(Created page with "==Summary== Gallbladder sarcomatoid carcinoma is a rare neoplasm characterized by the simultaneous presence of malignant epithelial and mesenchymal components. There is littl...") |
m (Scipediacontent moved page Draft Content 641771009 to Wong et al. 2015a) |
(No difference)
| |
Latest revision as of 11:28, 31 August 2016
Summary
Gallbladder sarcomatoid carcinoma is a rare neoplasm characterized by the simultaneous presence of malignant epithelial and mesenchymal components. There is little information about the clinical behavior and optimal treatment for these tumors. The current study examines the case of a 52-year-old female patient who presented with postprandial epigastric pain that had persisted for 1 year. A diagnosis of gallbladder sarcomatoid carcinoma was confirmed by pathology after radical resection. Peritoneal metastasis occurred 3 months later, and the patient died 6 months after diagnosis. This study found that the prognosis of gallbladder sarcomatoid carcinoma is poor even after curative resection and there is as yet no known effective adjuvant therapy.
Keywords
Carcinoma; Gallbladder; Outcome; Sarcomatoid carcinoma; Therapy
Introduction
Adenocarcinoma is the most common type of malignant tumor generated in the gallbladder, whereas sarcomatoid carcinoma (also called carcinosarcoma) is rare [1]. The diagnosis of sarcomatoid carcinoma is generally made pathologically and requires the simultaneous presence of both malignant epithelial and mesenchymal components. Immunohistochemically, the epithelial component is positive for cytokeratin and the mesenchymal component is positive for vimentin [2]. In a meta-analysis of sarcomatoid carcinoma, adenocarcinoma was found to be the most common pathology in the epithelial component, followed by mixed adenosquamous cell carcinoma and squamous cell carcinoma. In the mesenchymal component, spindle cell pathology occurred most frequently followed by combined spindle cell and chondroid features, and osteoid histology [3].
Sarcomatoid carcinoma of the gallbladder was first reported in 1907 by Landsteiner [4]. Since then, less than 100 further cases have been reported in the English literature [1]. Like gallbladder adenocarcinoma, gallbladder sarcomatoid carcinoma is most common in women. The female/male ratio is between 3.25:1 and 5:1 [1] and [3]. The age at diagnosis ranges from 45 to 91 years old [3]. Sarcomatoid carcinoma is rare at sites such as the lungs, kidneys, gastrointestinal tract and gallbladder, and occurs more commonly in the uterus where it is known as a malignant mixed Müllerian tumor [5].
Case report
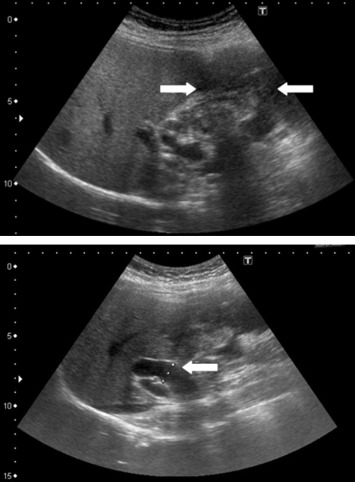
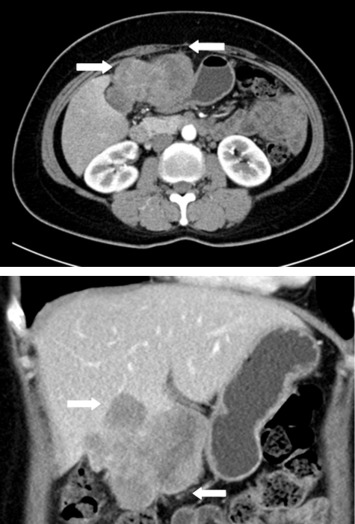
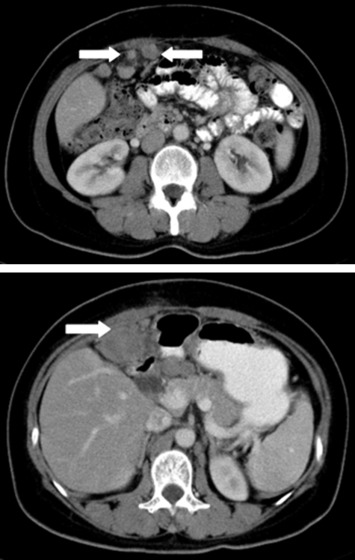
A 52-year-old female patient presented with postprandial epigastric pain that had persisted for 1 year. The pain was mild and improved after antacid treatment. She was seen at our hospital after being diagnosed with a hepatic tumor at a local clinic. Abdominal ultrasonography revealed one mixed echogenic tumor approximately 9.5 cm from the gallbladder sac and extending to S4–5 of the liver. The common bile duct was dilated to 1.4 cm (Fig. 1). Liver function tests and total bilirubin levels were within the normal range. However, carbohydrate antigen 19-9 (CA 19-9) was elevated to 60.9 U/mL. A follow-up abdominal computed tomography (CT) confirmed a 7.5 cm sized gallbladder cancer with liver invasion (Fig. 2), consistent with clinical Stage III cancer according to the TNM system (tumor-node-metastasis classification system).
|
|
|
Figure 1. Abdominal ultrasonography showing one mixed echogenic tumor approximately 9.5 cm from the head of the gallbladder sac. The common bile duct was dilated to 1.4 cm. |
|
|
|
Figure 2. Abdominal computed tomography confirmed a 7.5 cm gallbladder cancer with liver invasion. |
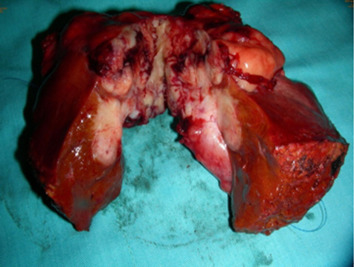
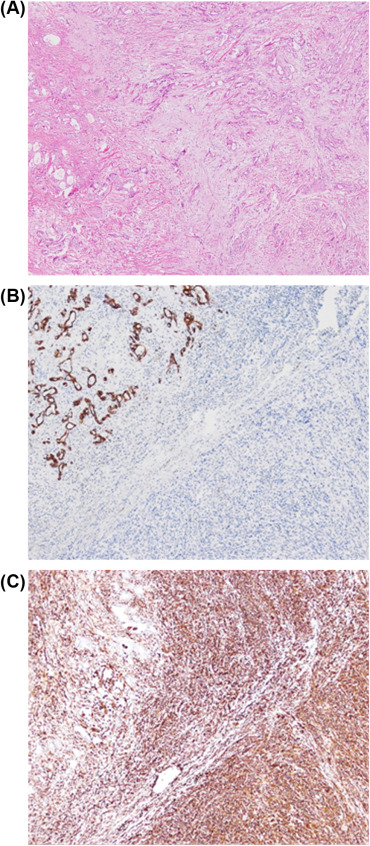
Cholecystectomy and segmental hepatectomy of segments S4b and S5 were performed. An 8 cm, friable gallbladder mass had grown outward and involved the liver (Fig. 3). Pathologically, the tumor was a sarcomatoid carcinoma in Stage III (Fig. 4). The surgical margin was free of vessel or cystic duct invasion. The tumor had an adenocarcinoma component with some glandular structures and showed positive staining for cytokeratin (AE1/AE3) and a sarcoma component with spindle cells staining positively for vimentin.
|
|
|
Figure 3. An 8 cm friable mass extending outward from the gallbladder and involving the liver. |
|
|
|
Figure 4. (A) Sarcomatoid carcinoma of the gallbladder. Immunohistochemical staining showing (B) cytokeratin-positive epithelial component; (C) vimentin-positive sarcomatous component. |
The postoperative CA 19-9 level decreased to 28.5 U/mL. Epigastric pain improved and the patient did not report any other discomfort. However, 3 months later, the follow-up abdominal CT revealed a peritoneal, poorly enhancing soft tissue mass over the anterior of the liver and variably sized nodules on the omentum (Fig. 5). A CT-guided biopsy confirmed metastatic peritoneal sarcomatoid carcinoma. The patient received chemotherapy consisting of three courses of cisplatin (30 mg/m2) and fluorouracil (2600 mg/m2). The patient died 6 months after diagnosis.
|
|
|
Figure 5. A metastatic peritoneal sarcomatoid carcinoma identified by abdominal computed tomography. |
Discussion
We reported the case of a 52-year-old female patient who presented with postprandial epigastric pain that had persisted for 1 year. Peritoneal metastasis occurred 3 months later, and the patient died 6 months after diagnosis. The prognosis of gallbladder sarcomatoid carcinoma is poor even after curative resection and there is as yet no known effective adjuvant therapy.
The risk factors for gallbladder sarcomatoid carcinoma are not clear. Risk factors for gallbladder carcinoma include adenomyomatosis, anomalous union of the pancreaticobiliary ductal system, cholelithiasis, chronic Salmonella typhi infection, a first-degree relative with gallbladder cancer, and exposure to carcinogens such as methylcholanthrene, o-aminoazotoluene and nitrosamines. Our patient did not have any of these risk factors. Patients with gallbladder sarcomatoid carcinoma often present with nonspecific symptoms and signs such as abdominal pain, nausea, anorexia, fever, jaundice, weight loss, or a palpable mass [1] and [6]. Varying CA 19-9 levels were noted among previous gallbladder sarcomatoid carcinoma cases [1] and [7]. Our patient was diagnosed at a late stage when she presented with abdominal pain and had an elevated CA 19-9 level that decreased after radical resection.
Because gallbladder sarcomatoid carcinoma is poorly understood, a definite staging system is not available. Most case series or reports are staged by the TNM classification of gallbladder carcinoma. The most important treatment is surgical resection. Our patient underwent a radical resection; adjuvant chemotherapy or radiotherapy were not used due to a lack of evidence supporting a therapeutic benefit [6] and [8]. The use of fluorouracil plus oxaliplatin for adjuvant therapy was previously documented in another case report [7]. In that case, the patient was diagnosed with Stage II disease and was still alive 6 months postoperatively.
Our patient had a poorly enhanced, peritoneal soft tissue mass over the anterior of the liver with differently sized nodules on the omentum 3 months after surgery. We used a regimen of fluorouracil plus cisplatin, similar to that used previously, for palliative chemotherapy. Fluorouracil is typically used for gallbladder carcinoma and oxaliplatin for sarcoma. The overall median survival for sarcomatoid carcinoma ranged from 2 months to 9 months [1], [3] and [9]. In one meta-analysis, it was found that patients with a tumor > 5 cm have a significantly worse outcome as determined by Kaplan-Meier survival analysis. The median survival was 11 months in patients with tumors < 5 cm but only 5 months in patients with tumors > 5 cm. This observation may be explained by the presence of a greater extent of local invasion with larger tumors due to the thinness of the gallbladder wall. Age, sex, epithelial and mesenchymal component types, and the presence of a gallstone did not significantly influence prognosis [3]. Prognosis was poor even with surgical resection with a median survival after surgery of 7 months. For patients with advanced Stage III or IV disease, the median survival after surgery was reduced to 4 months. Furthermore, the median time to recurrence was < 1 year and the median survival time was only 1.5 months after recurrence [6].
Our patient underwent radical resection of a Stage III, 8 cm gallbladder sarcomatoid carcinoma. The postoperative performance status was good. However, recurrence without any symptoms was noted after 3 months and the patient received 3 courses of chemotherapy (fluorouracil plus cisplatin). The gallbladder cancer may have spread directly or via lymphatic, vascular, neural, intraperitoneal, and intraductal pathways. Typical recurrence patterns include locoregional and distant spread [10]. Locoregional recurrence at sites such as the porta hepatis of the resection margin or the retroperitoneum is due to microscopic residual disease or lymphatic spread. However, distant or intrahepatic metastasis is due to hematogenous spread. Our patient had early peritoneal recurrence indicative of distant metastasis and it is likely that microscopic spread had already occurred. Because the tumor was > 5 cm (8 cm) and the disease was in late stage (Stage III) we performed a radical resection with a free surgical margin.
In conclusion, surgical resection is the only proven curative treatment for gallbladder sarcomatoid carcinoma and early diagnosis is the key to a better prognosis. We strongly suggest early imaging follow-up after radical resection for patients with advanced gallbladder sarcomatoid carcinomas > 5 cm and/or Stage III or IV disease which may be due to undetectable small metastases. Further evidence of survival benefit for pathology-based, combined adjuvant chemotherapy is needed as no adjuvant therapies have proven effective thus far.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] K.H. Liu; Surgical management of gallbladder sarcomatoid carcinoma; World J Gastroenterol, 15 (2009), pp. 1876–1879
- [2] H.H. Kim, Y.H. Hur, E.H. Jeong, E.K. Park, Y.S. Koh, J.C. Kim, et al.; Carcinosarcoma of the gallbladder: report of two cases; Surg Today, 42 (2012), pp. 670–675
- [3] L. Zhang, Z. Chen, M. Fukuma, L.Y. Lee, M. Wu; Prognostic significance of race and tumor size in carcinosarcoma of gallbladder: a meta-analysis of 68 cases; Int J Clin Exp Pathol, 1 (2008), pp. 75–83
- [4] K. Landsteiner; Plattenepithelkarzinom und sarkom der gallenblase in cinem falle von cholelithiasis; Zischr Klin Med, 62 (1907), pp. 427–433
- [5] L.E. Kernochan, R.L. Garcia; Carcinosarcomas (malignant mixed mullerian tumor) of the uterus: advances in elucidation of biologic and clinical characteristics; J Natl Compr Canc Netw, 5 (2009), pp. 550–556
- [6] T. Okabayashi; Surgical outcome of carcinosarcoma of the gall bladder: a review; World J Gastroenterol, 15 (2009), pp. 4877–4882
- [7] J.J. Pu, W. Wu; Gallbladder carcinosarcoma; BMJ Case Rep (2011) http://dx.doi.org/10.1136/bcr.05.2010.3009
- [8] T. Ajiki, T. Nakamura, Y. Fujino, Y. Suzuki, Y. Takeyama, Y. Ku, et al.; Carcinosarcoma of the gallbladder with chondroid differentiation; J Gastroenterol, 37 (2002), pp. 966–971
- [9] Z.H. Hu, Z.W. Li, L. Shen, M. Zhang, S.S. Zheng; Surgical therapy and prognosis of sarcomatoid carcinoma of the gallbladder; Hepatobiliary Pancreat Dis Int, 9 (2010), pp. 175–179
- [10] W.R. Jarnagin, L. Ruo, S.A. Little, D. Klimstra, M. D'Angelica, R.P. DeMatteo, et al.; Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies; Cancer, 98 (2003), pp. 1689–1700
Document information
Published on 31/08/16
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?