Abstract
Objective
Giant cell tumor of bone (GCT) is a primary, osteolytic, benign tumor of the bone. Surgery is the commonly used treatment; however, recurrence remains a problem. Receptor activator of nuclear factor kappa B (RANKL) is responsible for the formation of osteoclastic cells. Discovery of RANKL and its human monoclonal antibody, denosumab, led to use of denosumab for treatment of GCT. The aim of this study was to evaluate clinical and pathological results of treatment of GCT with denosumab and to assess adverse effect profile and recurrence rate.
Methods
Thirteen patients with 14 lesions were enrolled in the study. Mean age was 38.3 years. Patients were given subcutaneous injections of denosumab (120 mg) every 4 weeks (with additional doses on days 0, 8 and 15 in cycle 1 only) and were radiologically evaluated for tumor response. Pain and functional status were measured using Visual Analog Score (VAS) and Musculoskeletal Tumor Society Score (MSTS). Adverse effects were analyzed after each cycle.
Results
Participants were 5 men and 8 women. Mean follow-up was 17 months. One lesion was Campanacci grade I, 8 were grade II, and 5 were grade III. Eight lesions were recurrent, and remaining were primary lesions. After average of 9 cycles (range: 4–17 cycles), all tumors underwent radiological regression. Ten lesions were removed surgically. More than 90% of giant cells were found to have regressed in all pathological specimens. On last follow-up, average VAS was 1 and MSTS was 87%. Fatigue and joint and muscle pain after injections was reported by 46% of patients, and mild hypocalcaemia was seen in 1 patient.
Conclusion
Denosumab has been shown to be a successful drug in treatment of GCT. Denosumab can be used as neoadjuvant for all recurrent lesions, grade II lesions with high surgical risk, grade III lesions, and metastatic cases of GCT.
Level of evidence
Level IV, Therapeutic study
Keywords
Denosumab ; Recurrence ; Giant cell tumor
Giant cell tumor (GCT) is an aggressive, benign bone tumor. GCT, which was first defined by Cooper and Travers, can produce pulmonary metastasis, albeit rarely (1–6%).1 ; 2 GCT constitutes 5% of primary bone tumors and 20% of benign bone tumors. Although GCT settles in metaphyseal area of long bones, particularly distal femur and proximal tibia, followed by distal radius and proximal humerus in 85% of cases, it is also seen in axial skeleton (10%) and in small bones of hands and feet (5%). GCT is common in third and fourth decades of life, and frequently emerges in women.3
Radiologically, GCT is lytic lesion without mineralization that often extends to subchondral area and is located eccentrically in metaphysoephyseal region. It has no sclerotic edge, but is separated from normal bone with narrow transition zone. It leads to cortical thinning, expansion, or cortex destruction, and soft tissue components may be found in more aggressive lesions. Pathological findings of GCT consist of osteoclast-like giant cells and proliferating mononuclear round stromal cells, which are responsible for neoplasia.4 Although osteoclast-like giant cells control bone resorption, stromal cells direct monocytes by managing tumor pathology and ensure formation of giant cells.5
The classic treatment for GCT is surgery. Following aggressive curettage and high-speed burring, use of chemical adjuvants such as phenol, alcohol, hydrogen peroxide, and liquid nitrogen, as well as defect filling with grafts or bone cement are among most frequently used methods of treatment. Endoprosthetic reconstruction may also be used for grade III tumors with serious cortical destruction.6 Recurrence rate ranges between 5% and 56%, depending on cement and chemical adjuvants used during surgery.7 Recurrence still continues to be the major problem. In recurrent lesions, in lesions with wide soft tissue component, and in surgically difficult localizations like sacrum, spine, pelvis, and distal radius, methods such as embolization, radiotherapy, or bisphosphonates may be used.8 ; 9
In the 1990s, effects of receptor activator of nuclear factor kappa B ligand (RANKL) and receptor activator of nuclear factor kappa B (RANK), members of the tumor necrosis factor (TNF) family, on formation of osteoclasts and bone resorption were demonstrated.10 ; 11 It was seen that RANKL expression in stromal cells caused neoplasia and stimulated both formation of osteoclast-like giant cells and bone resorption with activation of RANK.12 ; 13 Recognition of RANKL in pathogenesis led to investigations of potential curative effect of suppression of this molecule. The result was a human monoclonal immunoglobulin G2 antibody for RANKL, denosumab. It was initially shown to decrease bone resorption in postmenopausal osteoporosis and metastatic bone lesions.14 ; 15 ; 16 Thomas et al reported successful clinical and radiological results with 120 mg denosumab dose in recurrent or inoperable GCT in a phase 2 study in 2010.17 Denosumab was licensed for use in unresectable lesions and relapse tumors and in lesions for which resection would cause serious morbidity by the United States Food and Drug Administration in 2013 and the European Medicines Agency in 2014.18
The purpose of the present study was to evaluate clinical and pathological results of 120 mg subcutaneous denosumab treatment in patients with GCT and examine recurrence rate and adverse effect profile.
Patients and methods
Thirteen patients who were diagnosed as having GCT of bone between April 2011 and January 2015 in our clinic were included in this prospective study, conducted with permission from the Ministry of Health. Patient demographic data, tumor localization, and previous treatments were recorded. All patients were radiologically classified using Campanacci classification19 after plain radiographies, bone scan, and computerized tomography (CT) of the lesion and thorax, and magnetic resonance imaging (MRI) of lesion. Grade I GCT is latent tumor in which the cortex is intact and its borders are clearly separated. Grade II GCT is an active tumor, whose borders can be separated, but there is no sclerotic edge; thinning is present with expansion in cortex. Grade III GCT is aggressive lesion that has ambiguous borders. Cortical destruction and soft tissue components are present. A closed-needle biopsy was performed on patients with primary tumors. Diagnoses were confirmed by re-evaluating paraffin-embedded blocks of recurrent lesions. One pathologist experienced in musculoskeletal system pathology undertook all pathological evaluations. Prior to drug use, calcium, phosphorus, and parathyroid hormone levels were measured in all patients. All measured levels were found to be normal.
All recurrent lesions, grade II lesions with high surgical risk, metastatic lesions, and grade III lesions were included in this study. Subcutaneous injections of denosumab (120 mg) were given every 4 weeks (with additional doses on days 0, 8 and 15 in cycle 1 only). Concomitantly, 1500 mg calcium carbonate+400 IU vitamin D were given daily.17 ; 20 The number of cycles was recorded. Adverse effect profile was examined after each dose.
Radiographs were used to evaluate lesion mineralization, septa formation, ossification of soft tissue component, and corticalization in each cycle of treatment, and CT in third month and at the end of treatment. Final status of lesion of patients who underwent surgery was examined with MRI after treatment. Postoperative patients were assessed in terms of recurrence using radiography, CT, and MRI every 3 months in the first year.
Curettage or resection material after treatment was classified using a 2-grade staging system developed for this study according to the tumor response. Grade 1 was grouped as fibrous tissue, inflammation, and a small amount of woven bone formation; Grade 2 was defined as woven bone, precancellous bone, and cancellous bone formation.
Pain relief was evaluated after treatment and at last follow-up. Pain scores of all patients were assessed using visual analog scale (VAS) ranging 0 to 10. Functional status was evaluated using Musculoskeletal Tumor Society (MSTS) Score at last follow-up.21
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences software version 16 (SPSS Inc., Chicago, IL, USA). Average and standard deviation were calculated for quantitative variables, and number of cases was calculated for categorical variables. Differences between pre- and post-treatment VAS scores were analyzed using t-test. P value less than 0.05 was considered significant.
Results
Fourteen lesions in 13 patients (5 men, 8 women; mean age: 38.3 years [range: 26–51 years]) diagnosed as GCT of bone and treated with denosumab were included in the study. Average length of follow-up was 17 months (range: 10–30 months). One patient had multifocal lesions in proximal and distal femur. One lesion was grade I (multifocal lesion with recurrent grade 2 lesion in distal femur), 8 were grade II, and 5 were grade III. Six lesions were primary, whereas 8 were recurrent. Two patients with recurrent lesions had lung metastasis; these patients had been under follow-up for GCT for more than 10 years. Demographic data, tumor localization, radiological grade, and previous treatments are shown in Table 1 .
| Patient # | Sex (M/F) | Age (years) | Tumor localization | Campanacci grade | Recurrent/Primary | Previous treatment |
|---|---|---|---|---|---|---|
| 1 | F | 26 | L proximal fibula | Grade III | Recurrent | Proximal fibula resection |
| 2 | M | 33 | L distal femur | Grade II | Recurrent | 2 times C/C Fixation of pathological fracture Lung metastases |
| 3 | F | 36 | Sacrum | Grade II | Primary | |
| 4a | F | 31 | R distal femur | Distal femur grade I | Distal femur recurrent | Distal femur C/C |
| R proximal femur | Proximal femur grade II | Proximal femur primary | ||||
| 5 | M | 36 | R distal femur | Grade II | Primary | |
| 6 | F | 48 | L proximal fibula | Grade III | Primary | |
| 7 | F | 51 | R proximal tibia | Grade II | Primary | |
| 8 | M | 37 | R proximal humerus | Grade III | Recurrent | 3 times C/C |
| 9 | F | 35 | L distal radius | Grade III | Recurrent | C/G |
| 10 | F | 39 | R distal femur | Grade II | Recurrent | 2 times C/C |
| 11 | M | 49 | R distal femur | Grade II | Recurrent | C/G Lung metastases |
| 12 | M | 50 | R distal radius | Grade III | Recurrent | C/G |
| 13 | F | 27 | L proximal tibia | Grade II | Primary |
C/C: Curettage-cementation; C/G: Curettage-grafting; L: Left; R: Right.
a. Multifocal lesion.
Due to availability of denosumab in our country, 4 patients received 1 × 120 mg (Xgeva; Amgen, CA, USA) and 9 patients had 2 × 60 mg (Prolia; Amgen, CA, USA). An average of 9 cycles (range: 4–17 cycles) were administered.
Ten lesions were surgically treated following denosumab administration, and resection/curettage specimens obtained were pathologically examined (Fig. 1 ; Fig. 2 ). Pathological evaluation revealed that giant cells had regressed >90% in all cases. In 4 patients, 90% ossification of lytic area was seen radiologically with regression of pain; 3 of these patients continued follow-up without treatment and 1 continues to receive denosumab treatment for lung metastases. It was observed that lung lesions were regressing and remained stable (Fig. 3 ). For patients who underwent curettage, physical and chemical adjuvants such as high-speed burring, electrocauterization, phenol, alcohol, or hydrogen peroxide were used. Surgical treatments, number of treatment cycles, and pathological evaluation status are presented in Table 2 . No recurrence has been observed in surgically-treated patients.
|
|
|
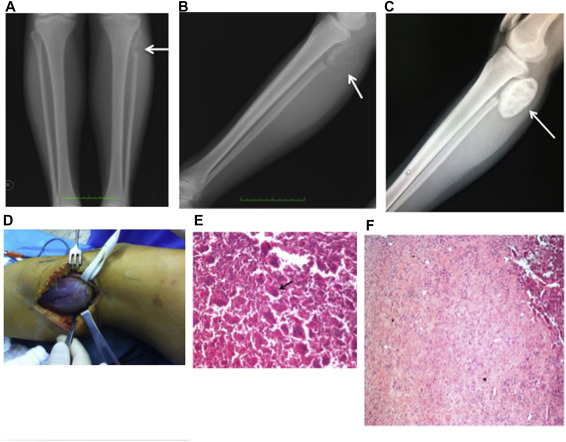
Fig. 1. 26-year-old female who underwent proximal fibula resection for giant cell tumor has recurrence in soft tissue. (a) Anteroposterior and (b) lateral radiographs show poorly ossified lesion in soft tissue (arrow), which showed high degree of ossification (arrow) after denosumab (c). Intraoperative view of resected lesion (d). 100× magnification with hematoxilen-eosin stain shows 100% regression of the giant cells (arrow) (e) before denasumab (f) after denasumab. |
|
|
|
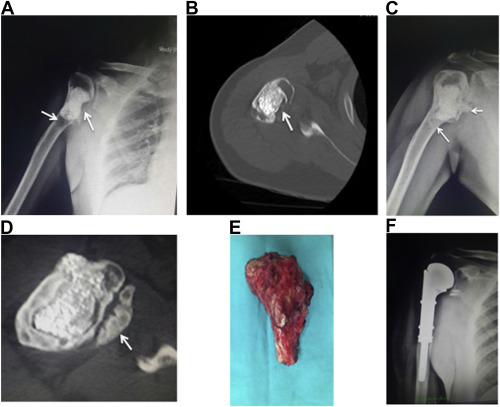
Fig. 2. 37-year-old man with giant cell tumor of proximal humerus previously treated with curettage and cementation. Anteroposterior radiograph (a) and computed tomography (b) before treatment (arrows show lytic areas) and (d,e) after treatment (arrows show ossification of lytic areas and soft tissue component). (e) Resection material and (f) postoperative anteroposterior radiograph. |
|
|
|
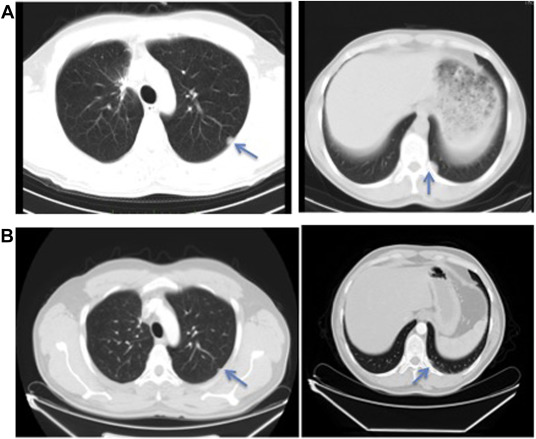
Fig. 3. Computed tomography images show regression of lung metastases. Arrows show (a) lesion before treatment and (b) after treatment. |
| Patient # | Treatment | Number of cycles | Pathologic evaluation |
|---|---|---|---|
| 1 | Resection of soft tissue recurrence | 12 | Grade II |
| 2 | Follow-up without surgery | 17 | |
| 3 | C/G | 4 | Grade II |
| 4 | Follow-up distal femur C/G and IF proximal femur | 8 | Grade I |
| 5 | C/C | 8 | Grade I |
| 6 | Follow-up without surgery | 10 | |
| 7 | C/C | 8 | Grade I |
| 8 | Resection and endoprosthetic reconstruction | 8 | Grade II |
| 9 | Resection and reconstruction with vascularized fibula | 8 | Grade II |
| 10 | Follow-up | 10 | |
| 11 | Resection and endoprosthetic reconstruction | 8 | Grade I |
| 12 | C/C | 8 | Grade II |
| 13 | C/C | 8 | Grade I |
C/C: Curettage-cementation; C/G: Curettage-grafting; IF: Internal fixation.
Average pre-treatment pain VAS of 7 (range: 3–10) was reduced to average of 2 after third cycle, and then 1 (range: 0–3) at last follow-up (p < 0.05). Average MSTS score at last follow-up was 87%. While assessing adverse effects, 6 patients (46%) reported fatigue, muscle and joint pain for a few days after the injections, and 1 patient had mild hypocalcaemia. No patients were found to have avascular necrosis of jaw, secondary infection, or other serious adverse effect.
When the planned and performed surgeries before and after denosumab were evaluated, no further surgical treatment applied in 1 proximal fibula lesion that were planned for resection and 3 recurrent distal femur lesions planed to have re-curettage and cementing. In the 10 patients who were surgically treated, the planned surgery did not change.
Discussion
Treatment of GCT has continued for the last 30 years with no perceptible change. Traditional treatments for grade I and II lesions include aggressive curettage and high-speed burring, followed by treatments with local adjuvants and defect-filling with graft or bone cement.6 Use of methyl methacrylate has advantages due to heat and cytotoxic effect, which acts as an adjuvant treatment and has led to less recurrence.22 Although local recurrence rates are reported to decrease with use of chemical adjuvants, it is still up to 56%.7 ; 23 In difficult localizations such as sacrum and distal radius, recurrence rate within soft tissue is quite high. For these lesions, embolization, radiotherapy, and use of interferon alfa and bisphosphonates such as zoledronic acid are also recommended.8 ; 9 ; 24 ; 25 ; 26
An increased understanding of GCT pathophysiology in recent years has identified RANKL in stromal cells, and the activity of this molecule in cytogenesis of giant cells similar to osteoclasts through osteoprotegerin, has made RANKL the real target of treatment.27 The first phase 2 study, conducted by Thomas et al in 2010, included a total of 37 patients from 8 centers with recurrent or unresectable tumors. After loading on days 0, 8, and 15, a monthly dose of 120 mg denosumab showed 86% good tumor response histologically and radiologically.17 Another multi-center study in 2013 reported 41% complete and partial response, and 58% stable disease after 7–20 doses of denosumab in group that could not be resected surgically, and 58% complete and partial response and 41% stable disease was achieved in group that was planned to undergo surgery.20 Present study showed 100% radiological and pathological response.
In this study, most frequently encountered adverse effects were constitutional symptoms such as pain in extremities and back, headache, fatigue, and mild hypocalcemia. Thomas et al reported that 1 patient developed secondary bone sarcoma and another patient developed mandibular osteonecrosis.17 Chawla et al reported 84% of patients encountered arthralgia, headache, nausea, fatigue, and back and extremity pain. Three patients were reported to have mandibular osteonecrosis, and hypocalcemia was found in 15% of patients. Three patients had new primary malignant lesions; however, only 1 of these was considered secondary malignant transformation.20 Malignant transformation rate of GCT has been reported to be 1%.18 Malignant degeneration may be caused by dedifferentiation of tumor or secondary to previous radiotherapy (50% of cases).28 Wojcik et al compared pathology samples of 9 patients treated with denosumab with samples of 9 patients of GCT with malignant transformation. In all patients, giant cells had been eradicated. It was also noted that increase in cellularity and accumulation of atypical and immature bone detected in patients in early stages of denosumab treatment might be mistaken for primary bone sarcoma. In GCT, it has been documented that less atypia and mitotic activity is seen and infiltrative growth pattern does not exist, which is dissimilar to novo and secondary sarcomas. Patients who receive longer-duration denosumab treatment have shown decrease in cellularity and maturation in bone.29 It should be kept in mind that less-experienced pathologists might evaluate such changes in favor of bone sarcomas. Aponte-Tineo et al reported that malignant transformation was encountered after denosumab in recurring GCTs.30
Although denosumab use in treatment of GCT causes fast symptomatic and radiological recovery, is easy to use, and has a good adverse effect profile, there are still questions to be answered. The first is with regard to cytotoxic effect of denosumab on stromal cells. In a study conducted by Lau et al, zoledronic acid was compared with denosumab in terms of cytotoxicity on stromal cells. Zoledronic acid was found to have caused decreased growth of stromal cells and dose-dependent apoptosis, whereas denosumab had minimal inhibitory effect without causing apoptosis.31 This raises the question as to whether denosumab is only effective while it is being used; there are no studies about length of denosumab treatment on unresectable or recurrent lesions that are followed-up without surgery. Furthermore, considering that RANK and RANKL are found in many systems of the body, effects of long-term inhibition on other organ systems are not yet known.
In studies that compared whether difference was achieved in planned and performed surgery, it was evaluated that among 222 patients who used denosumab for an average of 15.3 months, 38% underwent less morbid surgery with an 80% decrease in need for hemipelvectomy and amputations, and 85% success in preservation of joints. A 15% recurrence rate was reported during 13 months of follow-up. Risk was found to be higher when bone grafting had been performed and adjuvants had not been used.32 There is still no consensus about use of denosumab after surgery in the literature. Rutkowski et al. reported that 106 patients who did not have surgery were symptom free and had stable sclerosis on follow-up after an average of 22.5 doses of denosumab.32
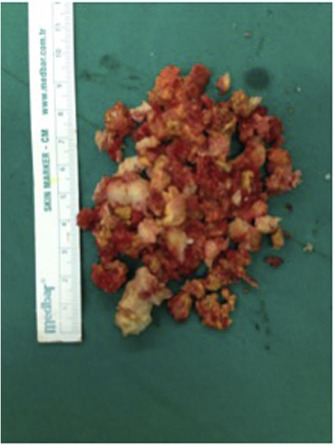
Although ossification of soft tissue component and lytic cortex, as well as less bleeding are factors that facilitate surgery, curettage of tough, fibrous, and sclerotic tissue that forms after denosumab treatment becomes quite difficult (Fig. 4 ). It is also difficult to separate pathological and normal tissue during curettage. It should be kept in mind that live cells may remain in developed bone septum walls and curettage should be performed with powerful equipment. Adjuvant treatments should not be forsaken.
|
|
|
Fig. 4. Fibro-osseous curettage specimen after denosumab treatment. |
The weaknesses of this study may be counted as the following: limitation of the number of patients and heterogeneous distribution of cases. To evaluate medical treatment efficiency of this rare primary bone tumor, multi-center studies with more homogenous groups must be planned. Prospective design, description of a new pathological classification, and detailing personal surgical experience are very strong points of this study.
Conclusion
Good clinical and radiological results can be achieved with denosumab use in GCT, and it has a safe adverse effect profile. We recommend that it be used as a neoadjuvant treatment in all recurrent patients, in grade II lesions with high surgical risk, metastatic lesions and grade III lesions.
References
- 1 M. Balke, L. Schemper, C. Gebert, et al.; Giant cell tumor of bone: treatment and outcome of 214 cases; J Cancer Res Clin Oncol, 134 (9) (2008), pp. 949–978
- 2 M. Dominkus, P. Ruggieri, F. Bertoni, et al.; Histologicallay verified lung metastases in benign giant cell tumors-14 cases from a single institution; Int Orthop, 30 (2006), pp. 499–504
- 3 R.E. Turcotte; Giant cell tumor of bone; Orthop Clin North Am, 37 (2006), pp. 35–51
- 4 W.M. Mendenhall, R.A. Zlotecki, M.T. Scarborough, C.P. Gibbs, N.P. Mendenhall; Giant cell tumor of bone; Am J Clin Oncol, 29 (1) (2006), pp. 96–99
- 5 R.W. Cowan, G. Singh; Giant cell tumor of bone: a basic science perpective; Bone, 52 (1) (2013), pp. 238–246
- 6 K.M. Skubitz; Giant cell tumor of bone: current treatment options; Curr Treat Options Oncol, 15 (3) (2014), pp. 507–518
- 7 G.H. Prosser, K.G. Baloch, R.M. Tillman, S.R. Carter, R.J. Grimer; Does curettage without adjuvan therapy provide low recurrence rates in giant cell tumors of bone?; Clin Orthop, 435 (2005), pp. 211–218
- 8 O. Micke, F. Bruns, H.T. Eich, et al.; Radiation therapy for giant cell tumor of bone:Long-term results of a multicenter study in Germany; Int J Radiat Oncol Biol Phys, 63 (2005), p. 108
- 9 H.S. Hosalkar, K.J. Jones, J.J. King, R.D. Lackman; Serial arterial embolization for large sacral giant-cell tumors: mid- to long-term results; Spine, 32 (2007), pp. 1107–1115
- 10 W.C. Daugall, M. Glaccum, K. Charrrier, et al.; RANK is essential for osteoclast and lymph node development; Genes Dev, 13 (1999), pp. 2412–2424
- 11 G.J. Atkins, P. Kostakis, C. Vincent, et al.; RANK expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow and giant cell tumor of bone; J Bone Min Res Off J Am Soc Bone Min Res, 21 (2006), pp. 1339–1349
- 12 T. Morgan, G.J. Atkins, M.K. Trivett, et al.; Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB; Am J Pathol, 167 (2005), pp. 117–128
- 13 K.M. Skubitz, E.Y. Cheng, Dr Clohisy, R.C. Thompson, A.P. Skubitz; Gene expression in giant -cell tumors; J Lab Clin Med, 144 (2004), pp. 193–200
- 14 M.R. McClung, E.M. Lewiecki, S.B. Cohen, et al.; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density; N Engl J Med, 354 (2006), pp. 821–831
- 15 S.R. Cummings, J. San MArtin, M.R. McClung, et al.; FREEDOM Trial. Denasumab for prevention of fractures in postmenopausal women with osteoporosis; N Engl J Med, 361 (2009), pp. 756–765
- 16 A.T. Stopeck, A. Lipton, J.J. Body, et al.; Denosumab compared with zolendronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double blind study; J Clin Oncol, 28 (2010), pp. 5132–5139
- 17 D. Thomas, R. Henshaw, K.M. Skubitz, et al.; Denosumab in patients with giant cell tumor of bone: an open label, phase 2 study; Lancet Oncol, 11 (2010), pp. 275–280
- 18 A. Lopez-Pousa, J.M. Broto, T. Garrido, J. Vazguez; Giant cell tumor of bone: new treatments in development; Clin Transl Oncol, 17 (2015), pp. 419–430
- 19 M. Campanacci, A. Giunti, R. Olmi; Metaphyseal and diaphyseal localization of giant cell tumors; Chir Organi Mov, 62 (1) (1975), pp. 29–34
- 20 S. Chawla, R. Henshaw, L. Seeger, et al.; Safety and efficacy of denosumab for adults and sklettaly mature adolescents with giant cell tumor of bone: interim analysis of an open-label, paralel-group, phase 2 study; Lancet Oncol, 14 (2013), pp. 901–908
- 21 W.F.1 Enneking, W. Dunham, M.C. Gebhardt, M. Malawar, D.J. Pritchard; A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system; Clin Orthop Relat Res, 286 (1993 Jan), pp. 241–246
- 22 Z.H. Gao, J.Q. Yin, X.B. Xie, et al.; Local control of giant cell tumors of the long bone after agressive curettage with and without bone cement; BMC Musculoskelet Disord, 15 (2) (2014), p. 330
- 23 L. van der Heijden, P.D. Dijkstra, M.A. van de Sante, et al.; The clinical approach toward giant cell tumor of bone; Oncologist, 19 (5) (2014), pp. 550–561
- 24 M. Emori, M. Kaya, M. Sasaki, T. Wada, T. Yamaguchi; YamashitaT. Preoperative selective arterial embolization as a neoadjuvant therapy for proximal humerus giant cell tumor of bone: radiological and histological evaluation; Jpn J Clin Oncol, 42 (2012), pp. 851–855
- 25 A.W. Yasko; Interferon therapy for giant cell tumor of bone; Curr Opin Orthop, 17 (2006), pp. 568–572
- 26 M. Balke, L. Campanacci, C. Gebert, et al.; Biphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumor of bone; BMC Cancer, 10 (2010), p. 462
- 27 D.M. Thomas; RANKL, denosumab and giant cell tumor of bone; Curr Opin Oncol, 24 (2012), pp. 397–403
- 28 F. Bertoni, P. Bacchini, E.L. Staals; Malignancy in giant cell tumor of bone; Cancer, 97 (2003), pp. 2520–2529
- 29 J. Wojcik, A.E. Rosenberg, M.A. Bredella, E. Choy, G.P. Nielsen, V. Deshpande; Denosumab-treated giant cell tumor of bone exhibits morphologic overlap with malignant giant cell tumor of bone; Am J Surg Pathol, 40 (1) (2016), pp. 72–80
- 30 L. Aponte-Tinao, N.S. Piuzzi, P. Roitman, G.L. Farfalli; A high grade sarcoma arising in a patient with recurrent benign giant cel tumor of the proximal tibia while recieving treatment with denosumab; Clin Orthop Relat Res, 473 (2015), pp. 3050–3055
- 31 C.P.Y. Lau, L. Huang, K.C. Wong, S.M. Kumta; Comparison of the anti-tumor effects of denosumab and zolendronic acid on the neoplastic stromal cells of giant cell tumor of bone; Connect Tissue Res, 54 (6) (2013), pp. 439–449
- 32 P. Rutkowski, S. Ferrari, R. Grimer, et al.; Surgical downstaging in an open-label plase II trial of denosumab in patients with giant cell tumor of bone; Ann Surg Oncol, 22 (2015), pp. 2860–2868
Document information
Published on 31/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?