Abstract
Background
With continued increase in the use of mechanical circulatory support, the incidence of device thrombus remains a challenge. This study is a retrospective analysis of data at a single center to assess the safety and efficacy of thrombolytic use in durable mechanical assist devices.
Methods
Data was analyzed retrospectively from 154 patients who underwent left ventricular assist device (LVAD) implantation from 1/1/2005 to 6/30/2014. The HMII device was implanted in 131 patients while 23 received the HVAD. LVAD thrombus was diagnosed when lactate dehydrogenase levels exceeded 1000 units/l accompanied by clinical signs of hemolysis and heart failure, echocardiographic data and surges in pump power. TPA (tissue plasminogen activator) protocol consisted of a 5 mg intravenous bolus followed by 3 mg/h infusion in normal saline for 10 h. If symptoms persisted another cycle of TPA at 1 mg/h was continued up to 48 h.
Results
The TPA group had a 70% success rate. Success was defined as complete resolution of hemolysis and clinical symptoms with no requirement for LVAD exchange at 30 days. 95% survival was noted at 30 days and 90% were free of a hemorrhagic stroke in the TPA group. The rates of hemorrhagic strokes in the TPA group and the control group were not different (OR = 0.92).
Conclusion
The TPA protocol described here was successful consistently. Though this study is limited by its size and retrospective nature it leads the way for larger studies to generate more robust comparisons between different types of mechanical assist devices as well as the tailored use of thrombolytics in this patient population.
Graphical abstract
Keywords
LVAD thrombus;Thrombolytics;TPA;CF pumps
1. Background
Left ventricular assist devices (LVADs) have rapidly evolved as a standard therapy for end stage heart failure either as bridge to transplant (BTT) or as destination therapy (DT). The continuous-flow LVADS are smaller, less audible and more durable than the older pulsatile pumps. The smaller size allows placement in patients with a smaller body habitus [1]. With the extension of life and the more frequent use of these devices, complications have also evolved in this population of patients. Of these, bleeding and thrombosis have been the two major challenges.
The HeartWare HVAD (centrifugal pump) and the HeartMate II (axial-flow) provide unloading of the left ventricle throughout the cardiac cycle. This property leads to low pulse pressures and predisposition to arteriovenous malformations in the gastrointestinal tract contributing to bleeding. Bleeding is one of the most common adverse events in the first month after implantation [2]. Additionally the shear forces generated by these pumps accentuate the bleeding risk due to acquired von Willebrand disease which is known to resolve after device explant [2]. Passive hepatic congestion secondary to biventricular failure can also predispose these individuals to increased bleeding complications.
Thrombus formation is the other complication that affects the mechanical circulatory support population. Mechanical causes of thrombosis include post-surgical ventricular debris, emboli secondary to clots in the left atrial appendage and endocardial surface of the LV as well as inflow cannula malposition [3]. Inadequate anti-coagulation or anti-platelet therapy can contribute to thrombus formation. Interaction of prosthetic material with blood, leads to significant prolongation of hematologic, inflammatory, or immunologic responses. Prolonged activation of endothelial and coagulation systems after continuous flow-VAD implantation also seem to contribute to observed thrombosis. Intercellular adhesion molecules, E-selectin, tissue factor and D-dimer have all been shown to be up regulated status post-implantation [4] ; [5].
Both HeartWare (HVAD) and HeartMate II (HMII) patients are susceptible to thrombosis. Increasing incidence of pump thrombosis has been well documented in literature [6]; [7] ; [8]. Pump thrombosis can cause life-threatening device malfunction and embolic strokes. Patients with end-stage heart failure also tend to be in a pro-inflammatory state which can promote thrombosis. Multiple mechanisms appear to contribute to pump thrombosis [3]; [4]; [5]; [6]; [7] ; [8].
Pump thrombosis is typically diagnosed by combination of increasing lactate dehydrogenase (LDH)/plasma free hemoglobin, low haptoglobin, hematuria, low flow, cardiogenic shock, inability to unload the left ventricle, pump power surges and sustained power elevations [3]. Imaging modalities, such as CT and echocardiogram, are often used to help identify thrombosis [9] ; [10]. The efficiency of these modalities to visualize the interior aspect of the pump is limited hence the entire clinical picture needs to be used to arrive at the diagnosis. There is limited data on the best therapy for pump thrombosis at present. Recommendations for management vary widely ranging from medical management to catheter-directed thrombolysis and/or pump exchange as the first option [7]; [8]; [9]; [10]; [11]; [12]; [13]; [14]; [15]; [16]; [17]; [18]; [19] ; [20].
The incidence of device thrombosis has been estimated as 2–13% in adult continuous-flow devices [3]; [6]; [8]; [21] ; [22]. Prevention of thrombosis would be the most ideal strategy, which seems far away in the present day. The optimal doses of antiplatelet and anticoagulation treatments are still undefined due to lack of well-designed/controlled clinical trials. Despite a fairly rigid regimen of anticoagulation and antiplatelet measures, pump thrombosis still occurs and has devastating consequences [23]; [24]; [25]; [26] ; [27]. In addition to the existing routinely used regimens, other anticoagulants/antiplatelet agents reported in the literature include dipyridamole, pentoxifylline, dextran, and fluindione [28]; [29]; [30] ; [31].
Several etiologies for pump thrombosis, and the dilemma faced with treatment options, make this an important area of ongoing research [6]; [8]; [22] ; [32]. Though much knowledge has been gained in the field of anticoagulation, it is still difficult to pinpoint the few factors that predispose the patient to a device thrombus. Hence, the definition of a universal robust anticoagulation protocol still remains ambiguous.
As the standard of care in most centers is LVAD exchange which is a procedure associated with a high mortality and morbidity, a trial of TPA thrombolysis may be appropriate prior to such a major surgical undertaking in the absence of any contraindications. This retrospective study therefore seeks to examine results of thrombolytic treatment administered peripherally for LVAD thrombosis in both HVAD and HMII patients at a single center. The specific aims are 1) to determine if device thrombosis can be successfully treated with tissue plasminogen activator (TPA) protocol used in this center 2) to assess complications associated with TPA treatment.
2. Methods
This retrospective study was approved by Institutional Review Board Spokane (IRB#1942) in Spokane, Washington. A retrospective chart review was performed on patients who had either received a HeartMate II LVAD [Thoratec Corp., Pleasanton, CA] or a HeartWare HVAD LVAD [HeartWare Inc., Framingham, MA] for BTT or DT during the time period of January 1st, 2005 to June 30th, 2014. Patients who did not survive implant or received biventricular assist devices were excluded from the analyses. The sample included both men and women, who were 18 years or older. Patients included in this analysis were hemodynamically stable and underwent VAD exchange if they failed TPA treatment. The anticoagulation protocol consisted of aspirin and coumadin as per manufacturers recommendations (target INR of 1.8 to 2.5 for HMII and 2.0 to 3.0 for HVAD). On admission PT/INR, PTT, CBC, complete metabolic panel, fibrinogen and a thromboelastogram with platelet mapping were obtained. A 2-D echocardiogram was also performed on admission.
2.1. Patient population and characteristics
Patient demographics collected included gender, age at LVAD implant, Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile, indication for LVAD (BTT or DT), type of LVAD and history of comorbidities such as ischemic cardiomyopathy, hypertension, diabetes mellitus, peripheral vascular disease, tobacco use, anemia or hypercoagulable syndrome.
Analyses of factors that predispose to LVAD thrombus included length of VAD support, type of LVAD, history of peripheral vascular disease (PVD), tobacco use, hypercoagulable state, anemia, hypertension and diabetes. Additional outcome variables were evaluated for patients who met the criteria for suspected LVAD thrombus, including time to thrombus event, time to the second thrombus event (if applicable), use of TPA, and LVAD exchange. Any differences in time to occurrence of suspected thrombus between the two LVAD types were also evaluated. Adverse events such as renal failure, hemorrhagic CVA, and right ventricular failure were evaluated in conjunction with TPA administration. Success with TPA administration was defined as resolution of hemolysis and no indication for LVAD exchange for 30 days.
3. Study design
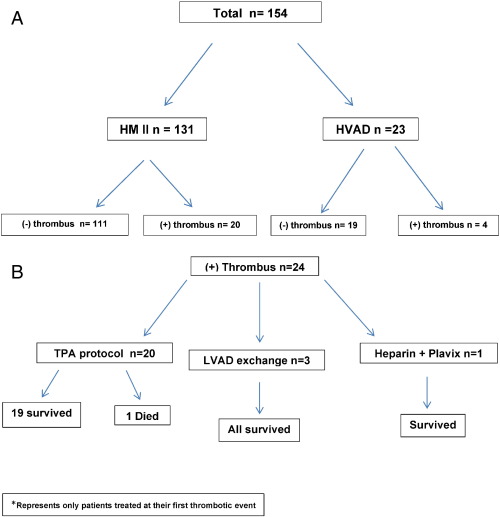
Retrospective analyses were performed on patient charts after IRB approval. The study population comprised of 154 patients who underwent left ventricular assist device (LVAD) implantation (131-HeartMate II and 23-HVAD) from 1/1/2005 to 6/30/2014. They were divided into four groups — HM II with device thrombosis, HVAD with device thrombosis, HM II with no device thrombosis, and HVAD with no device thrombosis (Fig. 1A). A total of 24 patients were diagnosed with LVAD thrombus. Of these, 20 patients received TPA infusions, 3 underwent LVAD exchange and 1 patient was placed on heparin and Plavix (Fig. 1B). Patients who were hemodynamically unstable underwent LVAD exchange.
|
|
|
Fig. 1. A shows the distribution of the two types of continuous flow pumps in the study population. B shows survival at 30 days. |
3.1. Diagnosis of thrombosis
Occurrence of LVAD thrombus was suspected with elevated LDH, hematuria, elevated quantitative plasma free hemoglobin. Diagnosis of LVAD thrombus was made when lactate dehydrogenase levels exceeded 1000 units/l in addition to clinical signs of hemolysis and heart failure and echocardiographic findings listed above. The plasma free hemoglobin was monitored concomitantly but absolute cut off values were not used to make a diagnosis due to high variability in the assay values at this center. A cannula flow velocity of > 2 m/s by echocardiography with more frequent opening of the aortic valve than at baseline in the context of increasing left ventricular internal dimension in diastole (LVIDd) without any manual speed changes was considered suggestive of a thrombus.
3.2. TPA protocol
TPA protocol typically consisted of a 5 mg intravenous bolus followed by 3 mg/h infusion in normal saline for 10 h. In cases where thrombus persisted as defined by laboratory data and clinical signs another cycle of TPA at 1 mg/h was continued up to 48 h. The total TPA dose never exceeded a maximum limit of 100 mg. All TPA infusions were performed via a peripherally inserted central intravenous line in the cardiac intensive care unit.
3.3. Statistical analysis
Descriptive statistics (mean, median, and standard deviation) were used to characterize the patient population, including baseline characteristics, thrombosis rates between devices, and results of TPA treatment and associated events. Success rates for TPA treatment were computed. Inferential statistics were used to test for independence between groups. A t-test was used to compare means for age and time to LVAD thrombosis, with confidence intervals denoted. The Chi-square test or Fischers exact test (for small sample size) was used to test for independence between categorical variables. The Cramers V test was utilized to calculate correlation. Odds ratios (OR) were calculated for categorical data. Statistical tests were performed using SPSS and Excel. Results were considered significant if p < 0.05.
4. Results
4.1. Characteristics of study population
Table 1 outlines the baseline characteristics of the patients. The study population had 21% females and 78.6% males. Ischemic and non-ischemic cardiomyopathies were equally represented. Hypertension and diabetes were noted in 43% and 35% of the study subjects respectively. Only 8.4% of the subjects had peripheral vascular disease. History of tobacco use was noted in 55% of the study population. A higher number of HM II LVADs were placed as compared to HVADs (85.1% versus 14.9%) in the period studied from 2005 to 2014. Approximately one tenth of the patients were in INTERMACS class 1.
| All study subjects (n = 154) | Patients without an LVAD | Patients with an LVAD | |
|---|---|---|---|
| Thrombus event (n = 130) | Thrombus event (n = 24) | ||
| Mean age at implant | 55.8 | 55.6 | 56.9 |
| Gender | 33 (21.4) F, 121 (78.6) M | 27 (20.8) F, 103 (79.2) M | 6 (25) F, 18 (75) M |
| Ischemic CM | 85 (55.2) | 71 (54.6) | 14 (58.3) |
| Non-ischemic CM | 69 (44.8) | 59 (45.4) | 10 (41.7) |
| HTN | 65 (42.2) | 54 (41.5} | 11 (45.8) |
| OM | 54 (35.1) | 44 (33.8) | 10 (41.7) |
| Peripheral vascular disease | 13 (8.4) | 10 (7.7) | 3 (12.5) |
| History of tobacco use | 85 (55.2) | 69 (53.1) | 16 (66.7) |
| HMII | 131 (85.1) | 111 (85.4) | 20 (83.3) |
| HVAD | 23 (14.9) | 19 (14.6) | 4 (16.7) |
| Length of pump support (days) | 550 + 475 | 526 + 494 | 682 + 330 |
| BTT | 110 (71.4) | 94 (72.3) | 16 (66.7) |
| DT | 44(28.6) | 36 (27.7) | 8 (33.3) |
| Pump exchange | 14(9.1) | 4(3.1) | 10 (41.7) |
| GI bleed | 40 (26) | 33 (25.4) | 7 (29.2) |
| Driveline infection | 24 (15.6) | 16 (12.3) | 8 (33.3) |
| INTERMACS Profile 1 | 15(9.7) | 15 (11.5) | 0 |
| INTERMACS Profile 2 | 54 (35.1) | 50 (38.5) | 4 (16.7) |
| INTERMACS Profile 3 | 40 (26.0) | 31 (23.8) | 9 (37.5) |
| INTERMACS Profile 4 | 30 (19.5) | 22 (16.9) | 8 (33.3) |
| INTERMACS Profile 5 | 8 (51.9} | 6 (4.6) | 2 (8.3) |
| INTERMACS Profile 6 | 6 (39) | 5 (3.8) | 1 (4.2) |
| INTERMACS Profile 7 | 1(0.6) | 1 (0.6) | 0 |
F—Female; M—Male.
4.2. Factors influencing VAD thrombus formation
Table 2 outlines factors that could contribute to pump thrombus. However there was no significance between the non-thrombus group and thrombus group patient demographics with respect to age (p = 0.637), gender (p = 0.64), history of ischemic cardiomyopathy (p = 0.73), hypertension (p = 0.7), diabetes mellitus (p = 0.46), peripheral vascular disease (p = 0.44), tobacco use (p = 0.22), and anemia (p = 0.11). Patients in the thrombus group were found to have a history for hypercoagulable syndrome with intermediate association strength (p < 0.000, V = 0.328). There were no significant differences between the non-thrombus group and thrombus group in operative characteristics with respect to LVAD indication for BTT or DT (p = 0.57) or LVAD type (p = 0.80). The INTERMACS profile was not significant in either group (p = 0.09). Patients included had INR within the recommended therapeutic range on presentation.
| (−) Thrombus N = 130 | (+) Thrombus N = 24 | p value | Correlation coefficient | |
|---|---|---|---|---|
| Characteristics | ||||
| Age (years) | 55.60% | 56.40% | 0.637 | 0.037 |
| Gender (female) | 27 | 6 | 0.266 | |
| HMII | 84.70% | 15.30% | 0.796 | 0.021 |
| HVAD | 82.60% | 17.40% | ||
| History of PVD | 7.70% | 12.50% | 0.436 | 0.063 |
| History of Tobacco Use | 53.10% | 66.70% | 0.219 | 0.99 |
| History of Hypercoagulable Syndrome | 0.00% | 12.50% | 0.000 | 0.328 |
| History of Anemia | 10.00% | 0.00% | 0.105 | 0.13 |
| History of Hypertension | 54 (41.5%) | 11 (45.8%) | 0.7 | 0.025 |
| History of Diabetes | 44 (33.8%) | 10 (41.7%) | 0.46 | 0.06 |
4.3. Average time to detection of pump thrombus
The average time to first thrombus event was 394 days (CI 256.6–531.3). The average time to first thrombus event for HMII LVAD patients was 357 days (CI 209.5–503.7). The average time to the first thrombus event for HVAD patients was 581 days (CI 81.6–1079.9). The average time from the first thrombus event to the second thrombus event was 314.7 days (CI 81.4, 548). No statistical significance was noted (Table 3).
| Time to 1st LVAD thrombus (HMII: n = 20, HVAD: n = 4) | Time from 1st to 2nd thrombus (HMII: n = 5, HVAD: n = 2) | |
|---|---|---|
| HMII & HVAD (n = 24) | 394 ± 326 | 315 ± 554 |
| HMII (n = 20) | 357 ± 315 | 382 ± 660 |
| HVAD (n = 4) | 581 ± 360 | 146 ± 124 |
| p = ns |
4.4. Patient outcome variables in the thrombus versus No thrombus groups
Patient outcome variables showed no significant differences between the non-thrombus group and thrombus group in relation to length of LVAD support (p = 0.06), occurrence of GI bleed (p = 0.70), driveline infection (non-thrombus group vs. thrombus group, at any time, p = 0.10), hemorrhagic CVA (p = 0.72), renal failure (p = 0.69), or right ventricular failure (p = 0.24). Analysis of the study groups showed no statistically significant differences in driveline infections or GI bleeding pre- and post-thrombus formation. Approximately 17% (n = 4) of the patients who experienced an LVAD thrombus had a driveline infection prior to the thrombus event, which was not significant in comparison to the non-thrombus patient group (p = 0.35).
4.5. Outcomes and adverse events with TPA protocol versus LVAD exchange
Table 4 ; Table 5 show outcomes and adverse events with TPA treatment versus LVAD exchange. Approximately 83.3% (n = 20) of patients received TPA. 1 patient did not survive until 30 days post-TPA administration. Of the 20 patients who received TPA for a first thrombus event, 10% experienced a related hemorrhagic CVA, 20% experienced renal failure, and 5% experienced right ventricular failure following TPA administration or throughout the remainder of the hospital admission. For those patients who received TPA for a first thrombus event, the corresponding non-thrombus group was more likely to have right ventricular failure (OR: 3.1). Renal failure did not increase in patients who experienced an LVAD thrombus (OR: 0.91). Hemorrhagic CVA did not increase in patients who experienced an LVAD thrombus (OR: 0.92). All the 4 HVAD patients who experienced a first thrombus event received TPA and were treated successfully. Of the 16 HMII LVAD patients who experienced a first thrombus event and received TPA, 63% were treated successfully. Sample size was too small for appropriate statistical analysis. Only 12.5% (n = 3) of patients received an LVAD exchange for the first suspected thrombus event, with all 3 patients surviving post-30 days of surgery. Of the 3 patients who received an LVAD exchange, 0% experienced hemorrhagic CVA, renal failure or right ventricular failure immediately following LVAD exchanges or throughout the remainder of the hospital stay. Sample size was too small for statistical analysis. 4.2% (n = 1) of patients did not receive either TPA or LVAD exchange, but instead received a combination of heparin and clopidogrel.
| 1st thrombus (n = 20) | 2nd thrombus (n = 4) | |
|---|---|---|
| TPA protocol | 83.3% | 57.1% |
| Free of hemorrhagic stroke following TPA | 90.0% | 100.0% |
| Alive at 30 days post-TPA | 95.0% | 50.0% |
| Alive at 1 year post-TPA or ongoing | 75% | 25% |
| 1st thrombus (n = 3) | 2nd thrombus (n = 3) | |
| VAD exchange | 12.5% | 42.9% |
| Free of hemorrhagic stroke following VAD exchange | 100.0% | 100.0% |
| Alive at 30 days post-surgery | 100.0% | 100.0% |
| (−) Thrombus | TPA | LVAD exchange | |||
|---|---|---|---|---|---|
| No thrombus (n = 130) | 1st thrombus event (n = 20) | 2nd thrombus event (n = 4) | 1st thrombus (n = 3) | 2nd thrombus (n = 3) | |
| Hemorrhagic stroke | 9.2% | 10.0% | 0.0% | 0.0% | 0.0% |
| Renal failure | 18.0% | 20.0% | 0.0% | 0.0% | 0.0% |
| RV failure | 14.0% | 5.0% | 25.0% | 0.0% | 33.3% |
Of the 29.2% (n = 7) of patients who experienced a second suspected thrombus event, 4 received TPA and 3 received an LVAD exchange. From the group treated with TPA, 1 of the 4 experienced right ventricular failure. None experienced hemorrhagic CVA or renal failure following TPA administration or throughout the remainder of the hospital admission. 2 patients did not survive until 30 days post-TPA administration. From the group treated with LVAD exchange, 1 of 3 experienced right ventricular failure. None experienced hemorrhagic CVA or renal failure following LVAD exchange or throughout the remainder of the hospital admission.
5. Discussion
This study is different from earlier investigations in that a single defined protocol was used to treat LVAD thrombosis Though the analyses was retrospective, the study showed a 95% survival rate with TPA at 30 days and 75% at 1 year for patients treated with TPA at the first incidence of pump thrombus. The number of patients treated at the second instance of pump thrombus was small, but a drop in survival was noted at 30 days at 50% and only 25% at 1 year. The sample sizes were too small to perform any meaningful statistical analyses. In our limited experience, TPA appeared to be the first choice instead of a pump exchange in hemodynamically stable individuals. A notable aspect of this protocol is that the adverse events were comparable in the thrombus and non-thrombus groups. The odds ratio points to a larger likelihood of developing right ventricular failure in the non-thrombus groups.
Some of the challenges in assessing VAD thrombus problems are the lack of adequate definition of the number of thrombi that are true versus suspected, and the lack of a unified anticoagulation/antiplatelet regimen. Additionally it is unclear if the length of pump support factors into the equation. The studies by Boyle et al. and Slaughter et al. [24] ; [25] showed that patients can be maintained at lower INR and without the heparin bridging for optimal management.
In a recent report by Najjar et al. medical management in general showed a 50% success rate and a 63% success rate with TPA treatment in particular [3]. Schlendorf et al. found a 37.5% success with thrombolytic therapy [16]. Our study shows a success rate of 70% with TPA including both HMII and HVAD patients. It should be noted that our study included a majority of HMII pumps and lower number of HVADS as compared to the by Najjar et al. which focused on the HVADS only as a bridge to transplant. Our study included destination therapy patients also in the analysis.
The hemorrhagic CVA noted in 10% of patients which included those with both HM II and HVAD. Though reports of associations between earlier bleeding events followed by incidence of pump thrombus exist in the literature [33] ; [34] a significant correlation between GI bleeds and pump thrombus events was not found in this retrospective analysis.
Patients with a history of hyper-coagulation syndromes were significantly predisposed to LVAD thrombosis. This is an area where rigorous investigation is required to define levels of anticoagulation. Anticoagulation requirements may be very different in these patients. Besides, hypercoagulability in the setting of heart failure may be more accentuated due to chronic inflammation and up regulation of coagulation factors in heart failure [35] ; [36]. Systematic studies on the pathogenesis of heart failure will shed light on subsets of patients who may have a higher risk for hypercoagulation in the context of up regulation of markers of inflammation, fibrosis, and coagulation. Such tailored risk assessment may be helpful in high risk populations. Better risk stratification will help in better prognostication and management. Genotype driven anticoagulation regimens could be another useful approach in this population [37].
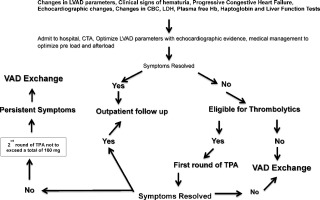
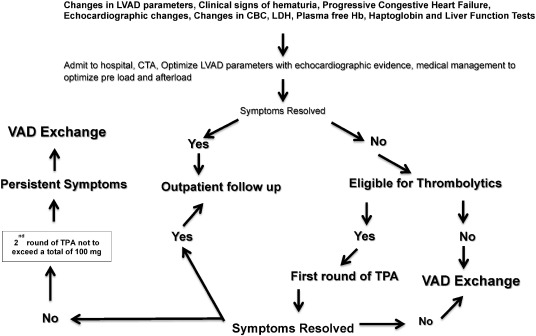
In this single center protocol thrombolytic treatment was used prior to initiating a VAD exchange if the patient had no contraindications. All patients were evaluated carefully for risk versus benefit before initiating a treatment protocol. A protocol for triaging patients with a suspected thrombus is shown in Fig. 2 which differs from the algorithm put forth by the ISHLT working group [26] in that the protocol presented here focuses on thrombolytics. The algorithm shown here is intended to help develop further investigations on these lines in larger studies using different types of mechanical pumps to gain further insight into optimal treatment options and outcome strategies.
|
|
|
Fig. 2. A suggested algorithm for use of thrombolytic therapy in the mechanical assist device population. |
6. Limitations
This is a small retrospective analysis which makes it difficult to perform meaningful statistics especially in terms of long term outcomes in comparison with LVAD exchange. The dilemma in definitively diagnosing a true thrombus versus signs and symptoms suggestive of a thrombus still remains. The data on the second thrombus event is too small for any comparison. The LVAD exchange group is also very small for any head to head comparison with the thrombolytic therapy group.
Being a retrospective study no log file analysis was done on the HVAD patients as this technique was not universally available at the time the patients were implanted. The hypercoagulable state noted in the study patients were only by history. No data was available regarding the types/etiology of the hypercoagulability in these patients. The percentage of patients in atrial fibrillation at the time of presentation could not be deciphered as this data was missing from the data set available for the analysis.
7. Conclusions
This was a retrospective study consisting of patient record analyses of 154 patients who underwent left ventricular assist device (LVAD) implantation (131-HM II and 23-HVAD) from 1/1/2005 to 6/30/2014. A single TPA protocol was used in this study population. The TPA protocol patients showed an overall 70% success including the HMII and HVAD pumps and the survival at 30 days post-thrombolytic therapy was 95%. At one year the survival was 75%. Success with VAD exchange was 100%. Survival at 30 days and 1 year was also a 100% in the VAD exchange population. The patients who underwent VAD exchange directly had contraindications to thrombolytic treatment. However the VAD exchange group was very small to perform any meaningful statistical analyses. A small group of patients underwent TPA treatment versus VAD exchange for a second thrombus event because these patients were poor surgical candidates. As the number of patients in the second thrombus group was small no statistical analyses was performed. Though this is a small retrospective study the TPA protocol presented here, when used appropriately appears to yield acceptable preliminary results in terms of safety and survival. The protocol needs validation in large multicenter trials for further definition and optimization in different patient subsets and varied anticoagulation patterns.
Disclosures
None of the authors have any disclosures that are relevant to this work.
Funding sources
None.
Acknowledgment
This paper is dedicated to the memory of our beloved colleague Dr. David Sandler, an astute cardiothoracic surgeon who dedicated his life to the field of surgical therapies for advanced heart failure.
References
- [1] M.S. Slaughter, F.D. Pagani, J.G. Rogers, et al.; Clinical management of continuous-flow left ventricular assist devices in advanced heart failure; J Heart Lung Transplant, 29 (4S) (2010), pp. S1–S39
- [2] J. Suarez, C.B. Patel, G.M. Felker, R. Becker, A.F. Hernandez, J.G. Rogers; Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices; Circ Heart Fail., 4 (2011), pp. 770–784
- [3] S.S. Najjar, M.S. Slaughter, R.D. Pagani, et al.; An analysis of pump thrombosis events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol; J Heart Lung Transplant, 33 (2014), pp. 23–34
- [4] P.M. Eckman, R. John; Bleeding and thrombosis in patients with continuous-flow ventricular assist devices; Circulation, 125 (2012), pp. 3038–3047
- [5] R. John, S. Panch, J. Hrabe, et al.; Activation of endothelial and coagulation systems in left ventricular assist device recipients; Ann. Thorac. Surg., 88 (2009), pp. 1171–1179
- [6] R.C. Starling, N. Moazani, S.C. Silvestry, et al.; Unexpected abrupt increase in left ventricular assist device thrombosis; N. Engl. J. Med., 370 (2014), pp. 33–40
- [7] G.H. Tang, M.C. Kim, S.P. Pinney, et al.; Failed repeated thrombolysis requiring left ventricular assist device pump exchange; Catheter. Cardiovasc. Interv., 81 (2013), pp. 1072–1074
- [8] M.R. Mehra, G.C. Stewart, P.A. Uber; The vexing problem of thrombosis in long-term mechanical circulatory support; J Heart Lung Transplant., 33 (2014), pp. 1–11
- [9] I. Mohamed, C.T. Lau, Bolen MA, et al.; Building a bridge to save a failing ventricle: radiologic evaluation of short- and long-term cardiac assist devices; Radiographics, 35 (2015), pp. 327–356
- [10] N.M. Fine, Y. Topilsky, Oh. JK, et al.; Role of echocardiography in patients with intravascular hemolysis due to suspected continuous-flow LVAD thrombosis; J. Am. Coll. Cardiol. Img., 6 (2013), pp. 1129–1140
- [11] E. Hohner, J. Crow, M.P. Moranville; Medication management for left ventricular assist device thrombosis; Am. J. Health Syst. Pharm., 72 (2015), pp. 1104–1113
- [12] C.R. Bartoli, G. Ailawadi, J.A. Kern; Diagnosis, nonsurgical management and prevention of LVAD thrombosis; J. Card. Surg., 29 (2014), pp. 83–94
- [13] J.M. Stulak, J. Cowger, J.W. Haft, et al.; Device exchange after primary left ventricular assist device implantation: indications and outcomes; Ann. Thorac. Surg., 95 (2013), pp. 1262–1267
- [14] M.F. Masood, L. Wang, M. Romano, et al.; Efficacy of intravenous tissue plasminogen activator (tPA) in treatment of device thrombus in continuous flow left ventricular assist devices with centrifugal design. (abstract); J. Heart Lung Transplant., 33 (2014), p. S132
- [15] T. Thenappan, A.S. Anderson, V. Jeevanadham, et al.; Treatment of left ventricular assist device thrombosis with extended catheter-directed intraventricular thromobolytic therapy; Circ. Heart Fail., 6 (2013), pp. e27–e29
- [16] K. Schlendorf, C.B. Patel, T. Gehrig, et al.; Thromobolytic therapy for thrombosis of continuous flow ventricular assist devices; J. Card. Fail., 20 (2014), pp. 91–97
- [17] N.K. Kapur, J. Upshaw, M.S. Kiernan, C.T. Pham; Left ventricular assist device thrombosis presenting as an acute coronary syndrome; J. Thorac. Cardiovasc. Surg., 147 (2014), pp. e72–e73
- [18] T. Hasin, S. Deo, J.J. Maleszewski, et al.; The role of medical management for acute intravascular hemolysis in patients supported on axial flow LVAD; ASAIO J., 60 (2014), pp. 9–14
- [19] M.S. Kiernan, D.T. Pham, D. DeNofrio, N.K. Kapur; Management of Heartware left ventricular assist device thrombosis using intracavitary thrombolytics; J. Thorac. Cardiovasc. Surg., 142 (2011), pp. 712–714
- [20] A. Kamouth, R. John, P. Eckman; Successful treatment of early thrombosis of HeartWare left ventricular assist device with intraventricular thrombolytics; Ann. Thorac. Surg., 94 (2012), pp. 281–283
- [21] S.J. Park, C.A. Milano, A.J. Tatooles, et al.; Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy; Circ. Heart Fail., 5 (2012), pp. 241–248
- [22] J.K. Kirklin, D.C. Naftel, R.L. Kormos, et al.; Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device; J. Heart Lung Transplant., 33 (2014), pp. 12–22
- [23] J.P. Slater, E.A. Rose, H.R. Levin, et al.; Low thromboembolic risk without anticoagulation using advanced-design left ventricular assist devices; Ann. Thorac. Surg., 62 (1996), pp. 1321–1327 (discussion 1328)
- [24] A.J. Boyle, S.D. Russell, Teuteberg JJ, et al.; The Heartmate II left ventricular assist device: analysis of outpatient; J. Heart Lung Transplant., 28 (2009), pp. 881–887
- [25] M.S. Slaughter, Y. Naka, R. John, et al.; Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy; J. Heart Lung Transplant., 29 (2010), pp. 616–624
- [26] D.J. Goldstein, R. John, C. Salerno, et al.; Algorithm for the diagnosis and management of suspected pump thrombus; J. Heart Lung Transplant., 32 (2013), pp. 667–670
- [27] M. Rossi, G.F. Serraino, F. Jiritano, A. Renzulli, et al.; What is the optimal anticoagulation in patients with a left ventricular assist device?; Interact. Cardiovasc. Thorac. Surg., 15 (2012), pp. 733–740
- [28] H. Copeland, P.E. Nolan, D. Covington, M. Gustafson, R. Smith, J.G. Copeland; A method for anticoagulation of children on mechanical circulatory support; Artif. Organs, 35 (2011), pp. 1018–1023
- [29] H. Tsukui, A. Abla, J.J. Teuteberg, et al.; Cerebrovascular accidents in patients with a ventricular assist device; J. Thorac. Cardiovasc. Surg., 134 (2007), pp. 114–123
- [30] P.Y. Litzler, H. Smail, V. Barbay, et al.; Is anti-platelet therapy needed in continuous flow left ventricular assist device patients? A single-centre experience; Eur. J. Cardiothorac. Surg., 45 (2014), pp. 55–59
- [31] A. Blitz; Pump thrombosis—a riddle wrapped in a mystery inside an enigma; Ann. Cardiothorac. Surg., 3 (2014), pp. 450–471
- [32] P. Shah, V.M. Mehta, J.A. Cowger, K.D. Aaronson, F.D. Pagani; Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support; J. Heart Lung Transplant., 33 (2014), pp. 102–104
- [33] T. Spanier, M. Oz, H. Levin, A. Weinberg, K. Stamatis, D. Stern, et al.; Activation of coagulation and fibrinolytic pathways in patients with left ventricular assist devices; J. Thorac. Cardiovasc. Surg., 112 (1996), pp. 1090–1097
- [34] J. Linneweber, T.W. Chow, M. Kawamura, J.L. Moake, Y. Nose; In vitro comparison of blood pump induced platelet microaggregates between a centrifugal and roller pump during cardiopulmonary bypass; Int. J. Artif. Organs, 25 (2002), pp. 549–555
- [35] A. Mongirdiene, L. Kursvietiene, A. Kasauskas; The coagulation system changes in patients with chronic heart failure; Medicina (Kaunas), 46 (2010), pp. 642–647
- [36] S.M. Jafri; Hypercoagulability in heart failure; Semin. Thromb. Hemost., 23 (1997), pp. 543–545
- [37] M. Awad, L.C.S. Czer, C. Soliman, A. Mirocha, A. Ruzza, J. Pinzas, et al.; Prevalence of warfarin genotype polymorphisms in patients with mechanical circulatory support; ASAIO J., 61 (2015), pp. 391–396
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?