerate biosynthesis. Other genes were tested such as alsS gene (from Bacillus subtilis) to further improve the alcohol yield while some genes responsible for by-product formation (adhE, ldhA, frdAB, fnr, pta) and pyruvate competition (pflB) were silenced. Overall, the isobutanol yield reached ~300 mmol/L (22 g/L) under microaerobic conditions.
The three presented strategies (microwave, pyrolysis, and bioengineering) represent some of the most important valorization methodologies. With the rapid advancement of these fields in waste valorization, it is expected that most industrial sustainability practices will have a different focus in various future scenarios.
Waste valorization is currently geared toward three sustainable paths: one would be on the production of fuel and energy to replace common fossil fuel sources and in parallel on the production of high-value platform chemicals as well as useful materials. Fossil-based fuels are clearly diminishing in supply and this has caused a global environmental concern due to rapidly rising emissions of fossil fuel by-products (both for processing and actual use). Because of this, waste valorization for energy and fuels are not only geared toward a sustainable fuel source but also toward a more benign fuel fit for an industrial up-scale. According to the Netherlands Environmental Assessment Agency, global CO2 emissions reached an all-time high in 2011 at around 34 billion tonnes of greenhouse gases (GHGs) [3]. Close to 90% of these emissions derive from fossil fuel combustion. Other toxic gases such as volatile organic compounds, nitrogen oxides (precursors of toxic ozone) and particulates come together with GHGs. In a more than likely scenario of a minimum of 2.5% energy demand growth per year, it is necessary to substitute fossil fuels progressively with cleaner fuel sources. Biomass combustion for electricity and heat production was reported to be less costly, providing at the same time a larger CO2-reduction potential [11]. Many studies also have shown convincing proof that the use of biomass for energy applications could be a highly interesting solution and cleaner technology for the future [12-14].
Another direction of waste valorization aims to produce high-value chemicals from residues including succinic acid (SA) [15], furfural and furans [16], phenolic compounds [17], and bioplastics [18]. These can be produced via chemical, chemo-enzymatic, and biotechnological approaches (e.g., solid state fermentation) but depending on the type of residue some compounds (e.g., essential oils, chemicals, etc.) can even be produced upon extraction and isolation [19]. The production of biomass-derived chemicals is a sustainable approach since it maximizes the use of resources and, at the same time, minimizes waste generation.
The major strength of biotechnology is its multidisciplinary nature and the broad range of scientific approaches that it encompasses. Among the broad range of technologies with the potential to reach the goal of sustainability, biotechnology could take an important place, especially in the fields of food production, renewable raw materials and energy, pollution prevention, and bioremediation. At present, the major application of biotechnology used in the environmental protection is to utilize microorganisms to control environmental contamination. Developing biotechnology could be a solution for these problems – this will also be given emphasis in this review.
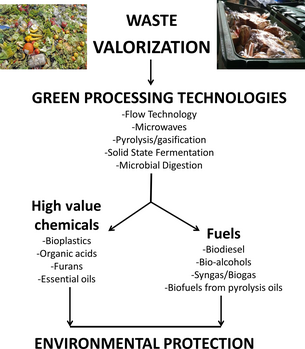
Although waste valorization is an attractive approach for sustainability, on a large scale perspective, the purification, processing, and even the degradation of stable natural polymers (e.g., lignin) into simple usable chemicals still remain a significant challenge (Fig. 1).
|
|
|
Figure 1. Valorization is essentially a concept of recycling waste into more usable industrial chemicals. Using established Green Processing technologies, various types of waste can be converted into high-value chemicals and fuels with the purpose of minimizing waste disposal volumes and eventually protecting the environment. |
In recent years, there have been increasing concerns in the disposal of food waste. The amount of food waste generated globally accounts for a staggering 1.3 billion tonnes per year. Apart from causing the loss of a potentially valuable food source or the regenerated resource, there are problems associated with the disposal of food waste into landfills. With this imminent waste management issue, food waste should be diverted from landfills to other processing facilities in the foreseeable future. In Hong Kong, there are 3600 tonnes of food waste generated (Table 1), 40% of which is made up of municipal solid waste (MSW). Fifty two percent (52%) of the MSW generated is dumped into landfills [20]. It is estimated that by 2018, all current landfill sites in Hong Kong will be exhausted.
| Waste | Tonnes/day |
|---|---|
| Municipal solid waste | 9000 |
| Domestic waste (including food waste) | 6000 (2550) |
| Commercial and industrial waste (including food waste) | 3000 (1050) |
| Construction waste | 3350 |
| Sewage sludge | 950 |
| Other waste | 200 |
| Total | 13,500 |
Although the problem of food waste is commonly found over the world, the systems of food waste processing can only be formulated at the local community level with the consideration of the area-specific characteristics. These include regional characteristics and composition of waste, land availability, peoples attitude and so forth. However, due to the lack of the local study concerning the suitability of food waste processing technologies for Hong Kong, this review is important to provide a few suggestions for the authorities to contemplate the adoption of a strategy on food waste disposal.
This contribution has been conceived to provide an overview on recent development of waste valorization strategies (with a particular emphasis on food waste) for the sustainable production of chemicals, materials, and fuels, highlighting key examples from recent research conducted by our groups. Reports on the development of green production strategies from waste and key insights into the recent legislation on management of wastes worldwide will also be discussed. The incorporation of these processes in future biorefineries for the production of value-added products and fuels will be an important contribution toward the worlds highest priority target of sustainable development.
Waste: Problems and Opportunities
In recent years, problems associated with the disposal of food waste to landfills lead to increased interest in searching for innovative alternatives due to the high proportion of organic matter in food waste,. First generation food waste processing technologies include waste to energy (e.g., anaerobic digestion), composting, and animal feed. Based on the characteristics of food waste, an integrated approach should be adopted with the focus on food waste reduction and separation, recycling commercial and industrial food waste, volume reduction of domestic food waste and energy recovery from food waste.
Sources, characterization, and composition of waste
The large amounts of waste generated globally present an attractive sustainable source for industrially important chemicals. Food waste including garbage, swill, and kitchen refuse [21], can be generally described as any by-product or waste product from the production, processing, distribution, and consumption of food [22].
The definition of food waste is, however, different in different countries or cities. In the European Union, food waste is defined as “any food substance, raw or cooked, which is discarded, or intended or required to be discarded.” The United States Environmental Protection Agency (EPA), on the other hand, defines food waste as “Uneaten food and food preparation waste from residences and commercial establishments such as grocery stores, restaurants, and produce stands, institutional cafeterias and kitchens, and industrial sources including employee lunchrooms.” In the United Nations, “Food waste” and “Food loss” are distinguished. Food losses refer to the decrease in food quantity or quality, which makes it unsuitable for human consumption [23] while food waste refer to food losses at the end of the food chain due to retailers’ and consumers’ behavior [24]. All in all, food waste includes not just wasted foodstuffs, but also uncooked raw materials or edible materials from groceries and wet market.
Food waste is generally characterized by a high diversity and variability, a high proportion of organic matter, and high moisture content. Table 1 summarizes some reported characteristics of food waste, indicating moisture content of 74–90%, volatile solids to total solids ratio (VS/TS) of 80–97%, and carbon to nitrogen ratio (C/N) of 14.7–36.4 [25]. Due to these properties, food waste disposal constitutes a significant problem due to the growth of pathogens and rapid autoxidation [26]. As there are already many different microorganisms in food waste, the high rate of microbial activity and the amount of nutrients in food wastes facilitate the growth of pathogens, which cause the concern for foul odor, sanitation problems, and could even lead to infectious diseases. The high moisture contents [23] also increase the cost of food waste transportation. Food waste with high lipid content is also susceptible to rapid oxidation. The release of foul-smelling fatty acids also adds difficulties to the storage of treatment of food waste (Table 2).
| Source | Characteristics | Country | ||
|---|---|---|---|---|
| Moisture content (%) | Volatile solid/total solid (%) | Carbon/nitrogen | ||
| ||||
| A dining hall | 80 | 95 | 14.7 | Korea |
| Universitys cafeteria | 80 | 94 | NAa | Korea |
| A dining hall | 93 | 94 | 18.3 | Korea |
| A dining hall | 84 | 96 | NA | Korea |
| Mixed municipal sources | 90 | 80 | NA | Germany |
| Mixed municipal sources | 74 | 90–97 | NA | Australia |
| Emanating from fruit and vegetable, markets, household and juices centers | 85 | 89 | 36.4 | India |
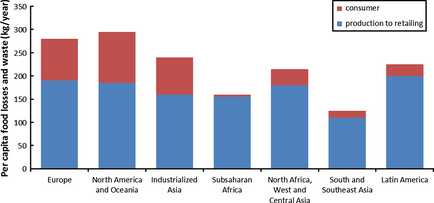
According to a study commissioned by the United Nations Food and Agriculture Organization (UNFAO) in 2011, 1.3 billion tonnes of food waste is generated per year and roughly one third of food produced for human consumption is lost or wasted globally. The report also noted that food waste of industrialized countries and developing countries have different characteristics. Firstly, increasingly important quantities of food waste are generated in industrialized countries as compared to volumes observed in developing countries on a per capita basis. Figure 2 shows the per capita food loss in Europe and North America is 280–300 kg/year. In contrast, the food loss per capita in sub-Saharan Africa and South/Southeast Asia accounts for 120–170 kg/year. Also, food waste is mainly generated at retail and consumer levels in industrialized countries. Comparatively, food waste is generated in developing countries mainly at postharvest and processing levels, supported by the per capita that is, food waste generated by consumer levels in Asia is only 6–11 kg/year [27].
|
|
|
Figure 2. Per capita food losses and waste, at consumption and pre-consumption stages, in different regions [19]. |
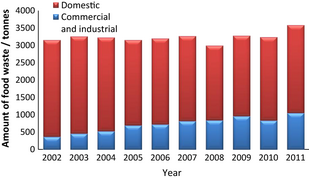
Interestingly, the amount of food waste generated for example in Hong Kong is staggering. Figure 3 shows an increasing trend of the food waste generated daily from 3155 tonnes in 2002 to 3484 tonnes in 2011. Although the disposal of food waste in landfills was found to be the most economical option [28], it causes numerous problems in landfill sites. As landfilling disposal generally buries and compacts waste under the ground, the decomposition of food waste produces methane, a GHG that is twenty-one times powerful than carbon dioxide (CO2) under anaerobic environment conditions. Such production can in fact remarkably affect the environment in the area as some reports indicated that around 30% of GHG produced in Hong Kong are generated in landfill sites [29]. Methane is also flammable and may lead to fires and explosions upon accumulation at certain concentrations. In addition, the decomposition of food waste develop unpleasant odor as well as leachates and organic salts that could damaging landfill liners, leaching out heavy metals and resulting in contamination of ground waters [30].
|
|
|
Figure 3. The amount of food waste generated daily in Hong Kong from 2002 to 2011 [25]. |
Valorization research has evolved through the years, with many techniques and developments achieved in recent decades. Waste feedstock including bread, wheat, orange peel residues, lignocellulosic sources, etc. are currently explored as sources of chemicals and fuels. On a recent review by Pfaltzgraff et al. [31], it was noted that the valorization of food wastes into fine chemicals is a more profitable and less energy consuming as compared to its possibilities for fuels production. Because of this, related waste processing technologies, particularly related to the production of fuels, have also been proposed to address energy efficiency and profitability from a range of different feedstocks. Toledano et al. [32, 33] reported a lignin deconstruction approach using a novel Ni-based heterogeneous catalyst under microwave irradiation. Different hydrogen donating solvents were explored for lignin depolymerization, finding formic acid as most effective hydrogen donating reagent due to the efficient generation of hydrogen for hydrogenolysis reactions (from its decomposition into CO, CO2, and H2) and its inherent acidic character that induces acidolytic cleavage of C-C bonds in lignin at the same time. The heterogeneous acidic support also acted as a Lewis acid, coordinating to lignin thereby promoting acidic protonation, and eventually dealkylation and deacylation reactions (Fig. 4). Figure 5 shows the structural complexity of the lignin biopolymer. Lignin deconstruction to simple aromatics including syringaldehyde, mesitol, and related compounds could serve the basis for a new generation of renewable gasolines [34].
|
|
|
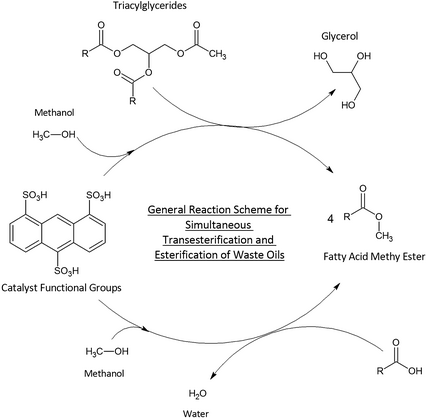
Figure 4. Simultaneous transesterification and esterification of waste oils using solid acid catalysts produced fatty acid methyl esters (a nonpolar component) along with water and glycerol (polar compounds) that separate out spontaneously from the reaction mixture forming two phases. |
|
|
|
Figure 5. The structural complexicity of lignin being composed of aromatic compounds show its potential in different applications such as for fuel, and in the production of high-value chemicals (Image adapted from Stewart et al., [40]). |
Simple phenolic compounds with potential antioxidant properties can also be derived from cauliflower by-products [35]. The proposed valorization strategy comprised a combined solvent-extraction step using an organic solvent together with a polystyrene resin (Amberlite XAD – 2) to recover most phenolics prior to high performance liquid chromatography (HPLC) analysis. Kaempferol-3-O-sophoroside-7-O-glucoside and its sinapoyl derivative kaempferol-3-O-(sinapoylsophoroside)-7-O-glucoside were obtained as main extracted components. A separate study by Sáiz et al. [36] also proved near-infrared (NIR) spectroscopy was a highly useful technique to characterization online of alcohol fermentation from onions. Along with multivariate calibration, this technique can lead to the analysis of samples with complex matrices without a prior sample preparation. One approach to a greener characterization method would be the coupling of a chromatographic technique to a flow instrument. This coupling has been shown to work in studies on metal analysis [37], online derivatization and separation of aspartic acid enantiomers [38], as well as for an enzyme inhibition assay [39], but it has not yet been shown to be successful for waste valorization.
Development of greener valorization strategies
There are numerous options for waste processing and/or recycling in the world. Composting, regenerated animal feed and bedding, incineration, anaerobic digestion, and related first generation strategies have been proposed and investigated for a long time. Some of these techniques have been successful in making their way to commercialization. Considering the storage problem and the large amount of food waste generated every day, food waste processing facilities have to be in a mega-scale size with enough treatment capacity to handle numerous tonnes of food wastes daily. It definitely requires a large initial investment for setting up the industrial scale facilities. Also, in case of off-site processing, the large volume and great weight of food waste adds difficulty since the collection of food waste significantly increases the transportation cost and time. Besides, the variation in composition of food wastes, affects the quality of regenerated products, such as compost and animal feed. Therefore, it decreases the products competiveness in the market.
As demonstrated in the above-mentioned examples, valorization may be carried out under different conditions depending on the target components needed. Before reaching an industrial upscale, enhancement of valorization product yield may be done by careful variation of the valorization strategies, in particular advanced protocols able to diversify on feedstock and end products obtained from them. Currently, an active area of research relates to catalytic valorization strategies using solid acid catalysts [41, 42]. One example of a green protocol on valorization of waste oils to biodiesel was provided by Fu et al. [43], in which a superacid was prepared by adding a sulfuric acid solution to zirconium hydroxide powder. Under optimum reaction conditions, 9:1 MeOH/oil molar ratio, 3% (w/w) catalyst, and 4 h reaction time at 120°C, biodiesel yield reached 93.2%. Apart from metal supports functionalized with acids, carbon-based catalysts for waste valorization are also attractively developed protocols. Aside from being an easily separable reaction component, functionalized carbonaceous materials can also be recyclable. In a study by Clark et al. [44], carbonaceous materials from porous starches (Starbons®, Department of Chemistry, University of York, York, UK) functionalized with sulfonated groups were found to have a catalytic activity 2–10 times greater to those of common microporous carbonaceous catalysts in a range of chemistries including biodiesel production from waste oils and SA transformations in a fermentation broth. A separate study by Luque et al. [45] employed carbonaceous residues of biomass gasification as catalysts for biodiesel synthesis. The results showed good ester conversion yields from fatty acids to methyl esters. The above-mentioned examples demonstrate that designer catalysts can be attractive options in the valorization of a range of waste feedstocks.
A promising sustainable approach would also be the use of ionic liquid-type compounds which can be derived from renewable feedstock such as the so-called deep-eutectic solvents [46-49] and even selected designer ionic liquids. These compounds are salts in their liquid states with very unique properties such as very low vapor pressure, thermal stability, and tunability based on different applications. A study by Ruiz et al. [50] presented a –SO3H functionalized Bronsted acid ionic liquids catalyzed synthesis of an important chemical precursor such as furfural from C5 sugars under microwave heating. Furfural yield varied from 40% to 85% depending on the type of Ionic liquid used and the feedstock employed in the process. It was shown that the ionic liquid 1-(4-Sulfonylbutyl)pyridinium tetrafluoroborate produced a yield of 95% for xylose conversion and 85% for furfural. Importantly, the protocol was amenable to the utilization of a biorefinery-derived syrup enriched in C5 oligomers, from which a 40–45% of furfural yield could be derived.
A separate study by Zhang et al. [51] showed that the direct conversion of monosaccharides, and polysaccharides to 5-hydroxymethylfurfural (5-HMF) may be accomplished using ionic liquids in the presence of Germanium (IV) chloride. Yields of the reaction could go as high as 92% depending on the reaction conditions used. The mechanism proposed by the researchers indicate the role of the GeCl4 as a Lewis acid catalyst for the ring opening of the sugars, which is immediately followed by several dehydration steps to produce 5-HMF.
As alternative to these catalytic strategies, photocatalytic approaches to waste valorization could also serve the basis of innovative and highly attractive future valorization protocols. A recent review by Colmenares et al. [52] addressed the potential and opportunities of photocatalysis to convert lignin biomass into fine chemicals using designer TiO2 nanocatalysts. These nanomaterials featuring doping agents (to lower the band gap of titania) have been shown to be effective in water splitting experiments (to form H2 and O2) to harness the potential of hydrogen as fuel. One of the earliest promising works of light-mediated degradation was shown by Stillings et al. [53] when they were able to degrade cellulose using Ultraviolet radiation. However, this has not been shown to be possible using visible light due to energy considerations. A photocatalytic approach to degradation may also be accomplished using functionalized graphenes (monolayers of sp2 carbon atoms in a honeycomb lattice known to have ballistic electron transport properties). Functionalized graphenes and composites with other semiconductors have been shown to exhibit degradation properties [54, 55], but this concept has not yet been applied to waste valorization strategies.
Recent legislation on waste management
Philippines
In Metro Manila at Philippines, almost 3.5 kg of solid waste is generated per capita every day. This amount includes food/kitchen waste, papers, polyethylene terephthalate bottles, metals, and cans. Although most Metro Manila residents do not practice the open burning of waste, a necessary waste segregation is performed for ease of collection. Being the countrys capital, and one of the worlds most densely populated cities, Metro Manila generates over 2400 tons of waste everyday, which equates to a government spending of Php 3.4 billion (63 Million Euros) in collection and disposal. Not much legislation is available in the Philippines in terms of waste management. Although Republic Act 9003 (Solid Waste Management Act) has been passed last 2000, a recent 2008 study showed that it has not been properly implemented [56].
Hong Kong
One third of the food waste generated in Hong Kong come from the Commercial and Industry (C&I) sector, with the remaining percentage coming from households. In recent years, the amount of disposal of food waste from C&I sectors remarkably increased by 280% from 373 tonnes in 2002 to 1050 tonnes in 2011. It is anticipated that the food waste generated in Hong Kong will continue to rise, driven by the significant increase of the C&I food waste generation. The disposal of food waste (an organic waste which decomposes easily) to landfills is not sustainable, as it leads to rapid depletion of the limited landfill space. From the 2013 Policy Address by the Office of the Chief Executive in Hong Kong [57], there was a special emphasis on “Reduction of Food Waste” as stated in Section 142 below:
“Food waste imposes a heavy burden on our landfills as it accounts for about 40% of total waste disposed of in landfills. In addition, odour from food waste creates nuisance to nearby residents. The Government has recently launched the “Food Wise Hong Kong Campaign” to mobilise the public as well as the industrial and commercial sectors to reduce food waste. We will build modern facilities in phases for recovery of organic waste so that it can be converted into energy, compost and other products.” [57].
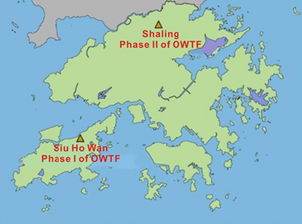
The Environmental Protection Department (EPD) has planned to develop Organic Waste Treatment Facilities (OWTF). Such facilities will adopt biological technologies – composting and anaerobic digestion to stabilize the organic waste and turn it into compost and biogas for recovery. The first phase of the OWTF will be constructed at Siu Ho Wan with a daily treatment capacity of 200 tonnes of source separated organic waste (Fig. 6). The second phase will be located at Sha Ling of North District with a daily treatment capacity of 300 tonnes of organic waste.
|
|
|
Figure 6. Map of Hong Kong indicates the location of the two organic waste treatment facility (OWTF) in Siu Ho Wan (Phase I) and Shaling (Phase II) [16]. |
Waste reduction at source should be the top priority so as to reduce the amount of food waste generated. Successful examples for the implementation of MSW charging scheme in Asian cities such as Taipei, Taiwan, and Seoul, South Korea could effectively reduce the total amount of MSW by 50% in 10 years [20]. These governments introduced quantity/volume-based charging scheme to create financial incentives to change publics food waste-generating behavior to achieve waste reduction at source. In addition, they introduced prepaid designated food waste bag charging system so as to achieve source separation. Food waste together with plastic bags can undergo treatment without extra separation step in the treatment facilities.
Waste Valorization Strategies: Case Studies
Biological treatment technologies including anaerobic digestion and composting have been reported extensively in past years. Under anaerobic digestion, biogas is generated as main product. Takata et al. [58] reported the production of 223 m3 biogas from 1 tonne of food waste. However, Bernstad et al. [59] reported that the yield of biogas production may vary depending on the composition of waste and the existence of detergent. Numerous studies show that the lack of enough nutrients limits the ability of enzymes to digest waste [21, 60]. This can divert waste from landfill, and thus prevent the emission of GHG to the environment. Also, the solid residues can be used as compost, which can reduce the amount of used chemical fertilizers. Economically, anaerobic digestion can generate electricity on-site and may reduce energy cost. Also, it can be adopted in sewage treatment facilities, thereby eliminating transportation costs. Another way to valorize waste is by incineration for energy recovery. However, burning food waste is an energy intensive process and may remove important functional groups from the treated feedstocks. The following sections report case studies of different feedstock in different countries to illustrate the potential of waste valorization for the production of materials, chemicals, and products.
Utilization of bakery waste in the biotechnological production of value-added products
Based on the large quantities of food waste generated at Hong Kong on a daily basis, Lin et al. have been recently focused on the valorization of unconsumed bakery products to valuable products via bio-processing in collaboration with retailer “Starbucks Hong Kong”. Research was initially set on the production of bio-plastics poly(3-hydroxybutyrate) (PHB) and platform chemicals (e.g., SA) via enzymatic hydrolysis of non pretreated bakery waste, followed by fungal solid state fermentation to break down carbohydrates into simple sugars for subsequent SA or PHB fermentation. In the proposed biotechnological process, bakery waste serve as the nutrient source, including starch, fructose, free amino nitrogen (FAN), and trace amount of subsidiary nutrients. The nutrient content is listed in Table 3 below.
| Content | Pastry | Cake | Wheat bran |
|---|---|---|---|
| |||
| Moisture | 34.5 g | 45.0 g | N/A |
| Starch (dry basis) | 44.6 g | 12.6 g | N/A |
| Carbohydrate | 33.5 g | 62.0 g | 15.0 g |
| Lipids | 35.2 g | 19.0 g | 6 g |
| Sucrose | 4.5 g | 22.7 g | N/A |
| Fructose | 2.3 g | 11.9 g | N/A |
| Free sugar | 1.5 g | ||
| Fiber | N/A | N/A | 50 g |
| Protein (TN × 5.7) (dry basis) | 7.1 g | 17.0 g | 14.0 g |
| Total phosphorus (dry basis) | 1.7 g | 1.5 g | N/A |
| Ash (dry basis) | 2.5 g | 1.6 g | N/A |
In general, pastries have larger starch and lipid content to those of cakes; whereas cakes have higher sugar (fructose and sucrose) and protein content. Nevertheless, both types of bakery waste were proved to serve as excellent nutritional substrates for fermentative production of SA or bioplastics after hydrolysis. Our groups previously demonstrated that SA could be produced from wheat-based renewable feedstock [63-65] and bread waste [66] via fermentation. Similarly, production of biopolymers from various types of food industrial waste and agricultural crops was shown to be techno-economically feasible for replacing petroleum-derived plastics [67].
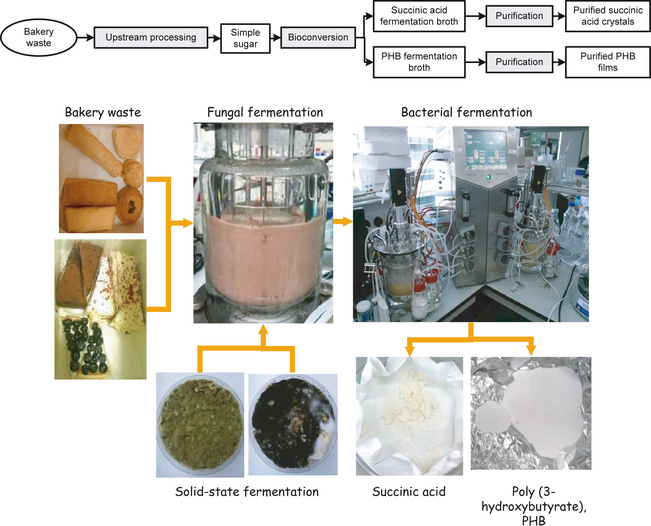
The key components in the project are illustrated in Figure 7. In the upstream processing, the bakery waste was collected from a Starbucks outlet in the Shatin New Town Plaza. A mixture of fungi comprising Asperillus awamori and Asperillus oryzae were utilized for the production of amylolytic and proteolytic enzymes, respectively. Macromolecules including starch and proteins contained in bakery waste were hydrolysed, expected to enrich the final solution in glucose and FAN. This hydrolysate was subsequently used as feedstock in a bioreaction by two different types of microorganisms (Actinobacillus succinogenes and Halomonas boliviensis) to produce (SA) and PHB, respectively.
|
|
|
Figure 7. Flow chart of a bakery-based food waste biorefinery development, from bakery waste as raw material to succinic acid and poly(3-hydroxybutyrate), PHB as final products. |
Although food waste is a no-cost nutritional source, the application of commercial enzymes in upstream processing might not be cost-efficient. To reduce process costs, the degradation of bread and bakery waste has been previously studied [61, 66]. In these studies, A. awamori and A. oryzae were the fungal secretors of glucoamylase protease and phosphatase as well as a range of other hydrolytic enzymes that does not require any external addition of commercial enzymes.
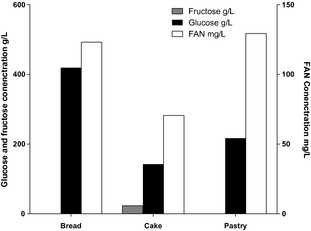
According to Figure 8, glucose (54.2 g/L) and FAN concentrations (758.5 mg/L) were achieved at 30% (w/v) pastry waste after enzymatic hydrolysis. On the other hand, sucrose present in cake was hydrolyzed to form 1 mole of glucose and 1 mole of fructose. The glucose (35.6 g/L), fructose (23.1 g/L), and FAN concentrations (685.5 mg/L) were achieved at 30% (w/v) cake waste. Among all, waste bread hydrolysate contained the highest glucose and FAN concentrations, which were 104.8 g/L and 492.6 mg/L, respectively. These results clearly demonstrate the potential of utilizing bakery hydrolysate as generic feedstock for fermentations.
|
|
|
Figure 8. Sugars and FAN concentrations achieved from enzymatic hydrolysis using different bakery waste (30%, w/v) with Aspergillus awamori and Aspergillus oryzae. |
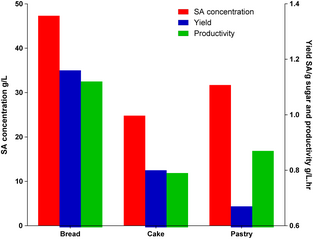
Batch fermentations on enzymatic hydrolysates were subsequently carried out to investigate the cell growth, glucose consumption as well as SA production. Cake hydrolysate consisting an initial sugar content of 23.1 g/L glucose and 18.5 g/L fructose, and pastry hydrolysate with an initial sugar content of 44.0 g/L glucose were both utilized as fermentation feedstock. At the end of fermentation, the remaining glucose was 5.2 g/L whereas fructose was 3.7 g/L. A final SA concentration of 24.8 g/L was obtained at the end point, which corresponded to a yield of 0.8 g SA/g total sugar and a productivity of 0.79 g/L.h (Fig. 9). The overall conversion of waste cake into SA was 0.28 g/g cake.
|
|
|
Figure 9. Succinic acid concentration, yield, and productivity in Actinobacillus succinogenes fermentations using different bakery hydrolysates. |
Compared with cake hydrolysates, pastry hydrolysates possessed larger concentrations of initial glucose (44.0 g/L). SA concentration continuously increased until sugar was depleted after 44 h. At the end of fermentation, the SA concentration reached 31.7 g/L, which corresponded to a yield of 0.67 g SA/g glucose and a productivity of 0.87 g/L.h.
SA production achieved from various food waste residues has been compared in Table 4. It is clear that SA yields obtained when using cake and pastry wastes as feedstock were comparable or higher to those of other food waste-derived media.
| Substrate | SA yield (g SA/g TS) | Overall SA yield (g SA/g substrate) | References |
|---|---|---|---|
| |||
| Wheat | 0.40 | 0.40 | [63] |
| Wheat flour milling by-product | 1.02 | 0.087 | [64] |
| Potatoes | N/A | N/A | [68] |
| Corncob | 0.58 | N/A | [69] |
| Rapeseed meala | 0.115 | N/A | [70] |
| Rapeseed mealb | N/A | N/A | [71] |
| Orange peel | 0.58 | Negligible | [72] |
| Bread | 1.16 | 0.55 | [66] |
| Cake | 0.80 | 0.28 | [61] |
| Pastry | 0.67 | 0.35 | [61] |
Biotechnological PHB production using bakery waste and seawater
Halomonas boliviensis has been utilized in fermentations for the bioconversion of bakery hydrolysate into PHB. This microorganism is a moderate halophilic and alkali tolerant bacterium that can produce PHB through fermentative processes under aerobic condition [73]. It was isolated from a Bolivian salt lake, and the rod-shaped H. boliviensis is able to survive and synthesize PHB under salty environment.
Table 5 shows a summary of PHB fermentation results using defined medium and bakery hydrolysates, namely cake and pastry hydrolysates. PHB yields suggested that a defined fermentation medium (40 g/L glucose, 5 g/L yeast extract) can provide an optimum PHB yield (72%). The lowest PHB yield (1–2%), as expected, was obtained under bakery hydrolysate fermentation media. This demonstrates that a defined medium with 40 g/L glucose and around 5 g/L yeast extract could provide sufficient nutrients for H. boliviensis to produce PHB efficiently.
| Batch no. | Fermentation medium | Fermentation mode | Feeding media | Fermentation time (h) | Glucose consumption (g) | CDW (g) | PHB production (g) | PHB content (%) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 1 | Defined (40 g/L glucose, 2 g/L yeast extract) | Batch | NIL | 64.0 | 13.0 | NIL | NIL | NIL |
| 2 | Defined (40 g/L glucose, 5 g/L yeast extract) | Batch | NIL | 88.0 | 24.0 | 24.9 | 17.4 | 17.4 |
| 3 | Defined (40 g/L glucose, 8 g/L yeast extract) | Batch | NIL | 75.0 | 59.9 | 9.2 | 4.3 | 4.3 |
| 4 | Pastry hydrolysate | Batch | NIL | 23.5 | 32.8 | NIL | NIL | NIL |
| 5 | Pastry hydrolysate | Fed-batch | Glucose solution | 135.5 | 112.3 | 5.7 | 2.1 | 2.1 |
| 6 | Pastry hydrolysate | Fed-batch | Pastry hydrolysate | 67.0 | 208.8 | 38.2 | 3.6 | 3.6 |
| 7 | Pastry hydrolysate | Fed-batch | Pastry hydrolysate | 87.0 | 359.9 | 15.6 | 0.6 | 0.59 |
| 8 | Cake hydrolysate | Fed-batch | Cake hydrolysate | 63.0 | 200.5 | 11.6 | 2.9 | 2.9 |
The overall glucose consumption for defined medium fermentation in the batch mode ranged from 13 to 60 g. High initial nitrogen source could hinder PHB production by 10 times, as indicated from PHB yield obtained by defined medium. A similar effect could possibly lead to the low PHB yield observed in bakery hydrolysate fermentation. With the continuous supply of nitrogen source, H. boliviensis consumed glucose in a faster rate for PHB production, maintenance, and synthesis of other metabolites (six times higher overall glucose consumption with the feeding of bakery hydrolysate). Halomonas boliviensis synthesizes ectonie and hydroxyectonie as osmolytes as NaCl concentration increases in the cells environment. Van-Thuoc et al. [74] reported the co-production of ectonie and PHB in a combined two-step fed-batch culture. Similarly, the formation of other primary metabolites such as ectoines in the bakery hydrolysate fermentation was observed in these studies. This consequently led to a lower PHB production when bakery hydrolysate and seawater were used as fermentation feedstocks. The highest overall yield of PHB production for the defined medium (with less glucose consumption and higher PHB production) was about 17% as compared to a rather low 3.5% observed for bakery hydrolysate.
In summary, this project is currently demonstrating the green credentials in the development of advanced food waste valorization practices to valuable products, which also include GHG reductions as well as the production of other air pollutants. Such a synergistic solution may be feasible for adoption by the Hong Kong Government as part of their strategy for tackling the food waste issue as well as for the environmentally friendly production of alternative platform chemicals and biodegradable plastics.
Chemical valorization of food waste for bioenergy production
The valorization of waste to important chemicals can be accomplished through different approaches as discussed. Another potentially interesting approach to advanced valorization practices would be the chemical utilization of various waste raw materials for conversion into high-value products.
A case study of such integrated valorization is a recent study on the conversion of corncob residues into functional catalysts for the preparation of fatty acid methyl esters (FAME) from waste oils [75]. The design of the catalyst involved an incomplete carbonization step under air to partially degrade the lignin materials mostly present in corncobs, followed by subsequent functionalization via sulfonation to generate –SO3H acidic sites. The solid acid catalyst was then subjected to conditioning prior to its utilization in the conversion of waste cooking oil with a high content of free fatty acids (FFA) to biodiesel-like biofuels. The advantage of the designed solid acid catalyst, apart from being derived from food waste, is the possibility to conduct a simultaneous esterification of FFA present in the waste oil as well as transesterification of the remaining triglycerides also present in the oil (Fig. 4).
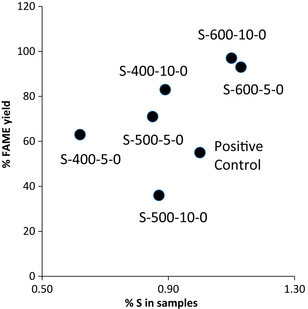
In this approach, the generation of two valuable products (a cheap solid acid catalyst and biodiesel-like biofuels) can be achieved starting from two food waste feedstock (corncobs and waste cooking oils). The solid acid catalysts were characterized using a range of techniques. Fourier transform infrared spectroscopy (FTIR) showed the presence of different functional groups including C=O, C-O, C-S, and aromatic C=C in the materials (Table 6). The catalytic activity of the solids also showed remarkable activity toward the conversion of waste cooking oils into biodiesel-like biofuels. A maximum of 98% yield to methyl esters could be obtained without prior purification of the oils. Importantly, kinetics of the transesterification reaction was significantly slower to those of the esterification of FFA present in the oil. Despite a low –SO3H loading (1 wt% S, 0.16 mmol/g –SO3H), the catalytic activity was still high, indicating a possibly different surface functionality (Fig. 10).
| Frequency of band | Corresponding functional group |
|---|---|
| 1700 cm−1 | C=O |
| 1597 cm−1 | C=C aromatic |
| 1219 cm−1 | S=O |
| 1029 cm−1 | C-S |
|
|
|
Figure 10. Plot of the FAME yield of the samples versus the %S content (A, material carbonized at 400°C for 5 h; B, carbonized at 400°C for 10 h; C, carbonized at 500°C for 5 h; D, carbonized at 500°C for 10 h; E, carbonized at 600°C for 5 h; F, carbonized at 600°C for 10 h). A higher degree of functionalization (higher %S) generally leads to improved FAME yields. Results also highlight the superior catalytic activities of sulfonated carbonaceous materials compared to blank (no conversion, data not shown) and the positive control referring to the homogeneously H2SO4 catalyzed reaction. |
The recyclability of the solid acid catalysts still, however, needs to be further optimized. Catalysts were found to deactivate quickly (after two uses) due to the aqueous promoted decomposition and hydrolysis of the sulfonated groups in the material [75]. Materials should be tested under different conditions of temperature, carbonizing atmosphere, and even pressure to improve the stability and robustness of the catalyst for the selected process. Nevertheless, this study provides a promising proof of concept of the potential of an integrated valorization of various waste raw material into valuable end products and biofuels as it avoids the pretreatment of the waste oils (generally required to reduce the high FFA content to allow the conventional base-transesterification process to take place avoiding the formation of undesirable soaps and emulsions) and generates a relatively pure biofuel from a residue using an environmentally friendly and cheap solid acid catalyst.
Tailored-made healing biopolymers from the meat industry
The meat industry generates enormous quantities of solid waste [76]. Managing such residues entails a significant problem for the sector as many of the generated by-products and residues are prone to degradation and microbial contamination. However, an important part of these residues are rich in various added value products, which upon extraction could constitute a source of interesting revenues for these industries. Among the most promising compounds from meat industry-derived by products, we can include oily fats and collagen [77]. Valorization of the aforementioned waste fats from slaughterhouses and meat processing industries to biodiesel-like biofuels has been studied via esterification/transesterification using different types of catalysts and protocols, which entailed in some cases a pretreatment and refining of the fat [78]. In principle, these will be, however, conducted in a similar way to that reported in the previously showcased study of waste cooking oils valorization.
Comparatively, collagen-containing residues (e.g., bovine hides) are increasingly important residues from the meat industry that are often derived to leather processing companies. Interestingly, the significant amounts of collagen present in such samples are not that well known [77].
Collagen is a ubiquitous and most relevant biopolymer in vertebrates [77-79], which possesses a highly interesting versatility to be employed in a wide range of applications in different areas from regenerative medicine to cosmetics and veterinary. Extraction and stabilization of collagenic biopolymers from waste, particularly related to their physical properties, (e.g., via cross-linking) constitutes an innovative pathway toward the production of novel potentially industrial products (e.g., tissue engineering, wound healing, antimicrobial aposits, etc.). Cross-linking methodologies can in principle generate additional bond formation to stabilize polymers with additional benefits on physical properties including swelling and flexibility.
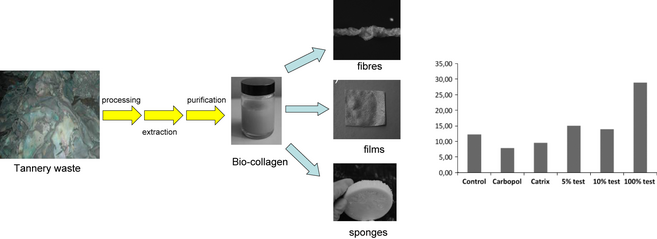
In the light of these premises, a recent example on the extraction, cross-linking and purification of collagenic biopolymers from splits (pickled hides) and the so-called wet-white hides (from tannery-derived hides treated with glutaraldehyde or phenolic compounds for chromium-free leather production) demonstrated the possibility to obtain valuable end products based on tailored-made biocollagen with improved mechanical properties, stabilized structures, and desired molecular weight ranges, which could be employed in wound healing acceleration in rats [80, 81]. Interestingly, these biopolymers could be easily shaped into various forms including fibers, sponges, and/or films, paving the way to the development of potentially novel biomaterials for different biomedical applications (Fig. 11). A simple hydrolytic process was able to extract the collagen, followed by subsequent cross-linking to stable biopolymers or direct application upon purification by ultrafiltration as unguent for induced wounds in rats [80, 81].
|
|
|
Figure 11. Meat and tannery-derived residues can be valorized to valuable collagenic biopolymers that can be formed into fibers, films, and sponges for various applications. Right plot depicts a comparison of activity in induced wounds in rats between pure and diluted collagen-extracted formulations (100%, 5% and 10% test, respectively) and a control sample (no treatment) and commercial formulations (Carbopol, Catrix). |
Maximum yields of biopolymer extracted were obtained at 0.25 mm grinding size and the use of diluted acetic acid as hydrolytic agent (24 h, room temperature), for which also a minimum swelling (better biopolymer properties) was also observed. Interestingly, samples obtained from splits exhibited a better desirability to those extracted from wet-white hides. Isolated biopolymers possessed molecular weight of ca. 300 kDa, in contrast to conventional collagen derivatives for which molecular weights are usually within 15–50 kDa for hydrolysates [82] and 50–200 kDa for gelatine [83].
Biocollagenic materials were found to be very attractive and highly useful in treatment of induced burns/wounds in mice (Fig. 11), showing in all cases improved tissue regeneration and wound healing as compared to untreated wounds and commercial formulations including Carbopol and Catrix. Even diluted formulations containing 5 wt% of the collagenic biopolymer (Fig. 11, right plot) were found to provide improved results.
Future Prospects and Conclusions
Excessive disposal of food and plastic waste are deteriorating the landfill issue in many parts of the World. Waste valorization is an attractive concept that has gained increasing popularity in many countries nowadays due to the rapid increase in generation of such waste residues. Because of this, researchers are not only developing valorization strategies but also focusing on the design of greener materials utilizing a range of green technologies. One example of this could be the synthesis of magnetically separable substances [84–87]. Not only are these able to catalyze the necessary conversion, they are also economically attractive due to their simple preparation [55, 84, 85]. Also, the production of carbon-based catalysts maybe continued for research, but greener preparations (such as microwave-mediated functionalization) to lessen the energy investment, should be explored. Furthermore, the emergence of graphene as catalyst in many reactions should also be noted for valorization purposes.
As previously mentioned, an interesting valorization protocol to develop would be a photocatalytic approach. To accomplish such photocatalytic strategies, TiO2, Pt/CdS/TiO2 composite materials [88], TiO2/Ni(OH)2 [84] clusters may be used depending on the target, samples, and reaction conditions. Photodegradation has been shown to be possible toward many environmental pollutants such as chlorofluorocarbons [55], CO2 [85], and NO [54], but whether these photoactive composites could degrade the stable polymeric structure of lignin/protein/carbohydrates is yet to be seen and perhaps understood. A recent study by Balu et al. [89], reports on the preparation of a TiO2-guanidine-(Ni,Co)Fe2O4 photoactive material. The addition of the guanidine was made to lower the band gap of the material hence making it active under visible light. Testing the material to a model chemical reaction, using malic acid and the synthesized photomaterial produced simpler chemicals such as formic acid, acetic acid, and oxalic acid with a selectivity of around 80%. This study provides proof of concept that band gap engineering of semiconductors can lead to the development of photoactive materials that may be used selectively for waste valorization. A photocatalytic approach will most importantly address one of the major drawbacks of industrial valorization which is on the relatively large amounts of energy needed for processing and purification of products.
The conversion of a range of feedstock into valuable products including chemicals, biomaterials, and fuels has been demonstrated in three essentially different case studies to highlight the significant potential of advanced waste valorization strategies.
The incorporation of these and similar processes in future biorefineries for the production of value-added products and fuels will be an important contribution toward the worlds highest priority target of sustainable development.
But perhaps the main and most important issue to be addressed for the sake of future generations, currently way overlooked, is society itself. The most extended perception of waste as a problem, as a residue, as something not valuable needs to give way to a general concienciation of society in waste as a valuable resource. A resource, which obviously entails a significant complexity (from its inherent diversity and variability), but one that can provide at the same time an infinite number of innovative solutions and alternatives to end products through advanced valorization strategies. These will need joint efforts from a range of disciplines from engineering to (bio)chemistry, bio(techno)logy, environmental sciences, legislation, and economics to come up with innovative alternatives that we hope to see leading the way toward a more sustainable bio-based society and economy.
Acknowledgments
R. D. Arancon thanks the Department of Chemistry of the Ateneo de Manila University in the Philippines for the wonderful opportunity to learn. Also, heartfelt thanks are due to Jhon Ralph Enterina (University of Alberta, Canada) and Jurgen Sanes (Simon Fraser University, Canada) for help with some articles. Carol Sze Ki LIN acknowledges the Biomass funding from the Ability R&D Energy Research Centre (AERC) at the School of Energy and Environment in the City University of Hong Kong. The authors are also grateful to the donation from the Coffee Concept (Hong Kong) Ltd. for the “Care for Our Planet” campaign, as well as a grant from the City University of Hong Kong (Project No. 7200248). C. S. K. Lin acknowledges the Industrial Technology Funding from the Innovation and Technology Commission (ITS/323/11) in Hong Kong. R. Luque gratefully acknowledges the Spanish MICINN for financial support via the concession of a RyC contract (ref: RYC–2009–04199) and funding under project CTQ2011–28954-C02-02. Consejeria de Ciencia e Innovacion, Junta de Andalucia is also gratefully acknowledged for funding project P10-FQM-6711. R. Luque is also indebted to Guohua Chen, the Department of Chemical and Biomolecular Engineering (CBME) and HKUST for the provision of a visiting professorship as Distinguished Engineering Fellow.
Conflict of Interest
None declared.
References
- Serrano-Ruiz, J. C., R. Luque, J. M. Campelo, and A. A. Romero. 2012. Continuous-flow processes in heterogeneously catalyzed transformations of biomass derivatives into fuels and chemicals. Challenges3:114–132.
- Glasnov, T. N., and C. O. Kappe. 2011. The microwave-to-flow paradigm: translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. Eur. J.17:11956–11968.
- PBL Netherland Environmental Assessment Agency. Trends in global CO2 emissions. http://edgar.jrc.ec.europa.eu/CO2REPORT2012.pdf (accessed 15 March 2013).
- Mohan, D., C. U. Pittman, and P. H. Steele. 2006. Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels20:848–889.
- Heo, H. S., H. J. Park, Y.-K. Park, C. Ryu, D. J. Suh, Y.-W. Suh, et al. 2010. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour. Technol.101:S91–S96.
- Cho, M.-H., S.-H. Jung, and J.-S. Kim. 2009. Pyrolysis of mixed plastic wastes for the recovery of benzene, toluene, and xylene (BTX) aromatics in a fluidized bed and chlorine removal by applying various additives. Energy Fuels24:1389–1395.
- Kantarelis, E., and A. Zabaniotou. 2009. Valorization of cotton stalks by fast pyrolysis and fixed bed air gasification for syngas production as precursor of second generation biofuels and sustainable agriculture. Bioresour. Technol.100:942–947.
- Luque, R., J. A. Menendez, A. Arenillas, and J. Cot. 2012. Microwave-assisted pyrolysis of biomass feedstocks: the way forward?Energy Environ. Sci.5:5481–5488.
- Wulff, N., H. Carrer, and S. Pascholati. 2006. Expression and purification of cellulase Xf818 from Xylella fastidiosa in Escherichia coli . Curr. Microbiol.53:198–203.
- Atsumi, S., T. Hanai, and J. C. Liao. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature451:86–89.
- Gustavsson, L., P. Börjesson, B. Johansson, and P. Svenningsson. 1995. Reducing CO2 emissions by substituting biomass for fossil fuels. Energy20:1097–1113.
- Lee, S. W., T. Herage, and B. Young. 2004. Emission reduction potential from the combustion of soy methyl ester fuel blended with petroleum distillate fuel. Fuel83:1607–1613.
- Gielen, D. J, A. J. M. Bos, M. A. R. C. de Feber, and T. Gerlagh. Biomass for greenhouse gas emission reduction. http://www.ecn.nl/docs/library/report/2000/c00001.pdf (accessed 15 March 2013).
- Gustavsson, L., J. Holmberg, V. Dornburg, R. Sathre, T. Eggers, K. Mahapatra, et al. 2007. Using biomass for climate change mitigation and oil use reduction. Energy Policy35:5671–5691.
- Chen, K., H. Zhang, Y. Miao, M. Jiang, and J. Chen. 2010. Succinic acid production from enzymatic hydrolysate of sake lees using Actinobacillus succinogenes 130Z. Enzyme Microb. Technol.47:236–240.
- Oliveira, L. S., and S. F. Adriana. 2009. From solid biowastes to liquid biofuels. Agriculture Issues and Policies Series: 265. Available at: http://www.demec.ufmg.br/disciplinas/eng032-BL/solid_biowastes_liquid_biofuels.pdf (accessed May 2013).
- Toledano, A., L. Serrano, A. M. Balu, R. Luque, A. Pineda, and J. Labidi. 2013. Fractionation of organosolv lignin from olive tree clippings and its valorization to simple phenolic compounds. ChemSusChem6:529–536.
- Du, C., J. Sabirova, W. Soetaert, and C. S. K. Lin. 2012. Polyhydroxyalkanoates production from low-cost sustainable raw materials. Curr. Chem. Biol.6:14–25.
- Balu, A. M., V. Budarin, P. S. Shuttleworth, L. A. Pfaltzgraff, K. Waldron, R. Luque, et al. 2012. Valorisation of orange peel residues: waste to biochemicals and nanoporous materials. ChemSusChem5:1694–1697.
- Au, E.2013. Food waste management and practice in Hong Kong in Commercial and Industrial (C&I) Food Waste Recycling Seminar, 8 February 2013, Food Education Association, The Hong Kong Polytechnic University, Hong Kong.
- Zhang, R., H. M. El-Mashad, K. Hartman, F. Wang, G. Liu, C. Choate, et al. 2006. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol.98:929–935.
- Russ, W., and R. Meyer-Pittroff. 2004. Utilizing waste products from the food production and processing industries. Crit. Rev. Food Sci. Nutr.44:57–62.
- Kornegay, E. T., G. W. Vander Noot, K. M. Barth, W. S. MacGrath, J. G. Welch, and E. D. Purkhiser. 1965. Nutritive value of garbage as a feed for swine. I. Chemical composition, digestibility and nitrogen utilization of various types of garbage. J. Anim. Sci.24:319–324.
- Westendorf, M. L.1996. Pp. 24–32inThe use of food waste as a feedstuff in swine diets. Proceeding of Food Waste Recycling Symp. Rutgers Coop. Ext., Rutgers Univ.-Cook College, New Brunswick, NJ.
- Grolleaud, M.2002. Post-harvest losses: discovering the full story. Overview of the phenomenon of losses during the post-harvest system. FAO, Agro Industries and Post-Harvest Management Service, Rome, Italy.
- Parfitt, J., M. Barthel, and S. Macnaughton. 2010. Food waste within food supply chains: quantification and potential for change to 2050. Philos. Trans. R. Soc. Lond. B Biol. Sci.365:3065–3081.
- Gustavsson, J., C. Cederberg, U. Sonesson, R. van Otterdijk, and A. Meybeck. 2011. Global food losses and food waste: extent, causes and prevention. FAO, Rome, Italy.
- Tatsi, A., and A. Zouboulis. 2002. A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Adv. Environ. Res.6:207–219.
- EPD (Environmental Protection Department of HKSAR). Monitoring of solid waste in Hong Kong 2011. https://www.wastereduction.gov.hk/chi/materials/info/msw2011tc.pdf (accessed October 2012).
- Abu-Rukah, Y., and O. Al-Kofahi. 2001. The assessment of the effect of landfill leachate on ground-water quality—a case study El-Akader landfill site-north Jordan. J. Arid Environ.49:615–630.
- Pfaltzgraff, L. A., M. De bruyn, E. C. Cooper, V. Budarin, and J. H. Clark. 2013. Food waste biomass: a resource for high-value chemicals. Green Chem.15:307–314.
- Toledano, A., L. Serrano, J. Labidi, A. Pineda, A. M. Balu, and R. Luque. 2013. Heterogeneously catalysed mild hydrogenolytic depolymerisation of lignin under microwave irradiation with hydrogen-donating solvents. ChemCatChem5:977–985.
- Toledano, A., L. Serrano, and J. Labidi. 2012. Process for olive tree pruning lignin revalorisation. Chem. Eng. J.193–194:396–403.
- Toledano, A., L. Serrano, A. Pineda, A. A. Romero, J. Labidi, and R. Luque. 2013. Microwave-assisted depolymerisation of organosolv lignin via mild hydrogen-free hydrogenolysis: catalyst screening. Appl. Catal. B. doi: 10.1016/j.apcatb.2012.10.015
- Llorach, R., J. C. Espín, F. A. Tomás-Barberán, and F. Ferreres. 2003. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J. Agric. Food Chem.51:2181–2187.
- González-Sáiz, J. M., C. Pizarro, I. Esteban-Díez, O. Ramírez, C. J. González-Navarro, M. J. Sáiz-Abajo, et al. 2007. Monitoring of alcoholic fermentation of onion juice by NIR spectroscopy: valorization of worthless onions. J. Agric. Food Chem.55:2930–2936.
- Dong, L.-M., X.-P. Yan, Y. Li, Y. Jiang, S.-W. Wang, and D.-Q. Jiang. 2004. On-line coupling of flow injection displacement sorption preconcentration to high-performance liquid chromatography for speciation analysis of mercury in seafood. J. Chromatogr. A1036:119–125.
- Cheng, Y., L. Fan, H. Chen, X. Chen, and Z. Hu. 2005. Method for on-line derivatization and separation of aspartic acid enantiomer in pharmaceuticals application by the coupling of flow injection with micellar electrokinetic chromatography. J. Chromatogr. A1072:259–265.
- de Boer, A. R., T. Letzel, D. A. van Elswijk, H. Lingeman, W. M. Niessen, and H. Irth. 2004. On-line coupling of high-performance liquid chromatography to a continuous-flow enzyme assay based on electrospray ionization mass spectrometry. Anal. Chem.76:3155–3161.
- Stewart, J. J., T. Akiyama, C. Chapple, J. Ralph, and S. D. Mansfield. 2009. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol.150:621–635.
- Sahu, R., and P. L. Dhepe. 2012. A one-pot method for the selective conversion of hemicellulose from crop waste into C5 sugars and furfural by using solid acid catalysts. ChemSusChem5:751–761.
- Chakraborty, R., S. Bepari, and A. Banerjee. 2010. Transesterification of soybean oil catalyzed by fly ash and egg shell derived solid catalysts. Chem. Eng. J.165:798–805.
- Fu, B., L. Gao, L. Niu, R. Wei, and G. Xiao. 2009. Biodiesel from waste cooking oil via heterogeneous superacid catalyst SO42−/ZrO2 . Energy Fuels23:569–572.
- Clark, J. H., V. Budarin, T. Dugmore, R. Luque, D. J. Macquarrie, and V. Strelko. 2008. Catalytic performance of carbonaceous materials in the esterification of succinic acid. Catal. Commun.9:1709–1714.
- Luque, R., A. Pineda, J. C. Colmenares, J. M. Campelo, A. A. Romero, J. C. Serrano-Ruiz, et al. 2012. Carbonaceous residues from biomass gasification as catalysts for biodiesel production. J. Nat. Gas Chem.21:246–250.
- Abbot, A. P., R. C. Harris, K. S. Ryder, C. D′Agostino, L. F. Gladden, and M. D. Mantle. 2011. Glycerol eutectics as sustainable solvent systems. Green Chem.13:82–90.
- Carriazo, D., M. C. Serrano, M. C. Gutierrez, M. L. Ferrer, and F. del Monte. 2012. Deep eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev.41:4996–5014.
- Zhang, Q., K. De Oliveira Vigier, S. Royer, and F. Jerome. 2012. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev.41:7108.
- Russ, C., and B. König. 2012. Low melting mixtures in organic synthesis- an alternative to ionic liquids?Green Chem.14:2969–2982.
- Serrano-Ruiz, J. C., J. M. Campelo, M. Francavilla, C. Menendez, A. B. Garcia, A. A. Romero, et al. 2012. Efficient microwave-assisted production of furfural from C5 sugars in aqueous media catalysed by Brönsted acidic ionic liquids. Catal. Sci. Technol.2:1828–1832.
- Zhang, Z., Q. Wang, H. Xie, W. Liu, and Z. K. Zhao. 2011. Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural by germanium (IV) chloride in ionic liquids. ChemSusChem4:131–138.
- Colmenares, J. C., R. Luque, J. M. Campelo, F. Colmenares, Z. Karpiński, and A. A. Romero. 2009. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: an overview. Materials2:2228–2258.
- Stillings, R. A., and R. J. V. Nostrand. 1944. The action of ultraviolet light upon cellulose. I. Irradiation effects. II. Post-irradiation effects1. J. Am. Chem. Soc.66:753–760.
- Ai, Z., W. Ho, and S. Lee. 2011. Efficient visible light photocatalytic removal of NO with BiOBr-graphene nanocomposites. J. Phys. Chem.115:25330–25337.
- Ismail, A. A., and D. W. Bahnemann. 2011. Mesostructured Pt/TiO2 nanocomposites as highly active photocatalysts for the photooxidation of dichloroacetic acid. J. Phys. Chem.115:5784–5791.
- Bernardo, E. C.2008. Solid-waste management practices of households in Manila, Philippines. Ann. NY Acad. Sci.1140:420–424.
- Office of the Chief Executive. 2013. Policy Address, 2013 (Office of the Chief Executive). The Hong Kong Government Special Administrative Region (HKSAR), Hong Kong. Available at http://www.policyaddress.gov.hk/2013/eng/p142.html (accessed 16 January 2013).
- Takata, M., K. Fukushima, N. Kino-Kimata, N. Nagao, C. Niwa, and T. Toda. 2012. The effects of recycling loops in food waste management in Japan: based on the environmental and economic evaluation of food recycling. Sci. Total Environ.432:309–317.
- Bernstad, A., and J. la Cour Jansen. 2012. Separate collection of household food waste for anaerobic degradation – Comparison of different techniques from a systems perspective. Waste Manage. (Oxford)32:806–815.
- Zhang, B., L.-L. Zhang, S.-C. Zhang, H.-Z. Shi, and W.-M. Cai. 2005. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ. Technol.26:329–340.
- Zhang, A. Y., Z. Sun, C. C. J. Leung, W. Han, K. Y. Lau, M. Li, et al. 2013. Valorisation of bakery waste for succinic acid production. Green Chem.15:690–695.
- Van-Thuoc, D., J. Quillaguamán, G. Mamo, and B. Mattiasson. 2008. Utilization of agricultural residues for poly(3-hydroxybutyrate) production by Halomonas boliviensis LC1. J. Appl. Microbiol.104:420–428.
- Du, C., S. K. C. Lin, A. Koutinas, R. Wang, P. Dorado, and C. Webb. 2008. A wheat biorefining strategy based on solid-state fermentation for fermentative production of succinic acid. Bioresour. Technol.99:8310–8315.
- Dorado, M. P., S. K. C. Lin, A. Koutinas, C. Du, R. Wang, and C. Webb. 2009. Cereal-based biorefinery development: utilisation of wheat milling by-products for the production of succinic acid. J. Biotechnol.143:51–59.
- Lin, C. S. K., R. Luque, J. H. Clark, C. Webb, and C. Du. 2012. Wheat-based biorefining strategy for fermentative production and chemical transformations of succinic acid. Biofuels Bioprod. Biorefin.6:88–104.
- Leung, C. C. J., A. S. Y. Cheung, A. Y.-Z. Zhang, K. F. Lam, and C. S. K. Lin. 2012. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J.65:10–15.
- García, I. L., J. A. López, M. P. Dorado, N. Kopsahelis, M. Alexandri, S. Papanikolaou, et al. 2013. Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator . Bioresour. Technol.130:16–22.
- Delgado, R., A. J. Castro, and M. Vázquez. 2009. A kinetic assessment of the enzymatic hydrolysis of potato (Solanum tuberosum). LWT Food Sci. Technol.42:797–804.
- Yu, J., Z. Li, Q. Ye, Y. Yang, and S. Chen. 2010. Development of succinic acid production from corncob hydrolysate by Actinobacillus succinogenes . J. Ind. Microbiol. Biotechnol.37:1033–1040.
- Chen, K., H. Zhang, Y. Miao, P. Wei, and J. Chen. 2011. Simultaneous saccharification and fermentation of acid-pretreated rapeseed meal for succinic acid production using Actinobacillus succinogenes . Enzyme Microb. Technol.48:339–344.
- Wang, R., L. C. Godoy, S. M. Shaarani, M. Melikoglu, A. Koutinas, and C. Webb. 2009. Improving wheat flour hydrolysis by an enzyme mixture from solid state fungal fermentation. Enzyme Microb. Technol.44:223–228.
- Li, Q., J. Siles, and I. Thompson. 2010. Succinic acid production from orange peel and wheat straw by batch fermentations of Fibrobacter succinogenes S85. Appl. Microbiol. Biotechnol.88:671–678.
- Quillaguamán, J., R. Hatti-Kaul, B. Mattiasson, M. T. Alvarez, and O. Delgado. 2004. Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile isolated from soil around a Bolivian hypersaline lake. Int. J. Syst. Evol. Microbiol.54:721–725.
- Van-Thuoc, D., H. Guzmán, J. Quillaguamán, and R. Hatti-Kaul. 2010. High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process. J. Biotechnol.147:46–51.
- Arancon, R. A., H. R. Barros Jr., A. M. Balu, C. Vargas, and R. Luque. 2011. Valorisation of corncob residues to functionalised porous carbonaceous materials for the simultaneous esterification/transesterification of waste oils. Green Chem.13:3162–3167.
- Cabeza, L., M. M. Taylor, G. L. DiMaio, E. Brown, W. N. Marmer, R. Carrió, et al. 1998. Processing of leather waste: pilot scale studies on chrome shavings. Isolation of potentially valuable protein products and chromium. Waste Manage. (Oxford)18:211–218.
- Gelse, K., E. Pöschl, and T. Aigner. 2003. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev.55:1531–1546.
- Mata, T. M., A. A. Martins, and N. S. Caetano. 2013. Valorization of waste frying oils and animal fats for biodiesel production. Pp. 671–693inJ. W. Lee, ed. Advanced biofuels and bioproducts. Springer, The Netherlands.
- Reis, R. L., N. M. Neves, J. F. Mano, M. E. Gomes, A. P. Marques, and H. S. Azevedo. 2008. Natural based polymers for biomedical applications. Woodhead Publishing, CRC Press, Cambridge, U.K.
- Catalina, M., J. Cot, M. Borras, J. de Lapuente, J. González, A. M. Balu, et al. 2013. From waste to healing biopolymers: biomedical applications of bio-collagenic materials extracted from industrial leather residues in wound healing. Materials6:1599–1607.
- Catalina, M., J. Cot, M. Borras, J. de Lapuente, J. González, A. M. Balu, et al. 2013. From waste to healing biopolymers: biomedical applications of bio-collagenic materials extracted from industrial leather residues in wound healing. Materials6:1599–1607.
- Langmaier, F., P. Mokrejs, R. Karnas, M. Mládek, and K. Kolomazník. 2006. Modification of chrome-tanned leather waste hydrolysate with epichlorhydrin. J. Soc. Leather Technol. Chem.90:29–34.
- Brown, E., C. Thompson, and M. M. Taylor. 1994. Molecular size and conformation of protein recovered from chrome shavings. J. Am. Leather Chem. Assoc.89:215–220.
- Yu, J., Y. Hai, and B. Cheng. 2011. Enhanced photocatalytic H2-production activity of TiO2 by Ni(OH)2 cluster modification. J. Phys. Chem. C115:4953–4958.
- Liang, Y. T., B. K. Vijayan, K. A. Gray, and M. C. Hersam. 2011. Minimizing graphene defects enhances titania nanocomposite-based photocatalytic reduction of CO2 for improved solar fuel production. Nano Lett.11:2865–2870.
- Polshettiwar, V., R. Luque, A. Fihri, H. Zhu, M. Bouhrara, and J. M. Basset. 2011. Magnetically recoverable nanocatalysts. Cheminform42:3036–3075.
- Liu, J., S. Z. Qiao, Q. H. Hu, and G. Q. Lu. 2011. Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small7:425–443.
- Daskalaki, V. M., M. Antoniadou, G. Li Puma, D. I. Kondarides, and P. Lianos. 2010. Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol.44:7200–7205.
- Balu, A. M., B. Baruwati, E. Serrano, J. Cot, J. Garcia-Martinez, R. S. Varma, et al. 2011. Magnetically separable nanocomposites with photocatalytic activity under visible light for the selective transformation of biomass-derived platform molecules. Green Chem.13:2750–2758.
References
- Zondag, H. A., D. W. De Vries, W. G. J. Van Helden, R. J. C. Van Zolingen, and A. A. Van Steenhoven. 2003. The yield of different combined pv-thermal collector designs. Sol. Energy74:253–269.
- Chow, T. T.2010. A review on photovoltaic/thermal hybrid solar technology. Appl. Energy87:365–379.
- Skoplaki, E., and J. A. Palyvos. 2009. On the temperature dependence of photovoltaic module electrical performance: a review of efficiency/power correlations. Sol. Energy83:614–624.
- Huang, B. J., T. H. Lin, W. C. Hung, and F. S. Sun. 2001. Performance evaluation of solar photovoltaic/thermal systems. Sol. Energy70:443–448.
- Bystrom, J. 2008. Iea shc task 35 subtask ada 1-2 outcome of pv/t market survey interviews. Available at http://archive.iea-shc.org/publications/task.aspx?Task=35 (accessed March 15, 2015).
- Tiwari, G. N., I. M. Al-Helal. 2015. Analytical expression of temperature dependent electrical efficiency of n-pvt water collectors connected in series. Sol. Energy114:61–76.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?