Abstract
A 14 year old patient with short stature, type I diabetes, and cataracts was referred for evaluation of avascular necrosis of the femoral head. Radiography was suggestive of spondyloepiphyseal dysplasia with decreased bone mineral density for age. Targeted molecular and biochemical testing were normal in this patient. Whole exome sequencing was performed and showed compound heterozygosity for previously reported pathogenic GALNS variants which were diagnostic of mucopolysaccharidosis, type IVA (Morquio A).
While this case describes neither a novel condition nor a new mutation, it does illustrate three important points in the diagnosis of patients with atypical forms of MPS IVA. First, that in many instances urine glycosaminoglycan analysis is not sufficient to rule out MPS IVA as a potential diagnosis. Patients in whom biochemical screening is advised should have measurement of leukocyte enzymatic activity. Second, that in patients with radiographic evidence of spondyloepiphyseal dysplasia with additional features or with normal targeted testing, MPS IVA should remain in the differential diagnosis. Third, that whole exome sequencing represents a viable diagnostic platform for evaluation of patients with unknown skeletal or metabolic disease.
Keywords
Mucopolysaccharidosis ; Morquio syndrome ; Whole exome sequencing ; Glycosaminoglycans
1. Introduction
Originally described by Morquio [9] , MPS IVA was noted to consist of skeletal abnormalities, corneal clouding, and cardiac valvular disease. Increased amounts of keratan sulfate were also observed in early reports [9] . It was eventually understood that the biochemical abnormalities of this condition resulted from a deficiency in the galactosamine-6-sulfatase enzyme [1] , which is caused by homozygous or compound heterozygous mutations in the GALNS gene located at 16q24.3 [11] .
This is a very rare disorder, with recent birth prevalence estimates varying by country from 1 in 71,000 to 1 in 500,000. Interestingly, there appears to be wide variability in how incidence and prevalence are reported, and the true incidence of MPS IVA remains uncertain [6] . Skeletal anomalies are a consistent feature of MPS IVA, although the exact nature of the abnormalities vary. Frequently, skeletal findings do not resemble the classic dysostosis multiplex seen in patients with other forms of MPS, complicating diagnosis. Patients with MPS IVA may have skeletal findings which resemble other more common acquired conditions such as Legg-Calve-Perthes disease. Skeletal findings may also be suggestive of a spondyloepiphyseal dysplasia [7] , or multiple epiphyseal dysplasia [15] , which can be particularly challenging in patient who presents with atypical findings of disease, especially if they do not have other compelling features to suggest a mucopolysaccharidosis.
We present a teenage female patient with a history of mildly short stature, type I diabetes, and subcapsular cataracts who was eventually diagnosed with MPS IVA on whole exome sequencing.
2. Clinical report
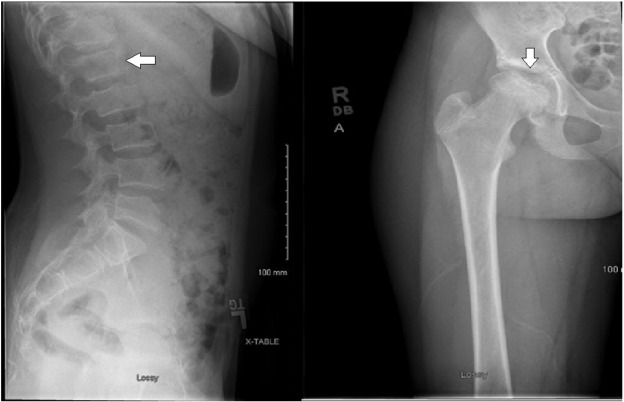
The patient is a now 15-year-old Caucasian female with normal early development, but postnatal onset of short stature with diminished growth velocity, although she did have an increase in growth velocity during her pubertal growth spurt of 9 cm between ages 12.85–13.80 years, which slowed to 5.5 cm between 13.80 years and reaching final adult height of 147.5 cm at 15.0 years (Fig. 1 ). Her adult height is 1.5 standard deviations above the mean for adult females with MPS IVA based on height data published by Montano and colleagues in 2007 [8] . This patient has had no cognitive or educational concerns and is a good student in school. She has above average intelligence and is quite social. She was in good health until eight months before initial presentation when she was diagnosed with type I diabetes and started on a typical insulin regimen. She was also found to have thyroid peroxidase and thyroglobulin antibodies, but has remained clinically euthyroid. Prior to presentation, the patient had been involved successfully with a competitive swimming team, but she began to experience lower back pain and right hip pain. Initially pain occurred with activity, but eventually was present even at rest. Radiography performed at that time showed irregularity of the right proximal femoral epiphysis along the subchondral surface, which was felt to be consistent with avascular necrosis of the femoral head. Similar features were found to a lesser degree on the left femoral head. Irregularity in the vertebral bodies was also noted, but without characteristic beaking of the anterior vertebral bodies. Repeat radiography at the time of presentation confirmed irregularity of the vertebral bodies, which was initially concerning for compression fracture, but later felt to represent platyspondyly (Fig. 2 ).
|
|
|
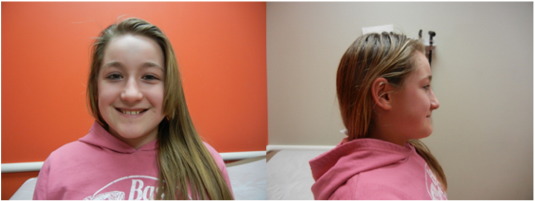
Fig. 1. Patient facial photographs from the front and laterally showing somewhat broad forehead with otherwise nondysmorphic facies and absence of coarseness associated with mucopolysaccharidosis. |
|
|
|
Fig. 2. Lateral lumbar spine radiograph showing end-plate irregularity and platyspondyly denoted by arrow. Right hip film showing somewhat shallow and sclerotic acetabulum with significant femoral head irregularity also denoted by arrow. |
On initial evaluation by metabolic specialist, the patient was noted to have short stature (Z = − 3.1). She was relatively macrocephalic (HC = 53.5 cm, 35th percentile), with somewhat prominent forehead. She did not appear coarse. Palpebral fissures were normal in length, with normal intercanthal distance. Nasal root was slightly high, with a straight nasal bridge, slightly bulbous nasal tip with slightly downhanging columella. All were typical for her family. No midfacial retrusion was noted. Upper and lower lip appeared normal, with good development of the philtrum. Chin was somewhat pointed, but without mandibular prognathism. Ears were normally formed and positioned. The patient exhibited mild rhizomelic shortening, although otherwise she appears fairly proportional.
Dual-energy X-ray absorptiometry (DXA) showed bone mineral density below expected for age with L1-L4 and left femoral neck 2.4 standard deviations below the mean for age. Given her findings, a diagnosis of spondyloepiphyseal dysplasia was entertained, and COL2A1 analysis was performed, which was normal. The patient was started on cyclic bisphosphonate infusions due to her low bone mineral density and pain caused by presumed avascular necrosis of the hip. The patient experienced significant improvement in her bone mineral density with modest improvement in pain. Repeat DXA one year after presentation showing lumbar spine (L1–L4) density at 0.5 standard deviations below the mean for age, and left femoral neck density at 1.2 standard deviations below the mean for age. Additional biochemical evaluation showed normal parathyroid level, vitamin D level. Urine glycosaminoglycans were 7.3 mg/mmol Cr (Normal 2.4–10.2 mg/mmol Cr) measured by spectroscopy. A specimen for leukocyte lysosomal enzyme activity was collected, but was unsatisfactory for analysis on receipt of the testing facility. With continued poor growth, and multiple health concerns including particularly brittle diabetes, cohesive diagnosis was considered important and whole exome sequencing was performed.
3. Mutation analysis
Exome sequencing was performed at Ambry Genetics, Aliso Viejo, California. Samples were prepared using the SeqCAp EZ VCRome 2.0 (Roche NimbleGen). Sequencing was sequenced using paired-end 100 cycle chemistry on the Illumnia HiSeq 2000 or HiSeq 2500. Initial data processing, base calling, alignments, and variant calls are generated by various bioinformatics tools. Data is annotated with the Ambry Variant Analyzer tool (AVA), including: nucleotide and amino acid conservation, biochemical nature of amino acid substitutions, population frequency, and predicted functional impact. Data analysis is focused on small insertions and deletions, canonical splice site alterations, and non-synonymous alterations. Previously described gene mutations and polymorphisms are referenced through the Human Gene Mutation Database, the Single Nucleotide Polymorphism database, 1000 genomes, HapMap data, and searchable literature. Variants are then filtered further based on family history and possible inheritance models. 96.55% of bases for the patient had coverage of greater than or equal to 20 ×, and the mean read depth for the patient was 229.04 ×. 191,735 variants were found in coding regions. A total of 168,015 single nucleotide variants were found, with 11,152 being synonymous, 9998 being missense and 118 other exonic single nucleotide mutations. 548 exonic deletions or insertions were found, with the balance of variants being intronic or intragenic.
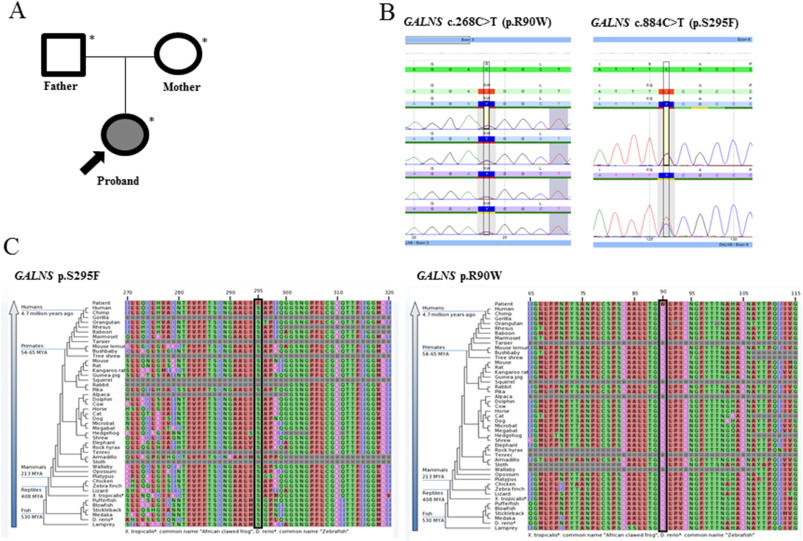
The patient was found to be heterozygous for two variants in the GALNS gene. The first such variant (c.884C > T, p.S295F) was maternally inherited and has been previously described as a pathogenic mutation [12] . The second variant (c.268C > T, p.R90W) was paternally inherited and has also been previously described as a pathogenic mutation [10] and [12] . Both variants have been found in compound heterozygous state in patients of Austrian ethnicity with diagnosis of MPS IVA, and the second variant has been found in compound heterozygous state in a patient of British ethnicity in a patient with documented low GALNS activity. Both are rare with the first variant having an ExAC frequency of 2.5 × 10− 5 . The second variant could not be found in publically available databases, further suggesting its rarity. Both variants occurred at highly conserved locations, in silico models predicted both to be deleterious, and both variants occurred in important functional domains (Fig. 3 ).
|
|
|
Fig. 3. (A) Pedigree. Shaded shapes indicate affected individuals. (B) An electropherogram of the c.884C > T (p.S295F) and c.268C > T (p.R90W) alterations in the proband. (C) Sequence conservation plots at the mutated site amino acid position across different species. |
4. Discussion
Patients with atypical diagnosis of MPS IVA may have significant delays in diagnosis, and it is common for other disorders to be initially considered. Patients with atypical presentation or patients who present later in life may lose some of the more specific features of MPS IVA such as anterior beaking of the vertebral bodies and may manifest platyspondyly, a nonspecific skeletal finding. As in our patient, initial investigation of the patient reported by Mendelsohn et al. was directed toward evaluation of collagenopathies, with sequencing of COL2A1 and COL11A2 and biochemical screening for MPS that were normal. It has been observed previously than in many patients with MPS IVA, urine glycosaminoglycans can be normal, which further complicates a challenging diagnosis. This has been further investigated, with evidence showing that patients with an attenuated form of MPS IVA have lower amount of urine glycosaminoglycan, particularly keratan sulfate (KS) levels, which in some cases were within the range of age-matched controls [2] , making the patients with the most atypical presentation the most challenging to accurately diagnose. Similarly, our patient had normal urine glycosaminoglycan analysis. Her co-diagnosis of type I diabetes also proved to be a confounding factor, as our diagnostic evaluation attempted to (unsuccessfully) incorporate this with her skeletal findings. Ultimately, in this patient with a highly unusual presentation of MPS IVA whole exome sequencing was required to make a definitive diagnosis. Since diagnosis, the patient has been commenced on elosulfase alfa therapy with some improvement in her subjective experience of skeletal pain and no obvious progression of disease.
Patients with MPS IVA, even attenuated or atypical versions of the disease, can have multisystemic health problems which can contribute to morbidity and mortality and may not be present at the time of diagnosis. Recent publication of guidelines for the management of MPS IVA have stressed the need for a cohesive strategy for management of disease including providers from multiple specialties and disciplines [4] and special emphasis is given to treatment in these guidelines given the 2014 approval by the United States Food and Drug Administration of elosulfase alfa for the treatment of patients with MPS IVA. Treatment in this condition is directed toward improvement of functional status of patients, using the six minute walk as an important end-point [3] .
Assessment by the Health-Related Quality of Life (HR-QoL) questionnaire shows significant decreases in quality of life for patients with MPS IVA which appear to mirror their ability for independent mobility. Patients who were ambulatory reported a mean HR-QoL that was significantly higher than those patients who used a wheelchair part of the time, and both scores were significantly higher than those patients who used a wheelchair all of the time [5] . The same study evaluated the mobility of patients and found that less than half of children (44.4%) required use of a wheelchair, while nearly all adults did (85.2%). These findings underscore not only the clinical heterogeneity of MPS IVA, but the fact that it is a progressive condition. Significant clinical variability exists among patients with this condition, with varying compromise of functional ability including ability for ambulation. While there is undoubted some correlation between severity of disease at diagnosis and subsequent clinical course, it is not at all clear that patients who have atypical MPS IVA consistent retain good functional status. For this reason, prompt diagnosis remains important in this group as well.
The first step to adequate treatment of MPS IVA is accurate diagnosis. Tradition in many centers has led to the use of biochemical screening for mucopolysaccharidoses, with glycosaminoglycans as the initial screen of choice. Our experience in this patient and other reported patients with attenuated disease strongly suggest that in a patient who might have attenuated MPS IVA, urine glycosaminoglycans should not be considered a screening modality of choice. It would be suggested that for biochemical screening, the best option would be evaluation of lysosomal enzyme activity on leukocytes as present guidelines suggest [13] . However, such testing requires proper handling and relatively quick transport to the testing facility. Alternately, in patients where a diagnosis of MPS IVA is more likely, it is becoming increasingly more reasonable to move directly to sequencing of the GALNS gene. DNA in blood for molecular analysis is far more stable than for enzyme analysis, which makes it more resistant to delays in handling or shipping. However, this provides its own set of issues as biallelic mutations are not always found patients with diagnosis of MPS IVA based on clinical and biochemical criteria. At least one report suggests that GALNS mutations will not be detected in 14% of alleles [14] .
However, whole exome sequencing has become an increasingly utilized tool in the armamentarium of the clinical geneticist. It remains an expensive diagnostic modality, and in most cases is not indicated as a first-line test. However, costs of this testing on a clinical basis are slowly decreasing, and it remains a reasonable choice for patients in whom a genetic diagnosis is suspected, but the phenotype does not resemble a known syndrome, particular when multiple rounds of biochemical and genetic testing have failed to arrive at a diagnosis.
In conclusion, patients such as this one who present with skeletal findings more suggestive of spondyloepiphyseal dysplasia, but also present with additional findings should be considered as potentially affected by MPS IVA. In such cases, leukocyte lysosomal enzyme levels would be the screening modality of choice. Additionally, in patients with skeletal dysplasias which are not able to be diagnosed radiologically or with targeted molecular testing, whole exome sequencing is a viable platform which is likely to result in diagnosis in a significant proportion of patients.
Compliance with ethics guidelines
Conflict of interest
Eric T. Rush, MD has performed consultative services and received speaker honoraria from Alexion Pharmaceutics, speaker honoraria from Biomarin Pharmaceuticals, and is involved in research which is supported by Amgen, Inc.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Details of the contributions of individual authors
Eric T. Rush, MD is solely responsible for the planning, conduct, and reporting of the work described in the following manuscript.
Funding
No funding was required for the generation of this manuscript.
Acknowledgements
The author thanks the personnel at Ambry Genetics for details of whole exome sequencing and is grateful to the patient and her family for their willingness to participate in this report.
References
- [1] N.M. DiFerrante, L.C. Ginsburg, P.V. Donnelly, et al.; Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses; Science, 199 (1978), pp. 79–81
- [2] V.C. Dũng, S. Tomatsu, A.M. Montaño; Mucopolysaccharidosis IVA: correlation between genotype, phenotype and keratan sulfate levels; Mol. Genet. Metab., 110 (1–2) (2013), pp. 129–138
- [3] C.J. Hendriksz, K.I. Berger, R. Giugliani, et al.; International guidelines for the management and treatment of Morquio A syndrome; Am. J. Med. Genet. A, 167A (1) (2015), pp. 11–25
- [4] C.J. Hendriksz, B. Burton, T.R. Fleming; Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study; J. Inherit. Metab. Dis., 37 (6) (2014), pp. 979–990
- [5] C.J. Hendriksz, C. Lavery, M. Coker; Burden of disease in patients with Morquio A syndrome: results from an international patient-reported outcomes survey; Orphanet J. Rare Dis., 9 (2014), p. 32
- [6] R.M. Leadley, S. Lang, K. Misso, et al.; A systematic review of the prevalence of Morquio A syndrome: challenges for study reporting in rare diseases; Orphanet J. Rare Dis., 9 (1) (2014), p. 173 (18)
- [7] N.J. Mendelsohn, T. Wood, R.A. Olson; Spondyloepiphyseal dysplasias and bilateral legg-calvé-perthes disease: diagnostic considerations for mucopolysaccharidoses; JIMD Rep., 11 (2013), pp. 125–132
- [8] A.M. Montaño, S. Tomatsu, G.S. Gottesman, M. Smith, T. Orii; International Morquio A Registry: clinical manifestation and natural course of Morquio A disease; J. Inherit. Metab. Dis., 30 (2) (Apr. 2007), pp. 165–174 (Epub 2007 Mar 8)
- [9] L. Morquio; Sur une forme de dystrophie osseuse familiale; Bull. Soc. Pediatr. (Paris), 27 (1929), pp. 145–152
- [10] A. Morrone, K.L. Tylee, M. Al-Sayed, A.C. Brusius-Facchin, et al.; Molecular testing of 163 patients with Morquio A (Mucopolysaccharidosis IVA) identifies 39 novel GALNS mutations; Mol. Genet. Metab., 112 (2) (2014), pp. 160–170
- [11] S. Tomatsu, S. Fukuda, M. Masue, et al.; Mucopolysaccharidosis type IVA: characterization and chromosomal localization of N -acetylgalactosamine-6-sulfate sulfatase gene and genetic heterogeneity ; Am. J. Hum. Genet., 51 (1992), p. A178 (suppl.)
- [12] S. Tomatsu, T. Nishioka, A.M. Montaño, et al.; Mucopolysaccharidosis IVA: identification of mutations and methylation study in GALNS gene; J. Med. Genet., 41 (7) (2004), Article e98
- [13] T.C. Wood, K. Harvey, M. Beck, et al.; Diagnosing mucopolysaccharidosis IVA; J. Inherit. Metab. Dis., 36 (2013), pp. 293–307
- [14] S. Tomatsu, A.M. Montano, T. Nishioka, et al.; Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A); Hum. Mutat., 26 (2005), pp. 500–512
- [15] R.S. Lachman, B.K. Burton, L.A. Clarke, S. Hoffinger, S. Ikegawa, D.K. Jin, H. Kano, O.H. Kim, C. Lampe, N.J. Mendelsohn, R. Shediac, P. Tanpaiboon, K.K. White; Mucopolysaccharidosis IVA (Morquio A syndrome) and VI (Maroteaux-Lamy syndrome): under-recognized and challenging to diagnose; Skeletal Radiol., 43 (3) (Mar. 2014), pp. 359–369
Document information
Published on 20/10/16
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?