Abstract
In patients with Brugada syndrome (BS), VF occurred predominantly during the nocturnal period. Some patients also developed ESs. In addition to the circadian rhythm, patients showed weekly and seasonal patterns. The patients with ESs had peak episodes of VF on Saturday and in the winter and spring, while episodes of VF in patients with single VF events occurred most often on Monday with smaller seasonal variation. Except for age, there was no difference in the clinical or ECG characteristics between the patients with ESs and those with single VF episodes.
Abbreviations
BS, Brugada syndrome;CRBBB, complete right bundle branch block;ECG, electrocardiogram;ES, electrical storm;ICD, implantable cardioverter defibrillator;IVF, idiopathic ventricular fibrillation;MRI, magnetic resonance imaging;SD, standard deviation;VF, ventricular fibrillation;VT, ventricular tachycardia
Keywords
Brugada syndrome;Rhythmicity;Ventricular fibrillation;Electrical storm;Sudden death
1. Introduction
Circadian, weekly, and seasonal variations are known to exist with fatal ventricular tachyarrhythmias (VTAs), as well as sudden death in patients with structural heart disease. In such patients, cardiac events peak during the morning hours, on Monday, and during the winter [1]; [2]; [3] ; [4]. In contrast, ventricular fibrillation (VF) in patients with Brugada syndrome (BS), an inherited arrhythmic syndrome generally lacking structural abnormalities, occurs at night [5]. A percentage of the patients with BS may develop electrical storms (ESs) due to VF. Recently, we reported a high prevalence of early repolarization in patients with BS who develop ESs [6]. Subsequently, we analyzed the rhythmic occurrence of ESs in a large sample of patients with BS with a history of ESs. The aim of this study was to identify the characteristic patterns of ESs in patients with BS to understand the pathogenesis underlying ESs in these patients and to ensure proper clinical management of this disorder.
2. Methods
2.1. Study population
We recruited 23 patients with Brugada syndrome and a history of ESs and 21 patients with Brugada who had only single VF events as a comparison group from multiple centers. The latter patients were recruited from 3 institutions during the last 7 years. ESs or VF occurred before or after the implantation of an implantable cardioverter defibrillator (ICD). BS was diagnosed according the published criteria [5].
After admission, patients were placed under intensive care and monitored for episodes of VF. Isoproterenol was administered to control recurrent VF[6]; [7] ; [8]. After informed consent was obtained from the patients and family members, an ICD was implanted before discharge. To control for possible recurrence of VF, antiarrhythmic drugs were prescribed based on the clinical judgment of the attending cardiologists.
2.2. ECG analysis
At the time of the first hospital admission, the ECGs were analyzed for the RR, PR and QT intervals, and the QT interval was corrected by Bazetts formula. When J waves were present, the type (notch or slurring), distribution pattern, and morphology of the ST segment following the J waves were determined [7] ; [8]. The ECGs were read by two cardiologists. When there was disagreement regarding the J waves, they discussed the results together to reach an agreement.
2.3. Data analysis
The ECG and other clinical features were compared between the patients with ESs and those with single episodes of VF. The exact time and day of the onset of the ES were obtained from the medical charts or ICD records, and the rhythmicity of the episodes of VF was analyzed and compared between the two patient groups. The acute and long-term clinical outcomes were also compared between the two groups.
2.4. Definitions
Patients with BS included in this study met the following inclusion criteria [5]: a typical coved type Brugada ECG pattern (≥ 0.2 mV elevation of the J point with a type 1 ST elevation in ≥ 1 right precordial lead) occurring spontaneously or after provocation with a sodium channel blocker [9]; normal cardiac structure and function confirmed by echocardiography, MRI or catheterization; negative serology for inflammatory disease and an absence of electrolyte imbalances.
Coronary spasms were excluded by a provocation test using either acetylcholine or ergonovine maleate when informed consent was obtained [10]. An ES was defined as ≥ 3 separate episodes of VF/24 h. For any early repolarization, J waves were diagnosed by the following criteria: 1) a notch or slur in the terminal portion of the QRS complex and 2) an amplitude of $_amp_$gt; 0.1 mV above the isoelectric line in at least two contiguous leads [11]; [12]; [13] ; [14].
As for the rhythmicity, VF occurring between 0:00 and 6:00 was defined as nocturnal, and VF occurring between 20:00 and 8:00 was defined as nighttime. Spring, summer, autumn and winter were defined as March to May, June to August, September to November, and December to February, respectively.
2.5. Statistical analysis
The continuous data are presented as the mean ± standard deviation (SD), and the categorical variables are expressed as absolute numbers or percentages. The statistical comparisons among the groups were made using a t-test or Mann–Whitney U-test for continuous variables and Pearsons chi-square test for categorical variables. The statistical analyses were performed with SPSS (Statistical Package for the Social Sciences), version 12.0 software (SPSS Inc., Chicago, IL, USA). A two-sided p of $_amp_$lt; 0.05 was considered statistically significant.
Written informed consent was obtained from all patients at each institution before the invasive procedures. This study was approved by the Ethics Committee of Keio University School of Medicine.
3. Results
3.1. Patient characteristics
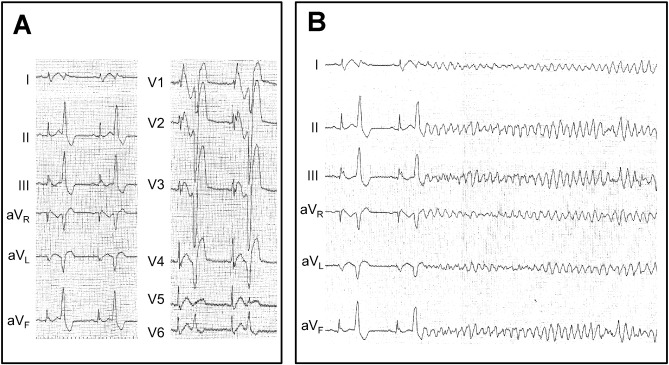
Forty-four patients who met the inclusion criteria were enrolled in this study. ESs developed in 23 patients, and 21 patients experienced single VF events. The mean age was significantly younger in the ES group (38 ± 13 vs. 48 ± 16 years, P = 0.004). Two patients in the ES group and one from the non-ES group had a family history of sudden cardiac death or Brugada syndrome (Table 2 ; Table 3). All episodes of ES and all single VF episodes occurred outside the hospital and were successfully defibrillated by an external defibrillator or ICD. The average number of VF events occurring in the ES patients was 4.8 ± 5.7 (Table 1 ; Table 2, P $_amp_$lt; 0.0001). Fig. 1 shows the onset of VF triggered by frequent premature ventricular complexes (patient no. 14, Table 2).
| Overall (n = 44) | ES (n = 23) | Single VF (n = 21) | P value | |
|---|---|---|---|---|
| Age | 42 ± 16 | 38 ± 13 | 48 ± 16 | 0.004 |
| Male sex, % | 42 (95.3) | 23(100) | 19 (90.5) | 0.080 |
| Family history, % | 5 (11.4) | 2 (8.7) | 3 (14.3) | 0.559 |
| Number of VF | 3.0 ± 4.5 | 4.8 ± 5.7 | 1 ± 0 | 0.004 |
| Spontaneous type 1 | 29 (65.9) | 18 (78.3) | 11(52.5) | 0.069 |
| J wave | 9 (27.3) | 6 (26.1) | 3 (14.3) | 0.328 |
| ECG parameters | ||||
| RR (s) | 0.91 ± 0.2 | 0.86 ± 0.2 | 0.92 ± 0.2 | 0.367 |
| PR (ms) | 161 ± 22 | 161 ± 22 | 160 ± 23 | 0.586 |
| QRS (ms) | 104 ± 14 | 104 ± 14 | 100 ± 12 | 0.062 |
| QT (ms) | 394 ± 35 | 394 ± 35 | 396 ± 39 | 0.309 |
| QTc (ms1/2) | 410 ± 26 | 410 ± 26 | 410 ± 29 | 0.939 |
| CRBBB, % | 3 (6.8) | 3 (13.0) | 0 (0) | 0.043 |
| Recurrence | 17 (38.6) | 11 (47.8) | 6 (28.6) | 0.188 |
| Time to recurrence, y | 2.1 ± 3.0 | 1.2 ± 1.6 | 4.3 ± 4.2 | 0.061 |
| Treatment for VF | ||||
| Bepridil, % | 12 (27.3) | 8 (34.8) | 4 (19.0) | 0.060 |
| RFCA, % | 2 (4.5) | 1 (4.3) | 1 (4.8) | 0.948 |
CRBBB: complete right bundle branch block. RFCA: radiofrequency catheter ablation.
| No. | Age | Sex | FH | No. of VF | Month | Week | Time | Status of VF onset | Pharmacological therapy | Recurrence (yrs after ES) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | SD | 3 | April | Saturday | 7:06 | nd | – | Yes (2.3) | Alive |
| 2 | 26 | M | – | 3 | December | Thursday | 21:37 | Awake | ISP/cilostazol | – | Alive |
| 3 | 42 | M | – | 30 | January | Friday | 3:00 | Sleep | – | – | Alive |
| 4 | 25 | M | – | 4 | January | Saturday | 21:20 | Alcohol | – | Yes (0.2) | Died |
| 5 | 21 | M | SD, BS | 3 | August | Wednesday | 2:38 | Sleep | – | Yes (0.5) | Alive |
| 6 | 21 | M | – | 3 | June | Saturday | 3:20 | Alcohol | – | Yes(0.1) | Alive |
| 7 | 36 | M | – | 9 | May | Tuesday | 2:13 | Sleep | – | – | Alive |
| 8 | 33 | M | – | 3 | July | Tuesday | 14:30 | Awake | – | Yes (0.25) | Alive |
| 9 | 53 | M | – | 3 | June | Thursday | 22:38 | nd | – | – | Alive |
| 10 | 38 | M | – | 4 | March | Monday | 1:43 | Sleep | – | – | Alive |
| 11 | 36 | M | – | 4 | December | Saturday | nd | nd | ISP/Bepridil | – | Alive |
| 12 | 61 | M | – | 7 | November | Saturday | 14:45 | nd | Bepridil | Yes (0.3) | Alive |
| 13 | 42 | M | – | 3 | May | Sunday | nd | nd | ISP/Bepridil | Yes (4.8) | Alive |
| 14 | 51 | M | – | 4 | February | Saturday | nd | nd | ISP/Bepridil | Yes (nd) | Alive |
| 15 | 39 | M | – | 3 | March | Tuesday | 4:27 | Sleep | ISP/Bepridil | Yes (0.7) | Alive |
| 16 | 30 | M | – | 3 | December | Thursday | 3:30 | Sleep | Quinidine | Yes (3.3) | Alive |
| 17 | 27 | M | – | 3 | July | Tuesday | 11:30 | nd | Disopyramide | Yes (0.1) | Alive |
| 18 | 70 | M | – | 3 | October | Sunday | 0:22 | Sleep | ISP/Denopamine | – | Alive |
| 19 | 29 | M | – | 3 | May | Sunday | 2:47 | Sleep | Quinidine | – | Alive |
| 20 | 42 | M | – | 3 | February | nd | 22:18 | nd | Bepridil | – | Alive |
| 21 | 38 | M | – | 3 | nd | nd | 3:22 | Sleep | Amiodarone | – | Alive |
| 22 | 50 | M | – | 3 | nd | nd | 16:28 | nd | Bepridil | – | Alive |
| 23 | 19 | M | – | 3 | April | nd | 21:18 | nd | Bepridil | – | Alive |
BS: Brugada syndrome, ISP: isoproterenol, M: male, nd: not determined. SD: sudden death, yrs: years.
|
|
|
Fig. 1. Frequent premature ventricular complexes and the onset of ventricular fibrillation. A: Twelve-lead ECG recorded prior to the onset of VF. Frequent PVCs originated from the right ventricular outflow tract region. B: VF follows a short–long–short sequence of premature ventricular complexes (patient no. 11). Both a coved type ST elevation in the right precordial leads, and J waves in the inferior leads are present. |
A spontaneous type 1 pattern was observed in 78.3% of patients in the ES group and in 52.4% of patients in the non-ES group (P = 0.069). The remaining patients underwent a provocation test for BS using pilsicainide, a class Ic drug, and the results were uniformly positive. The prevalence of J waves, the ST morphology following the J waves and their distribution patterns did not differ. There was no difference in the other ECG parameters, including the RR, PR, QRS, QT, or QTc intervals between the patients with ESs and those with single VF events, but complete right bundle branch block (CRBBB) was observed more frequently in the ES group (P = 0.043, Table 1). None of the patients developed any chest pain suggestive of myocardial ischemia prior to the onset of VF or an ES. There was no evidence of significant stenosis or coronary spasms in the patients who underwent a provocation test: 10 in the ES group and 20 in the non-ES group. Inflammatory disease and electrolyte imbalances were also ruled out by serological tests and blood chemistry.
3.2. Circadian pattern of the VF occurrence
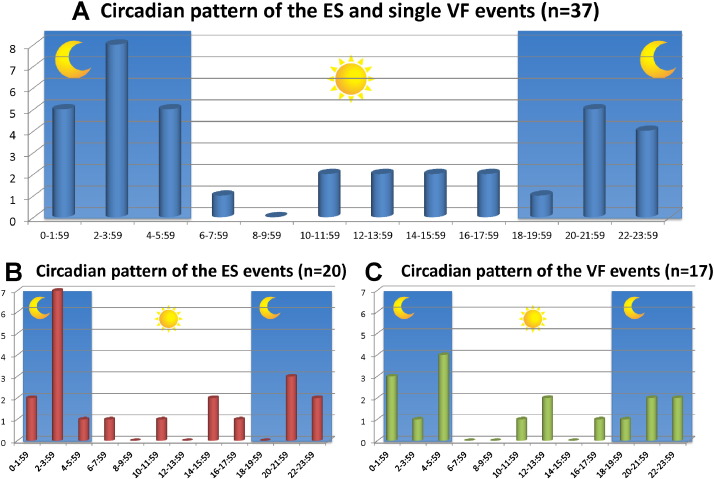
Fig. 2 shows the circadian distributions of the timing of VF events in 37 patients. Similar percentages of ESs and VF events occurred between 0:00 and 6:00 (nocturnal) in 10/20 (50.0%) vs. 8/17 (47.1%), and between 20:00 and 8:00 (nighttime) in 15/20 (75.0%) vs. 13/17 (76.5%), respectively (Fig. 2A and B). None of the patients who experienced these episodes were nighttime workers. Except for two patients who experienced an ES while drinking alcohol, the arrhythmic events occurred while the patients were at home and in a resting state or during sleep (Table 2).
|
|
|
Fig. 2. Circadian pattern of an ES and a single VF episode (n = 37). The overall circadian distribution shows a predominant occurrence of VF during the nocturnal period (A). There was no difference in the pattern of episodes of VF between the patients with an ES (B) and those with a single VF episode (C). |
3.3. Weekly pattern of VF
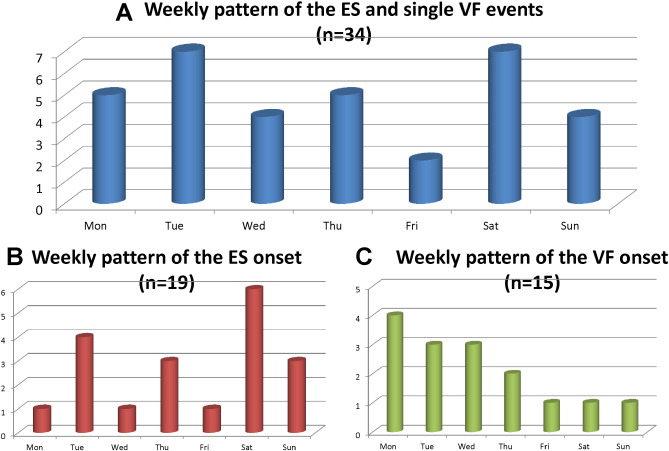
Fig. 3A shows the weekly distribution of the timing of the VF events in 34 patients. Overall, there were 2 peaks at the beginning of the week and on the weekend: Tuesday and Saturday. ESs peaked on Saturday (P $_amp_$lt; 0.001 vs. other weekdays), while single VF episodes peaked on Monday (P = 0.097 vs. other week days) (Fig. 3B, C). Episodes of VF occurred more frequently during the weekend (Saturday and Sunday) in the ES group compared with the single VF group: 47.4% vs. 12.3%, respectively (P = 0.035).
|
|
|
Fig. 3. Weekly pattern of episodes of VF (n = 34). Overall, there were 2 peaks on Tuesday and Saturday (A). ESs peaked on Saturday (P $_amp_$lt; 0.001 vs. other weekdays), while single VF episodes peaked on Monday (P = 0.097 vs. other weekdays) (B, C). VF occurred more often in the ES group during the weekend (Saturday and Sunday) compared to the single VF group: 47.4% vs. 12.3%, respectively (P = 0.035). |
3.4. Seasonal pattern of VF occurrences
Overall, the monthly distribution patterns in 36 patients showed 3 peaks in January, June and December (Fig. 4A). ESs peaked in May and December. The incidence of ESs was higher during the winter and spring compared with other seasons: 66.7% vs. 33.3% (P = 0.012, Fig. 4B). However, such seasonal differences were not observed in single VF episodes: 46.7% vs. 53.3% (P = 0.835, Fig. 4C).
|
|
|
Fig. 4. Seasonal pattern of ES and single VF events (n = 36). Overall, the monthly distribution patterns in 36 patients showed 3 peaks in January, June and December (A). ESs peaked in May and December, and incidence of ESs was higher during the winter and spring compared with other seasons: 66.7% vs. 33.3% (P = 0.012, B). However, such seasonal differences were not observed in single VF: 46.7% vs. 53.3% (P = 0.835, C). |
3.5. Management of VF and the outcomes
ESs were controlled mainly by intravenous administration of isoproterenol (Table 2). One patient in the ES group underwent catheter ablation of VF-triggering premature ventricular beats originating from the right ventricular outflow tract (Table 2, patient no. 2).
All 44 patients were implanted with an ICD and were discharged alive. For chronic therapy, the following antiarrhythmic drugs were prescribed to a limited number of patients based on the clinical judgment of their attending physician: bepridil (n = 8), quinidine (n = 3), denopamine (n = 2), disopyramide (n = 1), cilostazol (n = 1), or amiodarone (n = 1) in the ES patient group, and bepridil (n = 4), quinidine (n = 2) or mexiletine (n = 1) in the non-ES patient group (Table 2 ; Table 3). Bepridil was used most frequently for its Ito blocking effects.
| No. | Age | Sex | FH | No. of VF | Month | Week | Time | Status of VF onset | Pharmacological therapy | Recurrence (yrs after ES) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | F | – | 1 | October | Tuesday | 10:00 | Running? | – | – | Alive |
| 2 | 51 | M | – | 1 | January | Tuesday | 22:00 | Awake | – | – | Alive |
| 3 | 38 | M | – | 1 | July | Wednesday | 13:00 | Awake | – | – | Alive |
| 4 | 67 | M | – | 1 | June | Monday | 19:20 | Awake | – | – | Died |
| 5 | 45 | M | – | 1 | January | Monday | 0.00 | nd | Quinidine | Yes (0.2) | Alive |
| 6 | 45 | M | – | 1 | December | Monday | 4:00 | Awake | – | – | Alive |
| 7 | 47 | M | – | 1 | October | Sunday | 1:31 | nd | – | Yes (4.1) | Alive |
| 8 | 52 | M | – | 1 | January | Tuesday | 5:00 | Sleep | – | Yes (8.1) | Alive |
| 9 | 63 | M | – | 1 | nd | nd | 4:11 | Sleep | Bepridil/Mexiletine | – | Alive |
| 10 | 25 | M | – | 1 | nd | nd | 5:17 | Sleep | Quinidinea | – | Alive |
| 11 | 69 | M | – | 1 | nd | nd | 16:00 | Awake | Bepridil | – | Alive |
| 12 | 60 | M | – | 1 | nd | nd | 2:33 | nd | – | – | Alive |
| 13 | 34 | M | – | 1 | nd | nd | 22:00 | Awake | Bepridil | – | Alive |
| 14 | 37 | M | – | 1 | October | Friday | nd | Awake | Bepridil | – | Alive |
| 15 | 55 | M | SCD | 1 | July | Wednesday | 1:20 | nd | – | – | Alive |
| 16 | 44 | M | – | 1 | June | Wednesday | nd | Awake | – | – | Alive |
| 17 | 78 | F | – | 1 | June | Monday | nd | Awake | – | Yes (8.0) | Alive |
| 18 | 35 | M | – | 1 | May | Thursday | 13:40 | Awake | – | Yes (0.1) | Alive |
| 19 | 42 | M | – | 1 | March | Saturday | 20:40 | Awake | – | - | Alive |
| 20 | M | – | 1 | nd | nd | nd | nd | – | Yes (nd) | Alive | |
| 21 | 56 | M | – | 1 | December | Thursday | 21:00 | Awake | – | – | Alive |
a. Terminated because of skin rash. nd: not determined.
During the follow-up period of 6.2 ± 4.9 years, VF recurred in 11 patients (47.8%) in the ES group (Table 2) and 6 patients (28.6%) in the single VF group. The long-term recurrence rate was higher in the ES group, although not significantly (P = 0.188). Among 14 ES patients and 6 non-ES patients on antiarrhythmic drugs, VF recurred in 6/14 (42.9%) and 1/6 (16.7%), respectively, whereas among 9 ES and 15 non-ES patients on no antiarrhythmic drugs, VF recurred in 5 (55.6%) and 5 (33.3%), respectively. These differences were not significant. One patient who underwent catheter ablation was free from recurrent VF. The time to recurrence of VF was shorter for patients with ES compared with those with single VF, although this was not significant (1.2 ± 1.6 vs. 4.3 ± 4.2 years, P = 0.061). The time to recurrence of VF was not different between the patients with and without antiarrhythmic drugs (1.6 ± 1.0 vs. 2.7 ± 3.6 years, P = 0.480). One patient in each group died from refractory recurrent ESs followed by infection and heart failure (Table 2 ; Table 3).
4. Discussion
In patients with BS who experienced ≥ 1 VF episode, the patients with ESs were younger than those with single VF events, but there was no difference in other clinical features. Both ESs and single VF events occurred similarly during the night: between 0:00 and 06:00 or 20:00 and 08:00. However, episodes of ESs peaked on Saturday and occurred more often during the winter and spring, whereas single VF events peaked on Monday with smaller seasonal variation. Patients with Brugada syndrome and VF admitted over the weekend or in the winter and spring may require intensive care monitoring for possible recurrence of arrhythmias in the form of an ES.
4.1. Circadian rhythm of the VF occurrences
Matsuo et al. reported that in BS, VF occurs more frequently at night than at other times of the day and more frequently during sleep than during waking hours [15]. Subsequently, Takigawa et al. reported that VF associated with BS occurs more frequently between midnight and 6:00 am [18]. Consistent with earlier reports, in the present study, both ESs and single VF events in patients with BS occurred predominantly at night or during the nocturnal period (Fig. 2). The circadian rhythm of VF in BS is quite different from the pattern of sudden cardiac death or VTAs in patients with structural heart disease [1].
Mechanistically, vagal stimulation or withdrawal of sympathetic activity is shown to augment the transient outward currents (Ito) and suppress the ICa-L currents, which in turn lead to a shortening of the action potential duration or a loss of the dome of the action potential [19]. These changes in ionic currents are considered to exaggerate coved type ST elevation.
Clinically, it is well known that the ECG pattern is modulated by many factors, including cholinergic or adrenergic activities [20] ; [21]. Furthermore, the ECG exhibits a typical coved type ST elevation at the time that VF occurs. Unmasking of a typical ECG pattern in BS can also be induced during the recovery phase of exercise [22], during glucose-induced insulin secretion [16], and when the stomach is full after a large meal [17]; all conditions associated with increased vagal tone. Both the accentuated ECG pattern and the predominant occurrence of VF during the nocturnal period or at night confirm the close relationship between the increased vagal tone (with or without a withdrawal of sympathetic nerve activity) and the occurrence of VF. Although both ES and single VF episodes occurred similarly during the nocturnal period, patients who experienced ESs were younger. Young people are known to have more dominant vagal activity compared to older individuals, which can be a potential risk for the occurrence of VF in BS.
4.2. Weekly rhythm of the VF occurrences
We also made a first time observation that weekly and seasonal patterns were different between the ES and single VF events. ESs peaked on Saturday, while single VF events peaked on Monday (Fig. 3). These weekly patterns of episodes of VF are difficult to explain and may be related to working habits and social activities that modulate the autonomic tone and the arrhythmogenic substrate in BS. Vagal tone is higher in a relaxed state, which is more likely to be present during the weekend. Two patients also developed an ES during heavy alcohol intake on a weekend. The reason why single VF events mostly occurred on Monday requires further explanation (Fig. 3).
4.3. Seasonal rhythm of the VF occurrences
In the analysis of the data of appropriate ICD shock episodes for VF events in patients with BS, Takigawa et al. observed a seasonal peak between March and June [18]. This was in contrast to the report by Kim et al. who analyzed VF events in patients BS who had ICDs [19]. Although the patients with early repolarization syndrome had a seasonal peak from spring to summer, they observed no significant seasonal peak in patients with BS. In the current study, the overall seasonal VF events showed 3 peaks in January, June and December, and more ESs (66.7%) occurred during the winter and spring; however, 33.3% occurred during the rest of seasons (P = 0.012, Fig. 4). The reasons for the discrepant findings among the reports are not readily apparent, although there are probably several contributing factors, such as a difference in the patient populations, type of VF occurrence (ES vs. single VF episode), lifestyles, social activities and different climates. A further study that includes a larger number of patients is needed.
During cold winters, the plasma catecholamine levels are higher, which might trigger an acute myocardial infarction and sudden death in the general population [1]; [2]; [3] ; [4]; however, how this might be related to the occurrence of VF in BS is unknown. Febrile illnesses are also more common during cold seasons and can precipitate an episode of VF in BS [23]. None of the patients of the present study developed VF in association with a febrile illness. The difference in the seasonal patterns of episodes of VF between the patients with ESs and those with single VF episodes is another dilemma to be solved.
4.4. Future direction
Clinical studies with a larger number of patients are required to uncover the underlying mechanism driving the rhythmic pattern of episodes of VF in patients with BS. The existence of a master clock known as the suprachiasmatic nucleus with associated circadian genes may provide some insight into this mechanism (Per, Cry, Bmal1 and Clock). Evidence suggests that the circadian transcription of ion channels may contribute to the development of cardiac arrhythmias. For example, Klf15 (krüppel-like factor 15) controls the rhythmic expression of KChIP2 [20], and Bmal1 (Brain and Muscle Arnt-like 1) regulates SCN5A [21]. Future studies should investigate the circadian expressions of various ion channels in humans in general as well as in patients with BS.
4.5. Limitations
Although the present study included the largest number of ES episodes in patients with BS and studied the circadian patterns of VF events, further confirmation is still needed in a larger number of patients.
Except for age, there was no difference in the clinical and ECG features between the patients with BS and ESs and those with single VF episodes. We were unable to confirm specific factors involved in the development of ESs. Furthermore, the role of vagal tone in the specific weekday or season in producing a rhythmicity of VF was not evident. Although ECG findings can be variable, they were not analyzed in a serial manner. Several responsible genes have been reported in BS, and genetic studies may help deepen our understanding of the rhythmicity of ES and VF events in BS.
5. Conclusions
Both ES and single VF events occurred during the nocturnal period of the day in BS. However, ESs occurred most frequently on Saturday and in the winter and spring, while single VF events occurred more frequently on Monday with smaller seasonal variations. The pathogenesis and precipitating factors responsible for the differing rhythmicity of episodes of VF between ESs and single VF events must be determined to understand the pathogenesis of BS.
Funding sources
This work was supported by the Research Fund of Tachikawa Medical Center (Yoshifusa Aizawa), MEXT KAKENHI Grant Number 26461087 (Yoshiyasu Aizawa) and Grants from the Ministry of Health, Labor and Welfare of Japan for Clinical Research on Intractable Diseases (H26-040, to Keiichi Fukuda).
Conflict of interest for all authors
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] M.H. Ruwald, A.J. Moss, W. Zareba, C. Jons, A.C. Ruwald, S. McNitt, et al.; Circadian distribution of ventricular tachyarrhythmias and association with mortality in the MADIT-CRT trial; J. Cardiovasc. Electrophysiol., 26 (2015), pp. 291–299
- [2] E. Gonzalez Hernandez, A. Cabades O'Callaghan, J. Cebrian Domenech, V. Lopez Merino, R. Sanjuan Manez, I. Echanove Errazti, et al.; Seasonal variations in admissions for acute myocardial infarction. The PRIMVAC study; Rev. Esp. Cardiol., 57 (2004), pp. 12–19
- [3] M.C. Cohen, K.M. Rohtla, C.E. Lavery, J.E. Muller, M.A. Mittleman; Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death; Am. J. Cardiol., 79 (1997), pp. 1512–1516
- [4] F.A. Spencer, R.J. Goldberg, R.C. Becker, J.M. Gore; Seasonal distribution of acute myocardial infarction in the second National Registry of myocardial infarction; J. Am. Coll. Cardiol., 31 (1998), pp. 1226–1233
- [5] C. Antzelevitch, P. Brugada, M. Borggrefe, J. Brugada, R. Brugada, D. Corrado, et al.; Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association; Circulation, 111 (2005), pp. 659–670
- [6] Y. Kaneko, M. Horie, S. Niwano, K.F. Kusano, S. Takatsuki, T. Kurita, et al.; Electrical storm in patients with Brugada syndrome is associated with early repolarization; Circ. Arrhythm. Electrophysiol., 7 (2014), pp. 1122–1128
- [7] R. Rosso, E. Glikson, B. Belhassen, A. Katz, A. Halkin, A. Steinvil, et al.; Distinguishing “benign” from “malignant early repolarization”: the value of the ST-segment morphology; Heart Rhythm., 9 (2012), pp. 225–229
- [8] J.T. Tikkanen, M.J. Junttila, O. Anttonen, A.L. Aro, S. Luttinen, T. Kerola, et al.; Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome; Circulation, 123 (2011), pp. 2666–2673
- [9] A. Fujiki, M. Usui, H. Nagasawa, K. Mizumaki, H. Hayashi, H. Inoue; ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insight into the mechanism of Brugada syndrome; J. Cardiovasc. Electrophysiol., 10 (1999), pp. 214–218
- [10] Y. Igarashi, Y. Tamura, K. Suzuki, Y. Tanabe, T. Yamaguchi, T. Fujita, et al.; Coronary artery spasm is a major cause of sudden cardiac arrest in survivors without underlying heart disease; Coron. Artery Dis., 4 (1993), pp. 177–185
- [11] M. Haissaguerre, N. Derval, F. Sacher, L. Jesel, I. Deisenhofer, L. de Roy, et al.; Sudden cardiac arrest associated with early repolarization; N. Engl. J. Med., 358 (2008), pp. 2016–2023
- [12] Y. Aizawa, M. Chinushi, K. Hasegawa, N. Naiki, M. Horie, Y. Kaneko, et al.; Electrical storm in idiopathic ventricular fibrillation is associated with early repolarization; J. Am. Coll. Cardiol., 62 (2013), pp. 1015–1019
- [13] Y. Aizawa, A. Sato, H. Watanabe, M. Chinushi, H. Furushima, M. Horie, et al.; Dynamicity of the J-wave in idiopathic ventricular fibrillation with a special reference to pause-dependent augmentation of the J-wave; J. Am. Coll. Cardiol., 59 (2012), pp. 1948–1953
- [14] M. Haissaguerre, F. Sacher, A. Nogami, N. Komiya, A. Bernard, V. Probst, et al.; Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy; J. Am. Coll. Cardiol., 53 (2009), pp. 612–619
- [15] K. Matsuo, T. Kurita, M. Inagaki, M. Kakishita, N. Aihara, W. Shimizu, et al.; The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome; Eur. Heart J., 20 (1999), pp. 465–470
- [16] M. Nishizaki, H. Sakurada, T. Ashikaga, N. Yamawake, H. Fujii, M. Arita, et al.; Effects of glucose-induced insulin secretion on ST segment elevation in the Brugada syndrome; J. Cardiovasc. Electrophysiol., 14 (2003), pp. 243–249
- [17] T. Ikeda, A. Abe, S. Yusu, K. Nakamura, H. Ishiguro, H. Mera, et al.; The full stomach test as a novel diagnostic technique for identifying patients at risk of Brugada syndrome; J. Cardiovasc. Electrophysiol., 17 (2006), pp. 602–607
- [18] M. Takigawa, T. Noda, W. Shimizu, K. Miyamoto, H. Okamura, K. Satomi, et al.; Seasonal and circadian distributions of ventricular fibrillation in patients with Brugada syndrome; Heart Rhythm., 5 (2008), pp. 1523–1527
- [19] S.H. Kim, G.B. Nam, S. Baek, H.O. Choi, K.H. Kim, K.J. Choi, et al.; Circadian and seasonal variations of ventricular tachyarrhythmias in patients with early repolarization syndrome and Brugada syndrome: analysis of patients with implantable cardioverter defibrillator; J. Cardiovasc. Electrophysiol., 23 (2012), pp. 757–763
- [20] D. Jeyaraj, S.M. Haldar, X. Wan, M.D. McCauley, J.A. Ripperger, K. Hu, et al.; Circadian rhythms govern cardiac repolarization and arrhythmogenesis; Nature, 483 (2012), pp. 96–99
- [21] E.A. Schroder, M. Lefta, X. Zhang, D.C. Bartos, H.Z. Feng, Y. Zhao, et al.; The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility; Am. J. Physiol. Cell Physiol., 304 (2013), pp. C954–C965
- [22] H. Makimoto, E. Nakagawa, H. Takaki, Y. Yamada, H. Okamura, T. Noda, K. Satomi, K. Suyama, N. Aihara, T. Kurita, S. Kamakura, W. Shimizu; Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome; J. Am. Coll. Cardiol., 56 (2010), pp. 1576–1584
- [23] R. Dumaine, J.A. Towbin, P. Brugada; Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent; Circ. Res., 85 (1999), pp. 803–809
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?