Summary
Background
The aim of the present study was to demonstrate the antiulcer activity and mechanism of bezafibrate in a rat model of aspirin-induced gastric ulcer.
Methods
We used an aspirin-induced gastric ulcer model. Bezafibrate was administered orally in graded doses (10 mg/kg, 25 mg/kg, 50 mg/kg, 100 mg/kg, and 200 mg/kg) to detect the best effective antiulcer dose of bezafibrate. The parameters measured were: ulcer index, histopathological scoring of gastric ulcer, gastric juice analysis, gastric mucosal lipid peroxidation parameters, estimation of NO metabolite in blood, mRNA expression of inducible NO synthase iNOS and constitutive NO synthase (cNOS) in gastric mucosa, and gastric mucosal DNA fragmentation.
Results
The dose-dependent antiulcer activity of bezafibrate was shown by the ulcer index and histopathological score. Bezafibrate (100 mg/kg) significantly reduced total acidity, free acidity, and pepsin activity, and increased total hexoses and total proteins. Bezafibrate (100 mg/kg) also significantly reduced lipid peroxidation, inhibited iNOS expression, preserved cNOS expression, and inhibited DNA fragmentation.
Conclusion
Bezafibrate can decrease aspirin-induced gastric mucosal injury via reducing lipid peroxidation, inhibiting iNOS expression, preserving cNOS expression, and decreasing DNA fragmentation.
Keywords
Aspirin ; Bezafibrate ; Gastric ulcer ; Nitric oxide ; Rat
Introduction
Peptic ulcer is a common disorder of the gastrointestinal system and millions of people suffer from this disease worldwide. The medical cost of treating peptic ulcer and its complications amounts to billions of dollars annually. The pathogenesis of peptic ulcer disease is multifactorial, including Helicobacter pylori infection, chronic use of nonsteroidal anti-inflammatory drugs, cigarette smoking, alcohol, and reactive oxygen species (ROS) [1] ; [2] ; [3] . Peptic ulcer is produced by the imbalance between gastroduodenal mucosal defense mechanisms and offensive factors [4] . Recent studies also indicate that programmed cell death or apoptosis plays a significant role in gastric ulceration [5] ; [6] ; [7] . Nitric oxide (NO) is a crucial mediator of gastrointestinal mucosal defense, but, paradoxically, it also contributes to mucosal damage [8] . This can be illustrated by the ability of different NO concentrations to produce completely opposite effects on the same tissue [9] .
Nonsteroidal anti-inflammatory drugs such as aspirin is widely used as an anti-inflammatory, analgesic drug and in the prevention of cardiovascular events [10] . However, the major limitations of their clinical application are serious gastrointestinal side effects, especially peptic ulcerations and gastrointestinal bleeding [11] . Studies have demonstrated that the use of aspirin is associated with an elevated risk of symptomatic peptic ulcer [12] . The risk of peptic ulcer was elevated throughout treatment independently of its duration, was elevated with doses as low as 75 mg/d, and was no different from that with doses of 150 mg/d and 300 mg/d [13] ; [14] .
Bezafibrate, a peroxisome proliferator activated receptor (PPAR)α agonist, is often used in patients with diabetes mellitus and dyslipidemia. These patients are also taking aspirin for the prevention of cardiac events. So, we hypothesized that if bezafibrate demonstrated antiulcer activity against aspirin-induced gastric ulcer, then this combination can be used in patients with diabetes mellitus and dyslipidemia, with the additional benefit of the antigastric ulcer effect of bezafibrate.
Studies in animals have demonstrated the gastric antisecretory activity of PPAR-α agonists like ciprofibrate, bezafibrate, and clofibrate [15] ; [16] . Eason et al. [16] demonstrated significant inhibition of gastric secretion. Pathak et al. [15] demonstrated the effect of PPAR-α agonist, bezafibrate, on gastric secretion and gastric cytoprotection in various gastric ulcer models in rats, such as acetic-acid-induced chronic gastric ulcers, pylorus-ligation-induced gastric ulcers, ethanol-induced gastric ulcers, indomethacin-induced gastric ulcers, and ischemia–reperfusion-induced gastric ulcers. However, in the aspirin-induced gastric ulcer model, the precise mechanisms for antiulcer and gastric cytoprotective effects of bezafibrate have not been studied.
To the best of our knowledge, no study has been carried out to investigate the mechanism of the antigastric ulcer effect of bezafibrate. Therefore, the present study was contemplated with the aim of studying the mechanism of bezafibrate as an antigastric ulcer agent. Keeping in view the diversity of defensive mechanisms, the present study was limited to exploring the oxidative stress, apoptosis, and NO pathways and their involvement in the mechanism of the antigastric ulcer effect of bezafibrate.
Methods
Experimental animals
Wistar rats of either gender weighing 200–250 g were used for the present study. The animals were obtained from Central animal House, PGIMER, Sector 12, Chandigarh, India. The animals were maintained at 23 ± 2°C with a relative humidity of 65 ± 5% in a 12-hour light/dark cycle. The animals had free access to standard pellet chow diet and tap water ad libitum . The rats were acclimatized to laboratory conditions for at least 7 days. Food was withheld for 36 hours and water for 1 hour prior to commencement of the study.
Grouping
A total of 60 animals was divided into 10 groups (I–X) of six animals each as follows. Control Groups: Group I, normal control (normal saline); Group II, saline + aspirin (200 mg/kg); and Group III, ranitidine (50 mg/kg) + aspirin (200 mg/kg). For a dose–response study of bezafibrate to establish the best effective dose: Group IV, bezafibrate (10 mg/kg) + aspirin (200 mg/kg); Group V, bezafibrate (25 mg/kg) + aspirin (200 mg/kg); Group VI, bezafibrate (50 mg/kg) + aspirin (200 mg/kg); Group VII, bezafibrate (100 mg/kg) + aspirin (200 mg/kg); and Group VIII, bezafibrate (200 mg/kg) + aspirin (200 mg/kg). For mechanistic study with best effective dose: Group IX, best effective dose of bezafibrate (100 mg/kg) + aspirin (200 mg/kg); and Group X, L-NG -nitroarginine (L-NNA) (50 mg/kg) + best effective dose of bezafibrate (100 mg/kg) + aspirin (200 mg/kg).
Ethical permission
Institutional Animal Ethics Committee (IAEC, PGIMER, sector 12, Chandigarh) approval (No. 42/IAEC/163 dated 24.09.2008) was obtained before the start of the study and the study was carried out according to the ARRIVE guidelines.
Drug treatment
Aspirin was suspended in 1% carboxymethyl cellulose and administered orally at a dose of 200 mg/kg to produce gastric ulcer in animals in Groups II–X. Bezafibrate was dissolved in dimethyl sulfoxide (50 mg/kg) and administered at five different doses of 10 mg/kg, 25 mg/kg, 50 mg/kg, 100 mg/kg, and 200 mg/kg orally in animals of Groups IV–VIII 30 minutes before aspirin. Ranitidine was dissolved in water and given at a dose of 50 mg/kg orally in animals of Group III 30 minutes before aspirin. L-NNA (50 mg/kg) was dissolved in water and administered orally 30 minutes before bezafibrate (100 mg/kg) in animals of Group X.
Aspirin-induced gastric ulcers and collection of gastric juice
The animals were fasted for 24 hours before experimentation. They received water ad libitium . One hour before the experiments, water was withheld. In animals of Groups IV–VIII, bezafibrate was administered orally 30 minutes before aspirin administration, and in Group III, ranitidine was administered orally 30 minutes before aspirin administration (bezafibrate/ranitidine→aspirin→pyloric ligation). In animals of Group X, L-NNA was administered orally 30 minutes before bezafibrate administration and 30 minutes after bezafibrate administration, aspirin was administered (L-NNA→bezafibrate→aspirin→pyloric ligation). Thirty minutes after aspirin administration, animals were anesthetized with pentobarbital (40 mg intraperitoneally), the abdomen was opened, and the pyloric end of the stomach was ligated with thread, taking care of the blood vessels. The abdomen was closed and animals were kept individually in the metabolic cage provided with a platform with a wide mesh wire gauge to prevent coprophagy. Gastric juice was allowed to collect for 4 hours after pyloric ligation. After 4 hours pyloric ligation, rats were sacrificed with pentobarbitone overdose. The abdomen was opened and the cardiac end of the stomach was ligated. The stomach was quickly dissected out and cut open along the greater curvature and gastric juice was collected and centrifuged (2500 g, 5 minutes) to obtain clear gastric juice.
Dose–response study of bezafibrate to establish the best effective dose
A dose–response study of bezafibrate was done by administering five different doses (10 mg/kg, 25 mg/kg, 50 mg/kg, 100 mg/kg, and 200 mg/kg, orally) of bezafibrate in Groups IV–VIII. Bezafibrate was administered 30 minutes before aspirin administration. The doses of bezafibrate were selected on the basis of the study of Pathak el al [15] . The best effective dose of bezafibrate was selected on the basis of ulcer index and histopathological score as follows.
Ulcer index (damage score)
The damage score was assessed by grading the gastric injury on a scale of 0 to 4 based on the severity of mucosal hyperemia and hemorrhagic erosions according to Guha et al. [17] : 0 = almost normal mucosa; 0.5 = hyperemia; 1 = one or two hemorrhagic erosions; 2 = severe hemorrhagic erosions; 3 = very severe hemorrhagic erosions; and 4 = mucosa full of lesions (hyperemia, hemorrhagic erosions, or vascular congestion).
Histopathological study of gastric lesions
Longitudinal sections were taken from the gastric lesion (hyperaemia and erosions), along with the adjacent normal gastric mucosa, of different sizes and subjected to histopathological study using hematoxylin and eosin stain. The scoring of the ulcer was done as follows: 0 = no mucosal changes; 1 = changes limited to disruption of the surface lining epithelium or superficial layer of the mucosa, with no vascular congestion; 2 = half of the mucosal thickness showing tissue necrosis; 3 = more than two-thirds of the mucosal thickness destroyed, with marked tissue necrosis and vascular congestion; the muscularis mucosae remained intact; and 4 = complete destruction of the mucosa with necrosis and hemorrhage; muscularis mucosae not affected. In the dose–response study groups (IV–VIII), both gastric juice and tissue antioxidant parameters were analyzed.
Mechanistic study with best effective dose of bezafibrate (100 mg/kg)
Based on the ulcer index and histopathological scores, bezafibrate 100 mg/kg body weight, was selected as best effective dose. The detailed mechanistic studies were done in Groups IX and X as follows.
Analysis of gastric juice for offensive/defensive factors
Free and total acidity was estimated by the method of Mallika et al. [18] , and was expressed as mEq/h. Pepsin activity was measured according to the method of Newaz et al. [19] . Total hexoses were estimated by the method of Dische and Borenfreud [20] and expressed as μg/mL. Mucin content was estimated by the method of Sanyal et al. [21] and expressed as μg/g. Protein content was estimated by the method of Lowry et al. [22] and expressed as mg/mL.
The stomach tissue was homogenized in 50mM phosphate buffer (pH 7.2)/150mM NaCl (phosphate-buffered saline) at 4°C using a glass–teflon homogenizing tube. The homogenate was centrifuged (2500 g, 10 minutes). The supernatant was used for assessment of oxidative-stress-related parameters.
Tissue lipid peroxidation was evaluated by measurement of thiobarbituric acid reactive substances according to the method of Ohkawa et al. [23] . Superoxide dismutase (SOD) was estimated by the method of Kono [24] . Activity of the catalase was measured using the method of Luck [25] . Assay of reduced glutathione was performed in tissue homogenates by the method of Griffith [26] . For estimation of NO metabolite, 0.5 mL of blood was collected from each anesthetized animal by cardiac puncture and subjected to estimation of nitrate level by the method of Green et al. [27] .
Reverse-transcriptase polymerase chain reaction for inducible NO synthase mRNA in gastric mucosa
Total RNA was extracted from gastric mucosal samples by the method of Chomczynski and Sacchi [28] . RNA samples were stored at −80°C until analysis. Single-stranded cDNA was generated from 5 μg total cellular RNA by reverse transcriptase and oligo-(dT)- primers. Five micrograms of total RNA was uncoiled by heating (65°C for 5 minutes) and then reverse transcribed into cDNA. The resultant cDNA (2 μL) was amplified in a 50-μL reaction volume containing 2 U Taq polymerase, dNTP (200μM each), 1.5mM MgCl2 , 5 μL 10× polymerase chain reaction (PCR) buffer [10μM Tris/HCl (pH 8.3)/50μM KCl] and specific primer was used at a final concentration of 0.5μM. The PCR mixture was amplified in a DNA thermal cycler. The nucleotide sequences of the primer for inducible NO synthase (iNOS), constitutive NO synthase (cNOS), and β actin were based on the published cDNAs sequences (iNOS: sense 5′ CAG TGG CAA CAT CAG GTC and antisense 3′ GGT CTC GGA CTC CAA TCT, for cNOS: sense 5′ TAC TTG AGG ATG TGG CTG and antisense 3′ GTC TTC TTC CTG GTG ATG, for β actin: sense 5′ TTG TAA CCA ACT GGG ACG ATA TGG and antisense 3′ GAT CTT GAT CTT CAT GGT GCT AGG). A PCR product was detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. Location of predicted products was confirmed by using 100-bp ladder as a standard size marker.
Gastric mucosal DNA fragmentation study
DNA from gastric mucosa was isolated by phenol–chloroform extraction method and subjected to agarose gel electrophoresis. DNA ladder/smearing, if any, was visualized by UV-transilluminator and photographed.
Statistical analysis
Statistical analysis was assessed using one-way analysis of variance followed by Bonferroni comparison test. Ulcer index and histopathological scores were compared by Mann–Whitney U test. The values are expressed as mean ± standard deviation and p < 0.05 was considered statistically significant.
Results
Effect of bezafibrate on aspirin-induced gastric ulcer and ulcer index
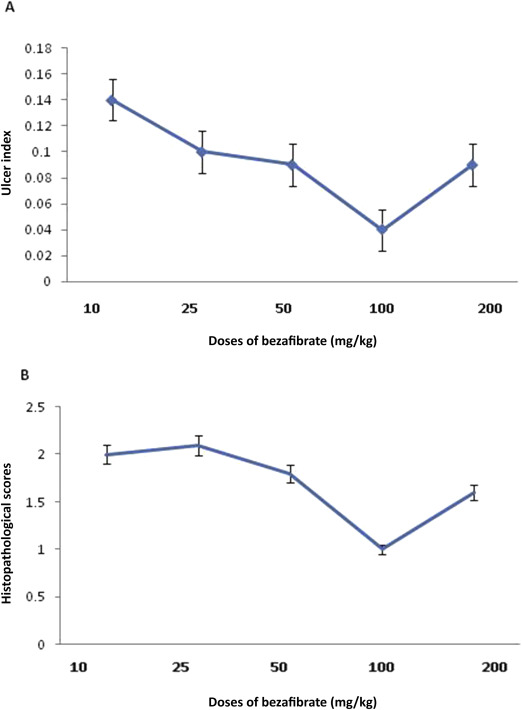
The macroscopic findings of the opened stomach showed that hemorrhagic gastric ulcers covered with coagulated blood were more apparent in the aspirin group than the control group. Coadministration of bezafibrate 100 mg/kg with aspirin inhibited aspirin-induced ulcer formation. Table 1 shows the effects of bezafibrate given in graded doses ranging from 10 mg/kg to 200 mg/kg intraperitoneally on the ulcer index in the rat model of aspirin-induced gastric ulcer. Aspirin caused a significant increase in the ulcer index as compared to that of the saline treated control group of animals. Administration of bezafibrate was associated with a significant decrease in the ulcer index compared to aspirin alone (Figure 1 A and Table 1 ). The most pronounced effect on ulcer index was observed at a dose of 100 mg/kg bezafibrate and this dose was used for further mechanistic studies. L-NNA pretreatment caused significant antagonism of the effect of bezafibrate (100 mg/kg) on the ulcer index (Table 1 ).
| Groups | Ulcer index | Histopathological scores | Gastric juice analysis | |||
|---|---|---|---|---|---|---|
| Free acidity (mEq/L) | Total acidity (mEq/L) | Total hexoses (μg/mL) | Total proteins (mg/mL) | |||
| Saline control | 0.03 ± 0.01 | 0.33 ± 0.09 | 3.66 ± 1.24 | 8.81 ± 0.99 | 4.16 ± 1.32 | 79.96 ± 3.92 |
| Aspirin + saline | 0.33 ± 0.04* | 2.83 ± 0.28* | 8.29 ± 1.42* | 13.76 ± 2.13* | 2.75 ± 1.05 | 49.48 ± 4.27* |
| Ranitidine + aspirin | 0.04 ± 0.02∗∗ | 1.5 ± 0.02** | 4.89 ± 0.45∗∗ | 10.79 ± 0.67∗∗ | 5.10 ± 0.43 | 77.07 ± 3.16∗∗ |
| Bezafibrate 10mg/kg + aspirin | 0.14 ± 0.03* | 2.0 ± 0.08* | 7.05 ± 1.23* | 12.32 ± 1.13* | 3.14 ± 0.78 | 64.79 ± 1.16*,** |
| Bezafibrate 25 mg/kg + aspirin | 0.10 ± 0.04* | 2.1 ± 0.05* | 6.93 ± 0.56* | 12.12 ± 0.57* | 3.34 ± 0.74 | 64.36 ± 1.4*,** |
| Bezafibrate 50 mg/kg + aspirin | 0.09 ± 0.02∗∗ | 1.8 ± 0.06∗∗ | 6.10 ± 0.71*,** | 11.29 ± 0.67*,** | 3.87 ± 0.52 | 65.81 ± 1.3*,** |
| Bezafibrate 100 mg/kg + aspirin | 0.04 ± 0.03∗∗ | 1.0 ± 0.05∗∗ | 5.50 ± 0.53*,** | 10.65 ± 1.31∗∗ | 4.49 ± 0.58 | 69.58 ± 2.36*,** |
| Bezafibrate 200 mg/kg + aspirin | 0.09 ± 0.04∗∗ | 1.6 ± 0.08∗∗ | 5.86 ± 0.86*,** | 11.66 ± 0.54* | 4.27 ± 0.21 | 68.95 ± 2.24*,** |
| LNNA + bezafibrate 100 mg/kg + aspirin | 0.24 ± 0.09∗∗∗ | 2.00 ± 1.78∗∗∗ | 7.61 ± 1.02∗∗∗ | 11.87 ± 1.11∗∗∗ | 3.74 ± 1.28 | 56.44 ± 3.29∗∗∗ |
Data are expressed as mean ± standard error; n = 6 in each group.
- p < 0.05 as compared to saline control group.
- p < 0.05 as compared to aspirin-treated group.
- p < 0.05 as compared to bezafibrate 100 mg/kg group.
LNNA = L-NG -nitroarginine
|
|
|
Figure 1. (A) Effect of graded doses of bezafibrate on ulcer index. (B) Effect of graded doses of bezafibrate on histopathological scores. Data are expressed as mean ± standard error (n = 6). |
Histopathological findings of gastric mucosa and scoring
The gastric mucosa obtained from the control rats showed normal mucosa, submucosa, muscularis and serosa. Aspirin administration showed ulcer formation with distorted gastric glands, and damaged mucosal epithelium and cell debris; however, coadministration of bezafibrate (100 mg/kg) with aspirin protected against these changes. Table 1 and Figure 1 B show the effects of graded doses of bezafibrate on the histopathological scores in aspirin-induced gastric ulcer. Administration of bezafibrate along with aspirin was associated with a significant decrease in the histopathological scores at a dose of 100 mg/kg and 200 mg/kg as compared to aspirin alone. The most pronounced effect was observed at a dose of 100 mg/kg bezafibrate. L-NNA pretreatment antagonized the effect of bezafibrate (100 mg/kg) on the histopathological score (Table 1 ).
Effect of bezafibrate on gastric juice parameters
Table 1 shows the effect of graded doses of bezafibrate on the defensive and aggressive factors in gastric juice in the rat model of aspirin-induced gastric ulcer. Bezafibrate (+ aspirin) at higher doses (50 mg/kg, 100 mg/kg, and 200mg/kg) and ranitidine (+ aspirin) showed significant reductions in the free and total acidity as compared to aspirin alone. All of the doses of bezafibrate and ranitidine produced a significant increase in total proteins as compared to aspirin alone (Table 1 ). None of the doses of bezafibrate produced any significant effect on total hexoses. The most pronounced effect on gastric juice parameters was observed at a dose of 100 mg/kg bezafibrate. LNNA pretreatment caused significant antagonism of the effects of bezafibrate (100 mg/kg) on all of the parameters except total hexoses (Table 1 ).
Effect of graded doses of bezafibrate on gastric mucosal lipid peroxidation and antioxidant system
Table 2 shows the effect of graded doses of bezafibrate on the gastric mucosal lipid peroxidation and antioxidant system. All the doses of bezafibrate and ranitidine showed significant decrease in malondialdehyde level and significant increase in SOD activity and reduced glutathione as compared to the aspirin-treated group. Bezafibrate (100 mg/kg) showed a significant increase in catalase activity. LNNA pretreatment caused significant antagonism of the antioxidant effect of bezafibrate (100 mg/kg; Table 2 ).
| Groups | MDA concentration (nmol/mg tissue) | SOD activity (U/g tissue) | Catalase activity (μmol of H2 O2 decomposed/min/mg protein) | GSH-Px activity (μ mol/min/mg protein) | Reduced glutathione (nM/mg protein) | Plasma nitrate (mg/mL) |
|---|---|---|---|---|---|---|
| Saline control | 9.24 ± 0.11 | 366.84 ± 6.32 | 7.65 ± 0.21 | 60.39 ± 0.62 | 75.05 ± 0.52 | 22.83 ± 2.08 |
| Aspirin + saline | 14.30 ± 0.46* | 263.18 ± 7.34* | 6.78 ± 0.10* | 55.48 ± 0.24* | 55.28 ± 1.13* | 28.37 ± 2.98* |
| Ranitidine + aspirin | 8.76 ± 0.44** | 316.25 ± 4.54*,** | 5.71 ± 0.25*,** | 64.11 ± 1.12*,** | 61.87 ± 1.34*,** | - |
| Bezafibrate 10 mg/kg + aspirin | 12.45 ± 0.64*,** | 322.68 ± 5.14*,** | 6.73 ± 0.35* | 58.97 ± 2.32 | 59.17 ± 1.37*,** | - |
| Bezafibrate 25 mg/kg + aspirin | 11.60 ± 0.46*,** | 326.54 ± 3.23*,** | 6.94 ± 0.21* | 59.15 ± 2.03** | 60.93 ± 1.27*,** | - |
| Bezafibrate 50 mg/kg + aspirin | 10.79 ± 0.67*,** | 340.65 ± 9.12*,** | 6.90 ± 0.36* | 59.87 ± 1.73** | 65.06 ± 1.21*,** | - |
| Bezafibrate 100 mg/kg + aspirin | 10.29 ± 1.01** | 356.52 ± 3.26** | 7.72 ± 0.97** | 64.63 ± 1.24*,** | 72.94 ± 1.56** | 23.33 ± 3.10 |
| Bezafibrate 200 mg/kg + aspirin | 11.53 ± 0.32*,** | 343.25 ± 5.53*,** | 6.85 ± 0.65* | 63.20 ± 1.10** | 67.20 ± 2.01*,** | - |
| LNNA + Bezafibrate 100 mg/kg + aspirin | 12.9 ± 1.11∗∗∗ | 295.50 ± 4.99∗∗∗ | 6.94 ± 0.23∗∗∗ | 56.66 ± 1.20∗∗∗ | 58.74 ± 6.99∗∗∗ | 22.86 ± 1.99 |
Data are expressed as mean ± standard error.
- p < 0.05 compared to the saline control group.
- p < 0.05 compared to the aspirin group.
- p < 0.05 as compared to the bezafibrate 100 mg/kg group.
LNNA = L-NG -nitroarginine.
Effect of bezafibrate on plasma nitrate level
Table 2 shows the effect of bezafibrate (100 mg/kg) alone and bezafibrate (100 mg/kg) + LNNA on plasma levels of the NO metabolite, nitrate, in aspirin-induced gastric ulcer in rats. In the aspirin-induced gastric ulcer group, there was a significant increase in plasma nitrate as compared to the saline control group (Table 2 ). When bezafibrate (100 mg/kg) was coadministered with aspirin there was a decrease in plasma nitrate level as compared to the aspirin-treated group. Pretreatment with LNNA did not cause a significant change in plasma nitrate level as compared to the saline control and bezafibrate (100 mg/kg) + aspirin-treated groups.
Effect of bezafibrate on mRNA expression of iNOS and cNOS in gastric mucosa
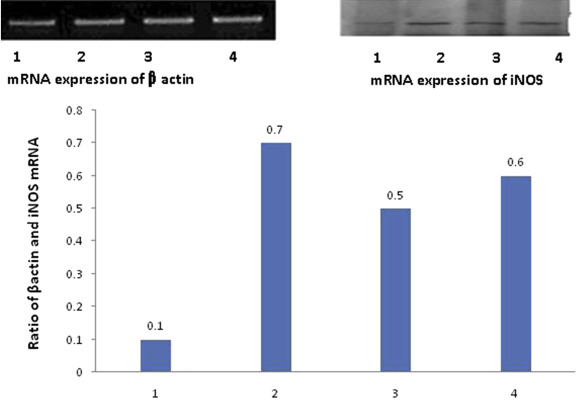
Figure 2 ; Figure 3 show the expression of mRNA of iNOS and cNOS in gastric mucosa. iNOS mRNA expression (Figure 2 ) was detected in the gastric mucosa of control animals. Following administration of aspirin, the ratio of iNOS to β-actin increased significantly, indicating that mRNA expression of iNOS was significantly upregulated by aspirin. In animals treated with bezafibrate (100 mg/kg) + aspirin, and LNNA + bezafibrate (100 mg/kg) + aspirin, this increase in iNOS protein expression was significantly attenuated, but was still higher than in the control animals (Figure 2 ).
|
|
|
Figure 2. Expression of mRNA for iNOS by reverse transcriptase polymerase chain reaction in gastric mucosa and the ratio of iNOS to β-actin mRNA in (1) control group, (2) aspirin group, (3) aspirin + bezafibrate 100 mg/kg group, and (4) aspirin + bezafibrate 100 mg/kg + L-NG -nitroarginine group in the upper panel. 1 = saline control; 2 = aspirin + saline; 3 = bezafibrate 100 mg/kg + aspirin; 4 = LNNA + bezafibrate 100 mg/kg + aspirin; iNOS = inducible NO synthase in the bar diagram. |
|
|
|
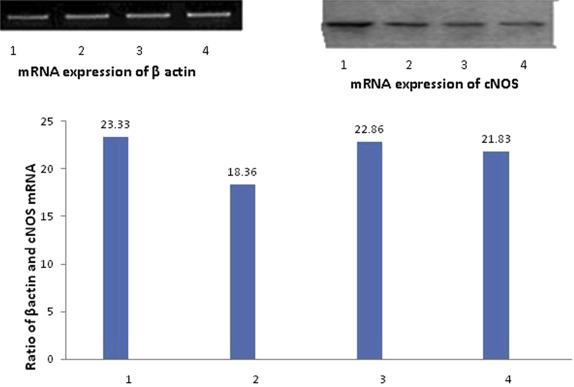
Figure 3. Expression of mRNA for cNOS by reverse transcriptase polymerase chain reaction in gastric mucosa and the ratio of cNOS to β-actin mRNA in (1) control group, (2) aspirin group, (3) aspirin + bezafibrate 100 mg/kg group, and (4) aspirin + bezafibrate 100 mg/kg + L-NG -nitroarginine group in the upper panel. 1 = saline control; 2 = aspirin + saline; 3 = bezafibrate 100 mg/kg + aspirin; 4 = LNNA + bezafibrate 100 mg/kg + aspirin; cNOS = constitutive NO synthase in the bar diagram. |
In all the control animals, cNOS was detected as a strong signal (Figure 3 ). Treatment with aspirin resulted in downregulation of mRNA expression for cNOS. In animals treated with bezafibrate (100 mg/kg) + aspirin and LNNA + bezafibrate (100 mg/kg) + aspirin, downregulation of mRNA expression of cNOS was not as prominent as with aspirin alone, however, expression was less than in the control group (Figure 3 ).
Effect of bezafibrate on DNA fragmentation in gastric mucosa
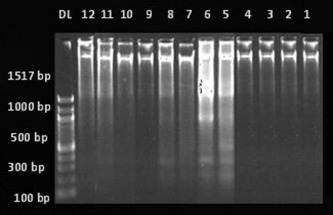
DNA extracted from gastric mucosa was subjected to agarose gel electrophoresis for qualitative estimation of DNA fragmentation. Figure 4 shows the DNA fragmentation in different experimental groups. Lane DL is a DNA fragment size marker indicating 100–2000 base pairs; Lanes 1–4 show DNA fragments in the control group; Lanes 5–8 show DNA fragments in the aspirin alone group; Lanes 9 and 10 show DNA fragments in the bezafibrate 100 mg/kg + aspirin group; and Lanes 11 and 12 show DNA fragments in the LNNA + bezafibrate 100 mg/kg + aspirin group. No ladder pattern was seen in the control group, suggesting there was no mucosal damage (Lanes 1–4). The aspirin-induced gastric ulcer group showed a laddering pattern with the formation of a large number of fragments ranging from 1517 base pairs to 100 base pair fragments, indicating DNA fragmentation (Lanes 5–8). Bezafibrate (100 mg/kg) + aspirin, and LNNA + bezafibrate (100 mg/kg) + aspirin groups had minimal DNA fragmentation, suggested by formation of fewer bands as compared to the aspirin-induced ulcer group (Lanes 9–12).
|
|
|
Figure 4. Agarose gel electrophoresis for qualitative estimation of DNA fragmentation in different experimental groups. Lane DL = DNA fragment size marker indicating 100–2000 base pairs; Lanes 1–4 = DNA fragments in the control group; Lanes 5–8 = DNA fragments in aspirin group; Lanes 9 and 10 = DNA fragments in bezafibrate 100 mg/kg + aspirin group; Lanes 11 and 12 = DNA fragments in L-NG -nitroarginine + bezafibrate 100 mg/kg + aspirin group. |
Discussion
We demonstrated that bezafibrate, a PPARα agonist, dose dependently produced antiulcer activity in a rat model of aspirin-induced gastric ulcer. Bezafibrate significantly decreased the total acidity and free acidity in the gastric juice. The total protein in the gastric juice was increased with bezafibrate treatment. Our results suggest that bezafibrate has both gastric antisecretory and gastroprotective effects and these results are consistent with previous reports with bezafibrate, ciprofibrate, and clofibrate [15] ; [16] . The present study histopathologically demonstrated the protective effect of bezafibrate on gastric mucosal injury induced by aspirin.
Ranitidine (50 mg/kg) was used as a positive control in the present study. The effect of ranitidine on ulcer index, histopathological scoring, and gastric juice parameters like total acidity, free acidity, total protein, and total hexoses were comparable to those of 100 mg/kg bezafibrate. This effect of ranitidine on aspirin-induced gastric ulcer has also been demonstrated by Nair et al. [29] .
The mechanistic studies with the best effective dose of bezafibrate (100 mg/kg) were also performed by exploring the mucosal lipid peroxidation and antioxidant system, apoptosis pathway, and NO pathway. Bezafibrate attenuated aspirin-induced gastric mucosal injury by significantly inhibiting thiobarbituric acid-reactive substance production, an index of lipid peroxidation, and inducing the antioxidant system, as there were increases in SOD activity, catalase activity and reduced glutathione level in the gastric mucosa. The results of the present study are in accordance with those of Naito et al. [30] . In this study, ranitidine also showed antioxidative effects similar to 100 mg/kg bezafibrate. The antioxidative effect of ranitidine has also been studied by Nair et al. [29] . Previous studies have demonstrated that the therapeutic effects of H2 blockers on peptic ulcers might be related to their antiradical/antioxidant capacity, suggesting that these drugs could be used for treatment of other diseases that are characterized by free-radical-mediated oxidative stress [31] ; [32] ; [33] . Ching et al. [32] showed that H2 blockers were also powerful hydroxyl radical scavengers. Another study showed that H2 blockers had powerful scavenging effects on hypochlorous acid and monochloramine, which arise from inflammatory cells such as neutrophils [33] .
Programmed cell death or apoptosis plays a significant role in gastric ulcer formation as demonstrated in previous studies [5] ; [6] ; [7] . In the present study, apoptosis was seen in the aspirin-treated gastric mucosa as there was a formation of a large number of DNA fragments in agarose gel electrophoresis. However, less DNA fragmentation (apoptosis) was seen in the bezafibrate + aspirin (100 mg/kg) group, as there were fewer bands formed in agarose gel electrophoresis of the gastric mucosa as compared to the aspirin alone group.
One of the mechanisms by which aspirin damages the gastric mucosa is the increased production of NO due to overexpression of iNOS [34] . NO is a mediator not only of gastrointestinal mucosal defense [35] but also of its damage [8] . It has been shown that different concentrations of NO have completely opposite effects on the same tissue [9] . In general, the mucosal and endothelial NOS isoforms produce low amounts of NO. However, the high quantity of NO produced by iNOS damages the epithelium [9] ; [33] ; [36] . The excessive release of NO from gastric epithelial cells induced by aspirin has been reported to exert detrimental effects [37] ; [38] . The present study demonstrates that one of the important mechanisms by which aspirin damages gastric mucosa is increased production of NO due to overexpression of iNOS. The excessive NO release has been reported to exert detrimental effects attributed to reactive nitrogen species, such as oxides of nitrogen and peroxynitrate, which are formed by the reaction of NO with oxygen and superoxide, respectively [39] . The addition of bezafibrate (100 mg/kg) to aspirin attenuated the plasma nitrate level, decreased the expression of iNOS, and prevented the decrease in cNOS expression in gastric mucosa. A similar result was demonstrated by Konturek et al. [40] with pioglitazone, a PPAR γ agonist. The mechanism by which bezafibrate attenuates iNOS expression and preserves cNOS expression remains to be clarified in further studies.
In conclusion, bezafibrate, a PPAR-α agonist, demonstrated dose-dependent antiulcer activity in rats. Bezafibrate also demonstrated antisecretory and gastroprotective action, reduced lipid peroxidation, inhibited iNOS expression, preserved cNOS expression, and inhibited DNA fragmentation in gastric mucosa. All these actions of bezafibrate contribute to its antiulcer effect in a rat model of aspirin-induced gastric ulcer. Therefore, bezafibrate, a hypotriglyceredemic agent, protects against aspirin-induced gastric ulcer in a rat model.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
The present study was funded by the Postgraduate Institute of Medical Education and Research, Chandigarh, India . (No.71/2-Edu/09/968 dated 3.9.2010)
References
- [1] D. Das, R.K. Banerjee; Effect of stress on the antioxidant enzymes and gastric ulceration; Mol Cell Biochem, 125 (1993), pp. 115–125
- [2] T. Mizui, M. Doteuchi; Lipid peroxidation: a possible role in gastric damage induced by ethanol in rats; Life Sci, 38 (1986), pp. 2163–2167
- [3] P.M. Vaananenn, J.B. Medding, J.L. Wallace; Role of oxygen-derived free radicals in indomethacin-induced gastric injury; Am J Physiol, 261 (1991), pp. G470–G475
- [4] J.L. Wallace, D.N. Granger; The cellular and molecular basis of gastric mucosal defense; FASEB J, 10 (1996), pp. 731–740
- [5] K. Kannan, S.K. Jain; Oxidative stress and apoptosis; Pathophysiology, 7 (2000), pp. 153–163
- [6] P.C. Konturek, T. Brzozowski, S.J. Konturek, R. Pajdo, J.E. Konturek, S. Kwiecien, et al.; Apoptosis in gastric mucosa with stress-induced gastric ulcers; J Physiol Pharmacol, 50 (1999), pp. 211–225
- [7] Y. Fuji, T. Matsura, M. Kai, H. Matsui, H. Kawasaki, K. Yamada; Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells ; Proc Soc Exp Biol Med, 224 (2000), pp. 102–108
- [8] M.N. Muscara, J.L. Wallace; Nitric oxide V: therapeutic potential of nitric oxide donors and inhibitors; Am J Physiol, 276 (1999), pp. G1313–G1316
- [9] J.L. Wallace, M.J. Miller; Nitric oxide in mucosal defense: a little goes a long way; Gastroenterology, 119 (2000), pp. 512–520
- [10] S.M. Weisman, D.Y. Graham; Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events; Arch Intern Med, 162 (2002), pp. 2197–2202
- [11] A. Lanas; Prevention and treatment of NSAID-induced gastroduodenal injury; Curr Treat Options Gastroenterol, 9 (2006), pp. 147–156
- [12] S. Shikawa, T. Inaba, M. Mizuno, H. Okada, K. Kuwaki, T. Kuzume, et al.; Incidence of serious upper gastrointestinal bleeding in patients taking non-steroidal anti-inflammatory drugs in Japan; Acta Med Okayama, 62 (2008), pp. 29–36
- [13] L.A. García Rodríguez, S. Hernández-Díaz; Risk of uncomplicated peptic ulcer among users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs; Am J Epidemiol, 159 (2004), pp. 23–31
- [14] P.C. Konturek, J. Kania, E.G. Hahn, J.W. Konturek; Ascorbic acid attenuates aspirin-induced gastric damage: role of inducible nitric oxide synthase; J Physiol Pharmacol, 57 (2006), pp. 125–136
- [15] R. Pathak, M. Asad, H.J. Harishikesheshavan, S. Prasad; Effect of peroxisome proliferator-activated receptor -α agonist (Bezafibrate) on gastric secretion and gastric cytoprotection in rats; Fund Clin Pharmacol, 21 (2007), pp. 291–296
- [16] C.T. Eason, A. Pattison, D.D. Howells, A.J. Spencer, F.W. Bonner; Assessment of gastric antisecretory effects of phenoxyisobutyrate derivatives in the rat and the mouse; Scand J Gastroenterol, 23 (1988), pp. 1063–1071
- [17] P. Guha, A. Dey, B. Sarkar, M.V. Dhyani, S. Chattopadhyay, S.K. Bandyopadhyay; Improved antiulcer and anticancer properties of a trans-resveratrol analog in mice; J Pharmacol Exp Ther, 328 (2009), pp. 829–838
- [18] M. Jainu, K.V. Mohan, C.S.S. Devi; Gastrorotective effect of Cissus quadrangularis extract in rats with experimentally induced ulcer ; Indian J Med Res, 123 (2006), pp. 799–806
- [19] M. Newaz, A. Blanton, P. Fidelis, A. Oyekan; NAD(P)H oxidase/nitric oxide interactions in peroxisome proliferator activated receptor (PPAR) alpha-mediated cardiovascular effects; Mutat Res, 579 (2005) 163–1
- [20] Z. Dische, E.A. Borenfreud; A spectroscopic method for the microdetermination of hexosamine; J Biol Chem, 184 (1950), pp. 517–522
- [21] A.K. Sanyal, P.K. Mitra, R.K. Goel; A modified method to estimate dissolved mucosubstances in gastric juice; Ind J Exp Biol, 21 (1983), pp. 78–80
- [22] O.H. Lowry, N.J. Rosenbrough, A.L. Farr, R.J. Randall; Protein measurement with the folin phenol reagent; J Biol Chem, 193 (1951), pp. 265–275
- [23] H. Ohkawa, N. Ohishi, K. Yagi; Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction; Anal Biochem, 95 (1979), pp. 351–358
- [24] Y. Kono; Generation of superoxide radical during auto-oxidation of hydroxylamine and an assay for superoxide dismutase; Arch Biochem Biophys, 186 (1978), pp. 189–195
- [25] Luck H. Catalase; H.W. Bergmeyer (Ed.), Methods of enzymatic analysis, Academic Press, New York (1963), pp. 885–894
- [26] O.W. Griffith; Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine; Anal Biochem, 106 (1980), pp. 207–212
- [27] L.C. Green, D.A. Wanger, J. Glogowski, P.L. Skipper, J.S. Wishnok, S.R. Tannenbaum; Analysis of nitrate, nitrite, and (15 N) nitrate in biological fluids ; Anal Biochem, 126 (1982), pp. 131–138
- [28] P. Chomczynski, N. Sacchi; Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on; Nat Protoc, 1 (2006), pp. 581–585
- [29] V. Nair, A. Arjuman, H.N. Gopalakrishna, P. Dorababu, P.V. Mirshad, D. Bhargavan, et al.; Evaluation of the anti-ulcer activity of NR-ANX-C (a polyherbal formulation) in aspirin & pyloric ligature induced gastric ulcers in albino rats; Indian J Med Res, 132 (2010), pp. 218–223
- [30] Y. Naito, T. Takagi, K. Matsuyama, N. Yoshida, T. Yoshikawa; Pioglitazone, a specific PPAR (ligand, inhibits aspirin- induced gastric mucosal injury in rats; Aliment Pharmacol Ther, 15 (2001), pp. 865–873
- [31] K. Kedziora-Kornatowska, W. Tkaczewski, J. Blaszcyk, A. Buczynski, J. Chojnacki, J. Kedziora; Effect of the H2- histamine receptor antagonist on oxygen metabolism in some morphotic blood elements in patients with ulcer disease; Hepatogastroenterology, 45 (1998), pp. 276–280
- [32] T.L. Ching, G.R. Haenen, A. Bast; Cimetidine and other H2 receptor antagonists as powerful hydroxyl radical scavengers; Chem Biol Interact, 86 (1993), pp. 119–127
- [33] D. Lapenna, S. De Gioia, A. Mezzetti, L. Grossi, D. Festi, L. Marzio, et al.; Eur J Clin Invest, 24 (1994), pp. 476–481
- [34] P.C. Konturek, J. Kania, E.G. Hahn, J.W. Konturek; Ascorbic acid attenuates aspirin-induced gastric damage: role of inducible nitric oxide synthase; J Physiol Pharmacol, 57 (2006), pp. 125–136
- [35] S. Calatayud, D. Barrachina, J.V. Esplugues; Nitric oxide: relation to integrity, injury, and healing of the gastric mucosa; Microsc Res Tech, 53 (2001), pp. 325–335
- [36] J. Piotrowski, A. Slomiany, B.L. Slomiany; Activation of apoptotic caspase-3 and nitric oxide synthase-2 in gastric mucosal injury induced by indomethacin; Scand J Gastroenterol, 34 (1999), pp. 129–134
- [37] B.J. Whittle; Gastrointestinal effects of nonsteroidal anti-inflammatory drugs; Fund Clin Pharmacol, 7 (2003), pp. 301–313
- [38] D.Z. Hsu, M.Y. Liu; Involvement of nitric oxide in gastric protection of epinephrine in endotoxin intoxication in rats; Toxicology, 204 (2004), pp. 203–208
- [39] D. Lamarque, B.J. Whittle; Involvement of peroxynitrite in the lipid peroxidation induced by nitric oxide in rat gastric mucosa; Eur J Pharmacol, 313 (1996), pp. R5–R7
- [40] P.C. Konturek, T. Brzozowski, J. Kania, S.J. Konturek, S. Kwiecien, R. Pajdo, et al.; Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, accelerates gastric ulcer healing in rat; Eur J Pharmacol, 472 (2003), pp. 213–220
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?