Abstract
The submission rates of feline uroliths to laboratories and the composition of uroliths have been reported in studies. The prevalence of uroliths reported on imaging findings has not been published. The objective of this retrospective study was to use imaging data to investigate the anatomical location and the prevalence of macroscopic in situ uroliths in cats. Radiographs, sonograms and imaging reports from two cohorts of cats (from New Zealand (n = 497) and the United States (n = 693)) from 2004-2013 were reviewed for the presence of in situ uroliths. Uroliths were categorized by their location in the lower or upper urinary tract. Radiographic studies were performed on 43% (212/497) of the cats from New Zealand and 50% (349/693) of the cats from the USA. Sonographic studies were performed on 57% (285/497) of the cats from New Zealand and 50% (344/693) of the cats from the USA. The total prevalence of uroliths was 3% in the New Zealand cohort and 13% in the USA cohort. Lower tract urolith prevalence in the New Zealand cohort was 2.4% (5/212) in cats ≤ 6y and 1.1% (3/285) in cats >6y. Upper tract urolith prevalence in the New Zealand cohort was 0.5% (1/212) in cats ≤ 6y and 1.8% (5/285) in cats >6y. Lower tract urolith prevalence in the United States cohort was 6.0% (11/183) in cats ≤ 6y and 2.9% (15/510) in cats >6y. Upper tract urolith prevalence in the United States cohort was 2.7% (5/183) in cats ≤ 6y and 10.2% (52/510) in cats >6y. The prevalence of uroliths in the upper tract or lower tract was low in the New Zealand cohort compared to that of cats in the USA cohort, irrespective of age category. Geographical location may be important when evaluating risk factors for feline urolithiasis.
Introduction
A urolith or calculus is a macroscopic concretion of mineral salts located within the urinary tract. Urolithiasis refers to the causes and effects of uroliths located at any site in the urinary tract (Osborne et al. 2009a). ‘Upper tract’ uroliths include nephroliths and ureteroliths, whereas ‘lower tract’ uroliths include cystoliths and urethroliths. It is important to note that uroliths are distinct from the more commonly diagnosed urethral plugs consisting of variable amounts of mineral in proportion to large quantities of matrix (Osborne et al. 1992, 2009a; Lund et al. 2013).
During the last 30 years, studies from many countries, mostly from diagnostic laboratories, have reported submission rates of feline uroliths, their composition and risk factors for their formation (Cannon et al. 2007; Picavet et al. 2007; Houston & Moore 2009; Osborne et al. 2009a).Recent reports draw attention to an increase in feline upper tract urolithiasis (Kyles et al. 2005; Lekcharoensuk et al. 2005; Palm & Westropp 2011; Zaid et al. 2011; Shipov & Segev 2013). In the last 30 years, only two reports on uroliths in cats living in New Zealand have been published; both analysing urolith composition (Jones et al. 1997, 1998). Additional unpublished data show the number of New Zealand cats from which uroliths were submitted for analysis declined recently (Beban 2014). The authors’ experience of feline urolithiasis in New Zealand is that macroscopic lower tract uroliths remain uncommon and upper tract uroliths are rare. This observation is of interest in light of the high rate of feline ownership and high numbers of feline patients in New Zealand receiving regular veterinary care (inc. NZCAC 2011). It is possible that urolithiasis may be an overlooked cause of morbidity in cats in New Zealand due to lack of presentation for care. Alternatively, the prevalence of uroliths in cats in New Zealand may be low. The first possibility is important as occult uroliths, particularly those in the upper tract, can be associated with significant morbidity (Kyles et al. 2005). The second possibility is also important as the predisposition of cats to form uroliths may be lower in New Zealand than in other countries. Both possibilities remain speculative as the prevalence of in situ uroliths has never been investigated in cats in New Zealand.
Previous studies in the USA, have reported the prevalence of urinary tract disorders in hospital populations of cats presented to veterinary teaching hospitals. From these cats with lower urinary signs or renal disorders the occurrence of urolithiasis was reported as a prevalence of feline urolithiasis (Kirk et al. 2001a) and nephrolithiasis (Kirk et al. 2001a) to be 0.3% and 0.01%, respectively. There are no studies to determine the prevalence of urolithiasis in all cats or hospitalized cats presented for imaging studies. Unless the background prevalence of uroliths in imaging studies is known, it will remain unclear as to whether the reported rates of feline urolithiasis reflect changing numbers of cats with uroliths or changing rates of detection, removal and submission of uroliths.
The aim of this retrospective study was to investigate the anatomical location and prevalence of uroliths in situ in two cohorts of hospitalized cats–one cohort from New Zealand, the other from the USA. Both study groups were composed of cats that underwent abdominal radiography or abdominal ultrasound examinations for a variety of reasons. We hypothesized that in cats with diverse clinical signs that led to imaging, the prevalence of uroliths, particularly that of upper tract uroliths, would be lower in a cohort of cats in New Zealand than in a cohort of cats in the USA.
Materials and methods
Cohort formation
A cohort of New Zealand cats was acquired by searching the veterinary hospital records for radiologists’ reports and accompanying images of all cats that underwent abdominal radiography or abdominal ultrasound imaging from 2004 to 2013 inclusive. Veterinary hospital medical records contained the only available source of the radiologists’ reports for cats in New Zealand. For the same period, a cohort of cats from the USA was then acquired by systematic random sampling (Dohoo et al. 2010; Kalton 1983) of imaging studies from a USA teleradiology company. Imaging reports on the teleradiology server were accessed by checking drop-down boxes for ‘Feline’, ‘Date’ and either ‘Radiology’ or ‘Ultrasound’. For each date and imaging modality, multiple screen pages, each with a list of 20 individual cases, were accessible. The third case from the third screen page of each month was selected. If the case met the inclusion criteria, the available images were reviewed, followed by review of the radiologists report. If the case did not meet the inclusion criteria, the next listed case that did was reviewed. The process was repeated for a date in the middle of the month and again 10 days later to distribute cases evenly through the study period.
Inclusion criteria
Cats were required to have a complete signalment and a radiologists report that included findings for both kidneys and the urinary bladder based on orthogonal abdominal radiographs or an abdominal ultrasound examination. Individual cats that were imaged multiple times were included once based on the first imaging method used. The age, sex, breed, presenting clinical signs and imaging modality used for each included cat were recorded.
Review of radiologist reports
The report for each included cat was reviewed for the finding of urolith(s). If a urolith was described, we classified it as either ‘nephrolith’, ‘ureterolith’, ‘cystolith’ or ‘urethrolith’ and classified its location as ‘upper tract’, ‘lower tract’ or ‘both’. On the basis of presenting clinical signs, we categorized cats with uroliths into one of five groups: ‘abdominal’ (e.g. vomiting, diarrhoea, abdominal mass, hepatobiliary disease), ‘pain’ (e.g. pain of unknown origin, abdominal pain, trauma pain), ‘respiratory’ (e.g. dyspnoea, cough), ‘urological’ (e.g. pollakiuria, haematuria, dysuria, or screening for renal disease, pregnancy or uterine pathology) and ‘unwell’ (e.g. anorexia, lethargy, depression, or unspecified presenting signs). The five categories were formed by author consensus (PW, KH, JB, RG) based on consideration of the variety of presenting reasons for imaging. One author (PW) then categorized cases, with equivocal cases categorized by consensus (PW, KH, RG).
Review of images
Two investigators (radiology resident and intern) reviewed all imaging studies accompanying the veterinary hospital reports, and 525 of the imaging studies accompanying the teleradiology reports (imaging studies for 168 reports were no longer available on the teleradiology server). The images were reviewed to determine whether any uroliths were overlooked or misdiagnosed in the original radiologists’ assessments. If the findings in an original report differed from the investigators interpretation, one of two board certified radiologists reviewed the images to either confirm the original diagnosis or re-classify the status of the urolith.
Statistical analysis
All statistical tests were selected and performed by two of the authors. Categorical and binary variables were described as counts and percentages, and the median and interquartile (IQR) range were calculated for non-parametric continuous data. Prevalence was calculated for each cohort and reported as the percentage of cats with a positive test result (number of cats with uroliths divided by the total number of included cats). The median age of the cats from New Zealand and the United States was compared using a Wilcoxon rank sum test. Within each cohort, categorical variables of interest among cats with uroliths were compared using appropriate Pearsons X2 or Fishers exact tests. The variables of interest included cat age and presenting clinical signs, and urolith location. The level for statistical significance was set at P < 0.05 and data were analysed using R v 3.1.0 (R Development CoreTeam, 2014: R Foundation for Statistical Computing, Vienna, Austria).
Results
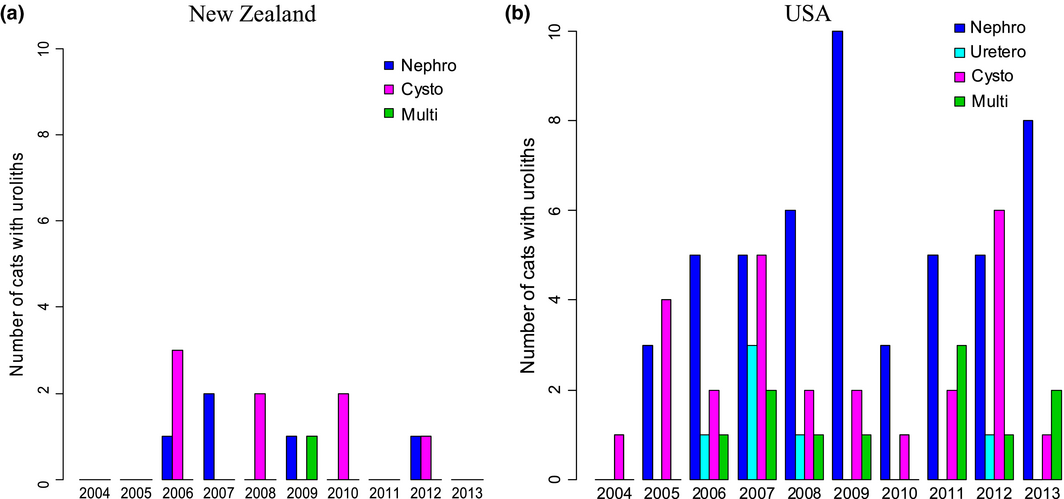
A total of 1190 cats met the inclusion criteria; 497 from New Zealand and 693 from the USA. Radiographic studies were performed on 43% (212/497) of the cats from New Zealand and 50% (349/693) of the cats from the USA. Sonographic studies were performed on 57% (285/497) of the cats from New Zealand and 50% (344/693) cats from the USA. The yearly proportions of radiographic and sonographic examinations performed in each cohort were similar during the study period (Table 1). In each cohort, the proportion of cats diagnosed with uroliths fluctuated through the study period (Fig. 1a, b). Uroliths were detected in radiographs from eight New Zealand cats and 36 USA cats, and in sonograms from six New Zealand cats and 57 USA cats (Table 1). Overall urolith prevalence was 3% (14/497) in the New Zealand cohort and 13% (93/693) in the United States cohort.
| New Zealand cats imaged | |||||
|---|---|---|---|---|---|
| Year | Total imaged per year | Radiographs (% of total per year) | Radiographs with uroliths (% of total radiograph) | Ultrasound (% of total per year) | US with urolith (% of total US) |
| 2004 | 21 | 7 (33.3) | 0 | 14 (66.7) | 0 |
| 2005 | 47 | 19 (40.4) | 0 | 28 (59.6) | 0 |
| 2006 | 47 | 21 (44.7) | 2 (9.5) | 26 (55.3) | 2 (7.7) |
| 2007 | 47 | 19 (40.4) | 2 (10.5) | 28 (59.6) | 0 |
| 2008 | 42 | 11 (26.2) | 0 | 31 (73.8) | 2 (6.5) |
| 2009 | 49 | 13 (26.5) | 0 | 36 (73.5) | 2 (5.5) |
| 2010 | 79 | 34 (43.0) | 2 (5.9) | 45 (57.0) | 0 |
| 2011 | 59 | 25 (42.4) | 0 | 34 (57.6) | 0 |
| 2012 | 56 | 26 (46.4) | 2 (7.7) | 30 (53.6) | 0 |
| 2013 | 50 | 37 (74.0) | 0 | 13 (26.0) | 0 |
| Total | 497 | 212 (43.0) | 8 (3.8) | 285 (57.0) | 6 (2.1) |
| United States cats imaged | |||||
|---|---|---|---|---|---|
| Year | Total imaged per year | Radiographs (% of total per year) | Radiographs with uroliths (% of total radiograph) | Ultrasound (% of total) | US with urolith (% of total US) |
| 2004 | 63 | 31 (49.2) | 0 | 32 (50.8) | 1 (3.1) |
| 2005 | 70 | 35 (50.0) | 2 (5.7) | 35 (50.0) | 5 (14.3) |
| 2006 | 71 | 35 (49.3) | 3 (8.6) | 36 (50.7) | 6 (16.7) |
| 2007 | 69 | 35 (50.7) | 9 (25.7) | 34 (49.3) | 6 (17.6) |
| 2008 | 68 | 33 (48.5) | 2 (6.1) | 35 (51.5) | 8 (22.9) |
| 2009 | 73 | 38 (52.1) | 4 (10.5) | 35 (47.9) | 9 (25.7) |
| 2010 | 71 | 36 (50.7) | 0 | 35 (49.3) | 4 (11.4) |
| 2011 | 70 | 36 (51.4) | 6 (16.7) | 34 (48.6) | 4 (11.8) |
| 2012 | 70 | 35 (50.0) | 5 (14.3) | 35 (50.0) | 8 (22.9) |
| 2013 | 68 | 35 (51.5) | 5 (14.3) | 33 (48.5) | 6 (18.2) |
| Total | 693 | 349 (50.0) | 36 (10.3) | 344 (50.0) | 57 (16.6) |
|
|
|
Figure 1. (a, b) Number of cats with uroliths at different anatomical locations year-by-year from the imaging study investigating in situ uroliths in hospitalized cats in New Zealand and in the United States. |
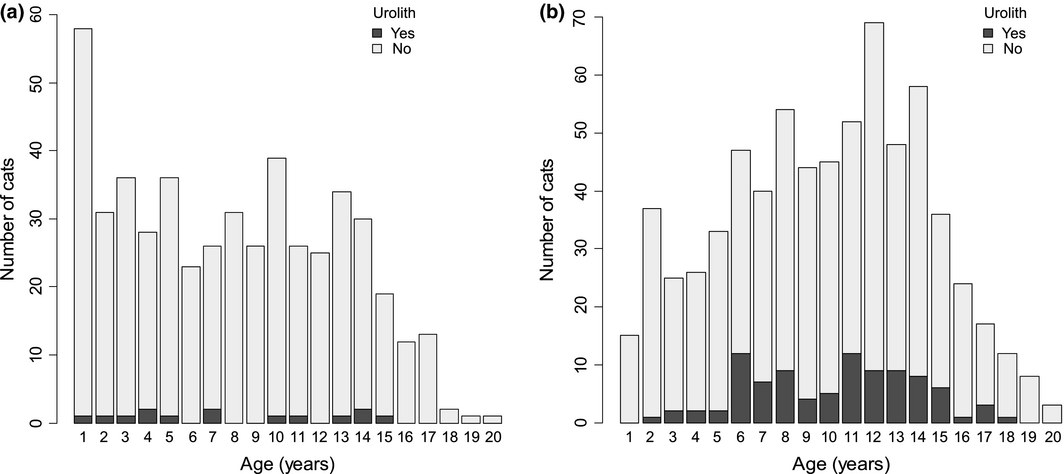
The median age of cats differed significantly between the two cohorts (Wilcoxon Rank Sum test, P < 0.001). The median age of cats was 8 years (range 1–20) in the New Zealand cohort, and 10 years (range 1–20) in the USA cohort. Because age of cat did not follow a normal distribution in both cohorts, age was categorized into two groups based on previously reported feline age categories (Lund et al. 2013; Paepe et al. 2013); ≤6y (‘junior to prime’) and >6y (‘mature to old’). The ≤6y category included 212 New Zealand cats and 183 USA cats. The >6y category included 285 New Zealand cats and 510 USA cats (Fig. 2a, b).
|
|
|
Figure 2. (a) Histogram of the ages of a cohort of cats hospitalized between 2004 and 2013 in New Zealand, plus age distribution of cats with uroliths, from the imaging study investigating in situ uroliths in hospitalized cats in New Zealand and in the United States. (b) Histogram of the ages of a cohort of cats hospitalized between 2004 and 2013 in the United States, plus age distribution of cats with uroliths from the imaging study investigating in situ uroliths in hospitalized cats in New Zealand and in the United States. |
Uroliths were present at a single location in 13/14 cats in the New Zealand cohort and 82/93 cats in the USA cohort. The most common site was the urinary bladder in New Zealand cats (8/13; 62%) and the kidneys in USA cats (50/82; 61%). In the combined study population more cats had uroliths confined to the upper tract than the lower tract (Table 2). Several cats had uroliths at multiple sites, including; a New Zealand cat with a nephrolith and a ureterolith, and 11 USA cats with nephroliths in conjunction with cystoliths (9 cats), ureteroliths (1 cat) and a single urethrolith (1 cat) (Fig. 2a, b). In each country, the number of cats with uroliths, and the anatomical location of the uroliths, varied yearly during the study period.
| New Zealand | United States | |||||||
|---|---|---|---|---|---|---|---|---|
| Presenting group | Cats (N) | Upper tract (N) | Lower tract (N) | Both (N) | Cats (N) | Upper tract (N) | Lower tract (N) | Both (N) |
| Abdominal | 141 | 1 | 1 | 0 | 266 | 21 | 5 | 4 |
| Pain | 63 | 0 | 0 | 0 | 33 | 1 | 2 | 1 |
| Respiratory | 16 | 0 | 0 | 0 | 30 | 2 | 0 | 1 |
| Urological | 60 | 2 | 7 | 0 | 112 | 10 | 16 | 3 |
| Unwell | 217 | 3 | 0 | 0 | 252 | 23 | 3 | 1 |
| Total | 497 | 6 | 8 | 0 | 693 | 57 | 26 | 10 |
| Overall prevalence | NZ 3% (14/497) | United States 13% (93/693) | ||||||
The presenting clinical signs of all cats in the New Zealand and USA cohorts, respectively, were categorized as ‘abdominal’ in 28% (141/497) and 38% (266/693), ‘pain’ in 13% (63/497) and 5% (33/693), ‘respiratory’ in 3% (16/497) and 4% (30/693), ‘urological’ in 12% (60/497) and 16% (112/693) and ‘unwell’ in 44% (217/497) and 36% (252/693) (Table 2).
Lower tract urolith prevalence in the New Zealand cohort was 2.4% (5/212) in cats ≤6y and 1.1% (3/285) in cats >6y. Lower tract urolith prevalence in the United States cohort was 6.0% (11/183) in cats ≤6y and 2.9% (15/510) in cats >6y. Upper tract urolith prevalence in the New Zealand cohort was 0.5% (1/212) in cats ≤6y and 1.8% (5/285) in cats >6y. Upper tract urolith prevalence in the United States cohort was 2.7% (5/183) in cats ≤6y and 10.2% (52/510) in cats >6y (Table 3).
In cats with uroliths, the main presenting clinical signs were categorized as ‘urological’ 64% (9/14) in the New Zealand cohort and either ‘abdominal’ 28% (26/93), ‘urological’ 28% (26/93) or ‘unwell’ 27% (25/93) in the USA cohort (Tables 2, 4). In each cohort, a significant association was present between a cats clinical signs and the location of a urolith in either the upper or lower urinary tract [New Zealand P = 0.03, USA P = 0.001 Fishers Exact Test for Count Data (for both)].
| Age category (N) | Upper tract (%) | Lower tract (%) | Both (%) |
|---|---|---|---|
| NZ ≤ 6y (212) | 1 (0.5) | 5 (2.3) | 0 |
| NZ > 6y (285) | 5 (1.8) | 3 (1.1) | 0 |
| USA ≤ 6y (183) | 5 (2.7) | 11 (6.0) | 3 (1.6) |
| USA > 6y (510) | 52 (10.2) | 15 (2.9) | 7 (1.4) |
| Nephrolith | Ureterolith | Cystolith | ||||
|---|---|---|---|---|---|---|
| Variable | New Zealand | United States | New Zealand | United States | New Zealand | United States |
| Number of cats | 5 | 50 | 0 | 6 | 8 | 26 |
| Age (years) | ||||||
| Median | 11 | 12 | – | 10.5 | 4.5 | 7.5 |
| Range | 2–14 | 5–17 | – | 6–16 | 1–15 | 2–15 |
| Age category (years) | ||||||
| ≤6 | 1 | 4 | 0 | 1 | 5 | 11 |
| >6 | 5 | 46 | 0 | 5 | 3 | 15 |
| Gender | ||||||
| Female | 0 | 3 | – | 0 | 2 | 2 |
| Female speyed | 2 | 12 | – | 4 | 3 | 12 |
| Male | 2 | 15 | – | 0 | 1 | 2 |
| Male neutered | 1 | 20 | – | 2 | 2 | 10 |
| Breed | ||||||
| Birman | 3 | 0 | – | 0 | 0 | 0 |
| DLH | 0 | 6 | – | 0 | 2 | 3 |
| DMH | 0 | 1 | – | 1 | 1 | 0 |
| DSH | 1 | 34 | – | 4 | 4 | 22 |
| Maine Coon | 0 | 3 | – | 0 | 0 | 0 |
| Persian | 0 | 1 | – | 0 | 1 | 0 |
| Ragdoll | 0 | 1 | – | 0 | 0 | 0 |
| Siamese | 1 | 3 | – | 0 | 0 | 1 |
| Other | 0 | 1 | – | 1 | 0 | 0 |
| Presenting category | ||||||
| Abdominal | 1 | 19 | – | 2 | 1 | 5 |
| Pain | 0 | 1 | – | 0 | 0 | 2 |
| Respiratory | 0 | 2 | – | 0 | 0 | 0 |
| Urological | 2 | 7 | – | 3 | 7 | 16 |
| Unwell | 2 | 21 | – | 1 | 0 | 3 |
| Modality | ||||||
| Ultrasound | 1 | 30 | – | 3 | 4 | 17 |
| X-ray | 4 | 20 | – | 3 | 4 | 9 |
In cats with uroliths in the New Zealand cohort, the association between age group and urolith location was not significant (P = 0.138). In cats with uroliths in the USA cohort, the association between age group and the urolith location was strong (P = 0.001), with upper tract uroliths being more common in cats >6y (52/57) than in cats ≤6y (5/57).
Urolith classification in the original radiologists’ reports correlated with the review of accompanying images in 496/497 of the cats from New Zealand and 524/525 cats with available images from the United States. Small nephroliths reported in one New Zealand cat were judged to be foci of dystrophic mineral, and a single ureterolith reported in a United States cat was reclassified as a urethrolith.
In each cohort, a wide range of breeds was represented with domestic cats predominating which is in accordance with the breed distributions in other studies of feline uroliths (Lekcharoensuk et al. 2000; Cannon et al. 2007; Picavet et al. 2007; Houston & Moore 2009) The distribution of male to female cats was almost identical between our two study cohorts and resembled feline gender distributions reported previously (Jones et al. 1997; Allan et al. 2000; Lund et al. 2005).
Discussion
Our finding that urolith prevalence was 3% in a hospital cohort of cats from New Zealand and 13% in a multi-hospital cohort of cats from the USA supports clinical impressions that the prevalence of uroliths in cats in New Zealand is relatively low. It also suggests there may be variance in feline urolith prevalence between countries–despite prevalence patterns for feline urolith composition being reported to be globally similar (Kirk & Bartges 2014).
This study is the first to determine the prevalence of uroliths in hospitalized cats presented for imaging studies at veterinary hospitals. The proportion of radiographic studies performed was slightly lower, and the proportion of sonographic studies performed was slightly higher, in the New Zealand cohort than in the USA cohort (Table 1). However, the proportion of cats detected with uroliths by either modality was lower in the New Zealand cohort than in the USA cohort. Accordingly, it appears unlikely that the prevalence difference we report between the cohorts resulted from differences in the proportions of studies performed with each imaging modality.
To the authors’ knowledge, this study is also the first to use data from a veterinary teleradiology company to investigate disease prevalence. The teleradiology database had an efficient and robust search facility, and the images and reports were easy to view. A drawback was the absence of some images from the early years of the study period. However, given the strong correlation between our review of available images and the radiologists’ reports, the absent images are unlikely to have contained findings that would significantly alter the prevalence figures we report.
Accurate determination of the prevalence of uroliths in the general feline population is difficult. Many studies of feline urolithiasis are based on populations of cats with clinical signs that led to the removal of the urolith. Data obtained from the laboratory analysis of the submitted uroliths provide valuable information on urolith composition and on risk factors for urolith formation. However, limited information is provided on urolith prevalence in wider populations as not all cats with uroliths are diagnosed or treated (Forrester & Roudebush 2007). In addition, uroliths submitted for analysis are more likely to be from the lower urinary tract than from the upper tract (Kyles et al. 2005; Picavet et al. 2007). Accordingly, reports based on urolith submissions may overestimate lower tract urolith prevalence, underestimate upper tract urolith prevalence and inaccurately represent urolith prevalence in the general cat population.
This study was based on commonly used imaging modalities capable of detecting in situ uroliths. Most feline uroliths are radiopaque (calcium oxalate, calcium phosphate and struvite) and are typically seen on survey radiographs when greater than approximately 2–3 mm in diameter (Lulich & Osborne 2009). Less radiopaque and non-radiopaque uroliths (urate, silica and cystine) are likely to be missed on survey radiographs although their detection may be possible with the enhanced contrast resolution of modern digital radiography systems (Hecht 2015). Ultrasonography typically detects radiopaque and non-radiopaque uroliths although their detection can be difficult if the urinary bladder is empty and the urolith is small with indistinct shadowing. In equivocal situations, urolith detection can be enhanced by observing for twinkling artefact during colour-Doppler sonography (Louvet 2006). Use of this artefact was not seen in any of the reviewed images. With either radiography or ultrasonography, intestinal contents superimposing the urinary tract can obscure uroliths. Small areas of dystrophic mineral, or mineral fragments within the intestine, may be mistaken for uroliths in radiographs, and dystrophic mineral in the kidneys may be mistaken for nephroliths in sonograms. None-the-less, despite their shortcomings, these imaging modalities offer practical methods for in situ urolith detection in hospital populations of cats.
The imaging appearances of in situ uroliths overlap to varying degrees making the accurate determination of their composition difficult. The authors are unaware of any studies specifically correlating the imaging appearance of in situ or in vitro feline uroliths with their chemical composition. Urolith composition may be predicted by correlating radiographic findings with knowledge of a patients diet and urine ph, sediment and culture (Osborne et al. 2009b). However, because the composition of the outer layer of a urolith may differ from that of the inner nidus, definitive knowledge of urolith composition can only be obtained by laboratory analysis.
The results of our study provide the first prevalence data from imaging data of urolithiasis in cats from New Zealand and the USA. Previous studies of hospital populations of cats have reported the prevalence of feline urolithiasis (Kirk et al. 2001a) and nephrolithiasis (Kirk et al. 2001a) to be 0.3 and 0.01%, respectively. Such studies were based on diagnostic codes and may have underestimated urolith prevalence in asymptomatic cats or in cats with non-urological signs, leading to different results from what we report based on imaging data. Our study has shown that cats with urolithiasis may present with a variety of clinical signs (Table 2) that are not classified as urological.
Most feline uroliths are reported to be located in the lower urinary tract (Bartges & Callens 2015). Our finding of more cats with uroliths confined to the upper urinary tract than the lower urinary tract is a likely consequence of our inclusion of cats with a variety of clinical signs rather than restricting our cohorts to cats with clinical signs supporting a diagnosis of lower urinary tract disease. This suggestion is supported by our finding of higher urolith prevalence in cats with non-urological signs than in cats with urological signs.
Despite the age disparity between the two cohorts, the prevalence of upper tract uroliths in cats >6y was 5.7 times higher in the USA cats than in the New Zealand cats. In addition, the prevalence of lower tract uroliths in cats ≤6y was 2.6 times higher in the USA cats than in the New Zealand cats. The association between the location of a urolith and a cats age category was significant in the USA cohort. However, no association with age was found in the New Zealand cohort, which may be due to a lack of statistical power to detect a difference.
In both cohorts, cats with uroliths had a broad range of clinical signs. Most cats with lower tract uroliths had clinical signs categorized as ‘urological’ and it is feasible that uroliths were anticipated in these cats at the time of imaging. In cats with uroliths, the significant association between presenting clinical signs and urolith location (P = 0.03 New Zealand, P = 0.001 USA) was likely driven by the high proportion of cats with urological signs that had lower tract uroliths. It is noteworthy that 7% (12/172) of cats with urological signs had uroliths confined to the upper tract. These uroliths may have been unexpected or overlooked if the clinicians focus was on the lower tract at the time of imaging.
Most cats in our study that had upper tract uroliths had clinical signs that were not specific for urinary tract disease. Some studies (Palm & Westropp 2011) report ureteroliths to be frequently associated with signs of pain whereas others (Kyles et al. 2005) record nonspecific signs in many cats with ureteroliths. In our study, no cats with ureteroliths had pain as a presenting sign. However, because cats are adept at hiding signs of pain, this finding may represent either a true lack of pain or an inability of clinicians to recognize signs of pain in cats at the time of presentation. Because of the effect of ureteral obstruction on renal function, it has been recommended that imaging be performed in cats with chronic nonspecific clinical signs, or in cats with renal failure, to rule out ureterolithiasis (Kyles et al. 2005). If such a recommendation had been followed in the practices that contributed cases to our study, the occurrence of uroliths might have been higher than what we have presented.
In this study, the prevalence of feline uroliths varied with geographical location. The primary providers of commercial cat foods differ between New Zealand (International E) and the USA (International E), and access to non-commercial food varies within and between countries. The influence of nutrition on urolith formation has been extensively described (Osborne et al. 2009b; Bartges & Callens 2015) and it is possible that differences in nutrition contributed to the variance in urolith prevalence we report. Differences in the genetic profile of the cats in each country, or metabolic differences, might have influenced regional differences in urolith formation. For example, urinary calcium oxalate excretion in cats may be related to endogenous calcium oxalate synthesis rather than to dietary factors involved in oxalate absorption (Dijcker et al. 2014). In a study of feline lower urinary tract disease there was a higher occurrence of uroliths in indoor cats compared with outdoor cats (Dorsch et al. 2014) and this effect may also have contributed to the geographical variance in urolith prevalence we report.
As a retrospective series, this study had several limitations. First, it was not possible to standardized image acquisition and quality. The decision to acquire images will have varied according to the clinical acumen of the attending clinician, and the image quality will have varied according to equipment quality and operator experience. Second, misclassification of cats' age, sex, breed or clinical signs, together with inter-operator variability during imaging, may have influenced the accuracy of data. Third, the sensitivity for diagnosis of feline ureteroliths is maximized when radiography and ultrasonography are performed concurrently (Kyles et al. 2005). In our study, imaging sensitivity was not maximized as the cats underwent radiography or ultrasonography rather than correlative imaging using both modalities. Fourth, bias was added to the study by selecting for cats whose presenting clinical signs and physical examination findings warranted abdominal imaging. Furthermore, detection bias may have been introduced by improved imaging techniques and heightened awareness of urolithiasis by clinicians during the period of the study. Finally, the relatively low number of cats found with uroliths limited the statistical power of comparisons between each cohort.
Conclusion
In cohorts composed of cats with diverse clinical signs, we found more cats with uroliths in the upper tract than in the lower tract. Most cats with upper tract uroliths had non-urological clinical signs, and none with ureteroliths had pain as a presenting clinical sign. These findings should remind clinicians to scrutinize the upper and lower tracts during imaging and to consider the possibility of urolith-associated morbidity in cats with non-urological signs.
Despite the morbidity and economic impact of feline urolithiasis in many parts of the world, reports documenting the prevalence of uroliths in the feline population presented to veterinary hospitals are lacking. In the cohort of cats presented for imaging at veterinary hospitals in this study, overall urolith prevalence in cats in New Zealand was less than one-quarter that of cats in the USA. The prevalence of uroliths was lower in the New Zealand cohort than in the USA cohort regardless of the anatomical location of the urolith or the age group of the cat. These findings support the authors' clinical experience that urolith prevalence was relatively low in New Zealand. Further studies involving cats matched for age, sex and breed would be useful to determine whether geographical location is a risk factor for feline urolithiasis in larger numbers of cats from different countries. Data for such studies could be acquired through collaboration with teleradiology companies. Such data would also allow monitoring of temporal trends in urolith prevalence akin to the temporal changes in urolith composition shown by analysis of uroliths submitted to diagnostic laboratories. If geographical variance in the tendency of cats to form uroliths is confirmed, additional epidemiological investigations would be worthwhile to provide insight into the factors responsible.
Acknowledgements
The authors gratefully acknowledge Professor Boyd Jones for his editorial advice and Antech Imaging Services for allowing access to their imaging database.
Source of Funding
None.
Conflicts of interest
Ron Green is employed by Antech Imaging.
Contributions
Paul F. Wightman- study concept, design, acquisition & analysis of data, MS write up and Revision. Kate E. Hill - study concept, design, analysis of data, MS write up and Revision. Janis Bridges and Charlotte F. Bolwell - data analysis, MS write up and Revision. John French and Brian A. Adler - acquisition and analysis of data. Eli B. Cohen - study concept, analysis of data. Ron Green - study concept, acquisition & analysis of data, Revision.
References
- Allan F.J., Pfeiffer D.U., Jones B.R., Esslemont D.H.B. & Wiseman M.S. (2000) A cross-sectional study of risk factors for obesity in cats in New Zealand. Preventive Veterinary Medicine46, 183–196. PubMed PMID: WOS:000088663800004.
- Bartges J.W. & Callens A.J. (2015) Urolithiasis. Veterinary Clinics of North America: Small Animal Practice45, 747–768.
- Beban H. (2014) Epidemiology of Canine and Feline Urolithiasis in New Zealand: 2009-12. VetScript ISSN 1170-280X. Vol XXV11(No 5 June 2014):18-20.
- Cannon A.B., Westropp J.L., Ruby A.L. & Kass P.H. (2007) Evaluation of trends in urolith composition in cats: 5,230 cases (1985-2004). Journal of the American Veterinary Medical Association231, 570–576. PubMed PMID: WOS:000248646100014.
- Dijcker J.C., Hagen-Plantinga E.A., Everts H., Queau Y., Biourge V. & Hendriks W.H. (2014) Factors contributing to the variation in feline urinary oxalate excretion rate. Journal of Animal Science92, 1029–1036. PubMed PMID: WOS:000332035900019.
- Dohoo I., Martin W., & Stryhn H. (2010) Veterinary Epidemiologic Research, 2010, pp 38. AVC Inc: Charlottetown, Prince Edward Island.
- Dorsch R., Remer C., Sauter-Louis C. & Hartmann K. (2014) Feline lower urinary tract disease in a German cat population a retrospective analysis of demographic data, causes and clinical signs. Tieraerztliche Praxis Ausgabe Kleintiere Heimtiere42, 231–239.
- Forrester S.D. & Roudebush P. (2007) Evidence-based management of feline lower urinary tract disease. The Veterinary Clinics of North America. Small animal practice37, 533–558. PubMed PMID: WOS:000246997100011.
- Hecht S. (2015) Diagnostic imaging of lower urinary tract disease. Veterinary Clinics of North America: Small Animal Practice45, 639–663.
- Houston D.M. & Moore A.E.P. (2009) Canine and feline urolithiasis: examination of over 50 000 urolith submissions to the Canadian Veterinary Urolith Centre from 1998 to 2008. Canadian Veterinary Journal-Revue Veterinaire Canadienne50, 1263–1268. PubMed PMID: WOS:000272725700006. English.
- International E (2015a) Cat Food in the US. Available at: http://www.euromonitor.com/cat-food-in-the-us/report2013 (Accessed October 1, 2015).
- International E (2015b) Cat Food in New Zealand. Available at: http://www.euromonitor.com/cat-food-in-new-zealand/report2013 (Accessed October 1, 2015).
- Jones B.R., Sanson R.L. & Morris R.S. (1997) Elucidating the risk factors of feline lower urinary tract disease. New Zealand Veterinary Journal45, 100–108. PubMed PMID: WOS:000071443400004.
- Jones B.R., Kirkman J.H., Hogan J. & Holmes S. (1998) Analysis of uroliths from cats and dogs in New Zealand, 1993-96. New Zealand Veterinary Journal46, 233–236. PubMed PMID: WOS:000077832000005.
- Kalton G. (1983) Introduction to Survey Sampling. Paper series on Quantitative Applications in the Social Sciences, series no 07-035 Beverly Hills, CA: Sage 1983;Series no.07-035:16-9.
- Kirk C.A., Bartges J.W. (2014) Feline Uroliths and Urethral Plugs: Epidemiology, Risk Factors and Pathogenesis. Available at: http://www.hillsvet.com. 15-22 (Accessed September 15, 2015).
- Kirk C., Lund E., Armstrong P. (eds) (2001a) Prevalence of Lower Urinary Tract Disorders of Dogs and Cats in the United States. Proceedings, Waltham International Symposium. Vancouver, Canada. Available at: https://www.waltham.com/dyn/_assets/_pdfs/winss/Abstracts_2001.pdf (Accessed September 10, 2015).
- Kirk C., Lund E., Armstrong P. (eds) (2001b) Prevalence of Renal Disorders of Dogs and Cats in the United States. Proceedings, Waltham International Symposium. Vancouver, Canada. Available at: https://www.waltham.com/dyn/_assets/_pdfs/winss/Abstracts_2001.pdf (Accessed September 10, 2015).
- Kyles A.E., Hardie E.M., Wooden B.G., Adin C.A., Stone E.A., Gregory C.R.et al. (2005) Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984-2002). Journal of the American Veterinary Medical Association226, 932–936. PubMed PMID: WOS:000227629500024.
- Lekcharoensuk C., Lulich J.P., Osborne C.A., Koehler L.A., Urlich L.K., Carpenter K.A.et al. (2000) Association between patient-related factors and risk of calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. Journal of the American Veterinary Medical Association217, 520–525.
- Lekcharoensuk C., Osborne C.A., Lulich J.P., Albasan H., Ulrich L.K., Koehler L.A.et al. (2005) Trends in the frequency of calcium oxalate uroliths in the upper urinary tract of cats. Journal of the American Animal Hospital Association41, 39–46. PubMed PMID: WOS:000238374800007.
- Louvet A. (2006) Twinkling artifact in small animal color-Doppler sonography. Veterinary Radiology & Ultrasound: The Official Journal of the American College of Veterinary Radiology and the International Veterinary Radiology Association47, 384–390. PubMed PMID: 16863058.
- Lulich J.P. & Osborne C.A. (2009) Changing paradigms in the diagnosis of urolithiasis. The Veterinary Clinics of North America Small Animal Practice39, 79–91. PubMed PMID: 19038652. Epub 2008/11/29. eng.
- Lund E.M., Armstrong P.J., Kirk C.A. & Klausner J.S. (2005) Prevalence and risk factors for obesity in adult cats from private US veterinary practices. International Journal of Applied Research in Veterinary Medicine3, 88–96. PubMed PMID: CABI:20053145556.
- Lund H.S., Krontveit R.I., Halvorsen I. & Eggertsdóttir A.V. (2013) Evaluation of urinalyses from untreated adult cats with lower urinary tract disease and healthy control cats: predictive abilities and clinical relevance. Journal of Feline Medicine and Surgery15, 1086–1097.
- inc. NZCAC (2011) Companion Animals in New Zealand July 2011. Available at: http://nzcac.org.nz/other-content-conference-contacts/nzcac-other-information/nzcac-publications (Accessed September 15, 2015).
- Osborne C.A., Kruger J.P., Lulich J.P., Bartges J.W., Polzin D.J., Molitor T.et al. (1992) Feline matrix-crystalline urethral plugs: a unifying hypothesis of causes. Journal of Small Animal Practice33, 172–177.
- Osborne C.A., Lulich J.P., Kruger J.M., Ulrich L.K. & Koehler L.A. (2009a) Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. The Veterinary Clinics of North America. Small Animal Practice39, 183–197. PubMed PMID: WOS:000262171600015. English.
- Osborne C.A., Lulich J.P., Forrester D. & Albasan H. (2009b) Paradigm changes in the role of nutrition for the management of canine and feline urolithiasis. Veterinary Clinics of North America: Small Animal Practice39, 127–141.
- Paepe D., Verjans G., Duchateau L., Piron K., Ghys L. & Daminet S. (2013) Routine health screening findings in apparently healthy middle-aged and old cats. Journal of Feline Medicine and Surgery15, 8–19.
- Palm C. & Westropp J. (2011) Cats and calcium oxalate strategies for managing lower and upper tract stone disease. Journal of Feline Medicine and Surgery13, 651–660. PubMed PMID: WOS:000294975200003.
- Picavet P., Detilleux J., Verschuren S., Sparkes A., Lulich J., Osborne C.et al. (2007) Analysis of 4495 canine and feline uroliths in the Benelux. A retrospective study: 1994-2004. Journal of Animal Physiology and Animal Nutrition91, 247–251. PubMed PMID: WOS:000246618500012.
- Shipov A. & Segev G. (2013) Ureteral obstruction in dogs and cats. Israel Journal of Veterinary Medicine68, 71–77. PubMed PMID: WOS:000319411900002.
- Zaid M.S., Berent A.C., Weisse C. & Caceres A. (2011) Feline ureteral strictures: 10 cases (2007-2009). Journal of Veterinary Internal Medicine25, 222–229. PubMed PMID: WOS:000288073800006.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?