(Created page with "==Summary== ====Purpose==== Bariatric surgery is an efficient procedure for the remission of type 2 diabetes (T2DM) from morbid obesity. However, in Asian countries, the mea...") |
m (Scipediacontent moved page Draft Content 499069670 to Jin-Kim et al 2013a) |
(No difference)
| |
Latest revision as of 12:22, 26 May 2017
Summary
Purpose
Bariatric surgery is an efficient procedure for the remission of type 2 diabetes (T2DM) from morbid obesity. However, in Asian countries, the mean body mass index (BMI) of T2DM patients is about 25 kg/m2. Various data on patients undergoing gastric bypass surgery suggest that the control of T2DM after surgery occurs rapidly. We hypothesized that even in nonobese patients with T2DM, the levels of incretin and insulin changed along with the improvement of T2DM as a consequence of the gastric bypass.
Materials and methods
From March to December 2011, 12 nonobese patients (mean BMI; 26.2 kg/m2) with poorly-controlled [mean glycated hemoglobin (HbA1C); 9.5%] diabetes underwent gastric bypass surgery. Values related to diabetes, including incretin [gastric inhibitory peptide (GIP) and glucagon-like peptide-1 (GLP-1)] levels were measured before and 1 month after surgery. All values were measured in response to a 75 g oral glucose tolerance test (OGTT).
Results
On average, the BMI decreased by 2.1 ± 0.7 kg/m2. Mean HbA1C level decreased by 1.6 ± 2%. Oral glucose-stimulated insulin levels increased and GLP-1 levels also increased significantly. Oral glucose-stimulated GIP levels decreased sharply.
Conclusion
Soon after gastric bypass in nonobese T2DM patients, control of T2DM is achieved. The incretin release after oral glucose is improved. This could be a consequence of changes of the enteroinsular axis, particularly in the incretins.
Keywords
gastric bypass;incretin;metabolic surgery;type 2 diabetes mellitus
1. Introduction
The number of people with type 2 diabetes mellitus (T2DM) is increasing at an alarming rate in every country. In 2011, the global prevalence of T2DM was estimated at 366 million (8.3%) of the adult population (age 20–79 years). It is expected to rise to 552 million (9.9%) by 2030.1
In Western countries, the association between obesity and T2DM is well established, because 90% of patients with T2DM show excess body weight.2 However, according to a recent survey by the Ministry of Health and Welfare of Korea, the mean body mass index (BMI) of T2DM patients in Korea is 24.9 ± 3.3 kg/m2.3
Some types of weight loss surgery, especially Roux-en-Y gastric bypass (RYGB) and biliopancreatic diversion (BPD), generally result in a loss of about 70% of excess body weight (68.1% and 72%, respectively) and cure diabetes in > 80% of the patients.4 It is noted that glycemic control after weight loss surgery often occurs before a significant weight loss.5 The recovery of T2DM is due not only to weight loss and decreased food intake, but also to other factors closely related to the rerouting of the gastrointestinal tract, the exclusion of the duodenum and jejunum, and rapid transit of food to the distal part of the small intestine.6; 7 ; 8
It has been proposed that the enteroinsular axis could be a major part of the mechanism related to antidiabetic effects of certain types of bariatric surgery.9 The enteroinsular axis means the connection between the gut and the pancreatic islets. The hormonal part of the axis, known as “incretin”, is released by nutrients and stimulates insulin secretion. The two main incretins are glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (gastric inhibitory peptide, GIP).10 ; 11
Both GLP-1 and GIP enhance meal-related insulin secretion, known as the “incretin effect”.12; 13; 14; 15 ; 16 In normal individuals, the release of both GLP-1 and GIP contributes to the stimulation of insulin secretion after a meal and to store glucose in the peripheral tissues. However, in all T2DM patients, the loss of this effect, defined as enteroinsular impairment, is characteristic.17 GLP-1 levels are blunted. Contrary to GLP-1, GIP levels are normal or overproduced, but the effect of GIP on insulin secretion is blunted.18
Several studies resulted in successful treatment of T2DM in nonmorbidly obese diabetes patients after single anastomosis gastric bypass surgery.19 ; 20 However, none of these studies measure incretins such as GLP-1 and GIP, or report the incretin effect on insulin secretion in nonmorbidly obese patients. We aimed to investigate the changes of the incretin levels in response to oral glucose in nonmorbidly obese T2DM patients before and 1 month after single anastomosis gastric bypass.
2. Patients and methods
The inclusion criteria for patients in this prospective study were the following: aged 20–65 years, BMI > 23.0 kg/m2 but < 30.0 kg/m2 (normal to overweight BMI based on the WHO definition), fasting C-peptide ≥ 1.0 ng/mL, and glycated hemoglobin (HbA1C) > 7.0% after 6 months of medical treatment. We have not stated the duration of diabetes limits. The exclusion criteria were: type 1 diabetes mellitus, any contraindication to general anesthesia, any history of previous gastrointestinal surgery, and the inability to comply with study protocols or regular follow-up. All patients gave informed consent and this prospective study was approved by the institutional review board for Human Research of Soonchunhyang University Hospital (Number 2011-69).
2.1. Single anastomosis gastric bypass
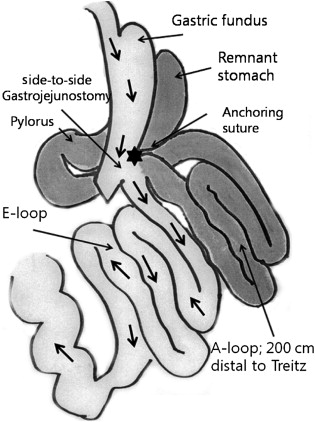
All patients underwent a laparoscopic single anastomosis gastric bypass with the 5-trocar method: four 12 mm trocars and one 5 mm trocar. At first, a long vertical and relatively large gastric tube was made by using endo-staplers (Endo GIA 60 mm Articulating Medium/Thick Reload with Tri-Staple Technology, Covidien Autosuture, Mansfield, MA, USA) along the lesser curvature from 2 cm proximal to the pylorus aiming towards the gastric fundus. We did not consider determining the exact size of the gastric pouch. The volume of the gastric pouch was roughly adjusted according to the patients BMI. We preserved the gastric fundus in every patient, because there is no need to restrict food intake in nonmorbid obese patients and because we need to secure the simplicity and reversibility of the procedure. We divided the stomach from the distal lesser curvature that is 2 cm proximal to the pylorus and made a vertical gastric tube. In patients with BMI > 25 kg/m2, we made a gastric tube from the distal lesser curvature, passing the upper part of the gastric angle, aiming towards the gastric fundus. In patients with BMI < 25 kg/m2, we made a gastric tube aiming towards the gastric upper body. After the formation of the vertical gastric pouch, the small intestine, 200 cm distal from the ligament of Treitz, was anastomosed with a gastric pouch using an endo-stapler (Endo GIA 45 mm Articulating Vascular/Medium Reload with Tri-Staple Technology, Covidien Autosuture) in a side-to-side, antecolic, isoperistaltic fashion. Consequently, part of the gastric antrum, duodenum, and proximal jejunum were bypassed and a side-to-side gastrojejunostomy was made (Fig. 1). An anchoring suture was made between the gastric pouch and the afferent limb for acute angulation of the afferent limb, maintaining a parallel line between the long gastric pouch and the efferent limb. An intraoperative leak test was performed using air and blue dye, to confirm a negative leak at the anastomosis.
|
|
|
Figure 1. Illustration of single anastomosis gastric bypass surgery. The surgeon made a vertical gastric tube and anastomosed it with jejunum 200 cm distal from the ligament of Treitz. |
2.2. Measurement of insulin, C-peptide and incretin after 75 g oral glucose tolerance test
2.2.1. Insulin secretion after oral glucose load
All patients underwent 2-hour oral glucose tolerance tests (OGTTs) with 75 g of glucose in a total volume of 225 mL before and 1 month after surgery. Blood samples, collected in chilled EDTA tubes with added aprotinin (500 kallikrein inhibitory units/mL blood) and dipeptidyl-peptidase IV (DPPIV) inhibitor (Linco Research Inc., St. Charles, MO, USA) (10 μL/mL blood), were centrifuged at 4°C before being stored at –70°C. Plasma concentrations of glucose, insulin, C-peptide, total and active GLP-1, and GIP were measured at 0, 15, 30, 60, 90, and 120 minutes during the 75 g OGTT.
2.2.2. Incretin assay
GLP-1, an indicator of secretion, was measured by ELISA [Multispecies GLP-1 Total, Millipore (Linco), Billerica, MA, USA] after plasma ethanol extraction. The intra-assay and inter-assay coefficients of variation (CVs) were < 5% and < 12%, respectively. Active GLP-1, an indicator of the potential action, and total GIP were measured by the multiplexing method [Milliplex MAP Human Gut Hormone Panel, Millipore (Linco)]. The intra-assay and inter-assay CVs were < 11% and < 19%, respectively.
2.3. Statistical analysis
The outcome variables were serum glucose and plasma insulin, C-peptide, GLP-1, and GIP concentrations. Data are presented as means ± SD unless otherwise stated. The total area under the curve (AUC) values 0–120 minutes for outcome variables were calculated using the trapezoidal method. Wilcoxon signed rank-sum tests were used to compare the data from before and after the surgery. Statistical significance was set at p < 0.05. Data were analyzed using the SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patients' characteristics
The patients' characteristics are shown in Table 1. Twelve patients (7 males and 5 females), with a mean age of 45.4 years (range 28–63 years), with T2DM of 9.1 ± 8 years duration (range 1–25 years) were studied before and 1 month after single anastomosis gastric bypass surgery. The mean BMI was 26.2 ± 2.1 kg/m2 (range 23.1–30.0 kg/m2). Two patients were treated with insulin and the others with more than one oral hypoglycemic agent.
| Before surgery | After surgery | p (Δ) | |
|---|---|---|---|
| Age (y) | 45.4 ± 10.2 | ||
| Duration (y) | 9.1 ± 8.2 (1–25) | ||
| Weight (kg) | 73.2 ± 7.8 | 66.7 ± 7.2 | 0.002 |
| BMI (kg/m2) | 26.2 ± 2.1 | 24.1 ± 2.2 | 0.002 |
| HbA1C (%) | 9.5 ± 1.6 | 7.9 ± 1.0 | 0.045 |
| Fasting glucose (mg/dL) | 170.3 ± 45.1 | 171.0 ± 62.4 | 0.875 |
| Fasting insulin (μIU/mL) | 15.8 ± 11.4 | 12.6 ± 5.8 | 0.347 |
| HOMA-IR | 6.35 ± 4.37 | 5.23 ± 2.61 | 0.469 |
| 120-min glucose (mg/dL) | 317.2 ± 57.4 | 232.3 ± 103.3 | 0.041 |
| Peak insulin (μIU/mL) | 49.3 ± 50.0 | 91.9 ± 75.2 | 0.136 |
| AUC glucose (mg/dL min−1) | 281.7 ± 31.7 | 252.4 ± 80.9 | 0.308 |
| AUC insulin (uIU/mL min−1) | 36.0 ± 36.0 | 57.0 ± 49.0 | 0.239 |
| AUC total GLP-1 (pmol/L min−1) | 27.6 ± 13.4 | 59.1 ± 17.1 | <0.0001 |
| AUC active GLP-1 (pg/mL min−1) | 108.0 ± 63.0 | 311.0 ± 178.0 | <0.0001 |
| AUC GIP (pg/mL min−1) | 184.0 ± 70.0 | 98.0 ± 35.0 | 0.002 |
Data are mean ± SD. Peak (during 120-minute value) and AUC (total area under the curve, 120 minutes) values were obtained during the 75 g OGTT.
Δ = difference between pre- and post-gastric bypass surgery. BMI = body mass index; GIP = gastric inhibitory peptide; GLP-1 = glucagon-like peptide-1; HbA1C = glycated hemoglobin; HOMA-IR = homeostasis model assessment-insulin resistance.
3.2. Side effects
No patients experienced severe adverse effects during OGTTs. No patients experienced stomach cramping or discomfort, nausea, sweating, flushing or palpitations early after the ingestion of glucose.
3.3. Changes in body weight and HbA1C levels
The average body weight of the 12 patients was reduced. The difference in body weight between pre- and post-gastric bypass surgery was −6.5 ± 2.1 kg in the 1st month (from 73.2 ± 7.8 kg to 66.7 ± 7.2 kg, p = 0.002). The difference in BMI between pre- and post-gastric bypass surgery was –2.1 ± 0.7 kg/m2 in the 1st month (from 26.2 ± 2.1 kg/m2 to 24.1 ± 2.2 kg/m2).
One month after surgery, the HbA1C levels decreased significantly (from 9.5 ± 1.6% to 7.9 ± 1.0%, p = 0.045).
3.4. Changes in requirements for antidiabetic medications
Notably, at 1 month after surgery, no patients needed insulin injections. Six patients, including two patients who had used insulin for glucose control, used a decreased dosage of antidiabetic medications after surgery.
3.5. Glucose and insulin levels during the OGTT
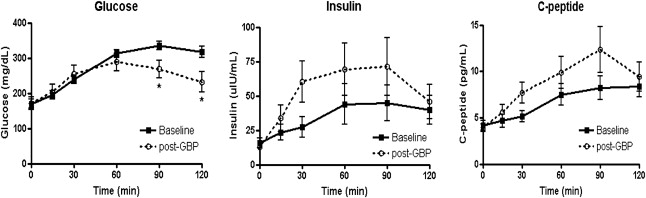
Changes in the outcome variable after surgery are shown in Table 1. Fasting glucose levels did not change, while 120-minute glucose levels decreased significantly. Peak insulin levels and C-peptide levels tended to be more enhanced than those at baseline (Fig. 2). The AUC of glucose levels were lowered 1 month after surgery, and the AUC of insulin 1 month after surgery tended to be higher than those at baseline, although the difference was not significant. The homeostasis model assessment-insulin resistance (HOMA-IR), indicator of insulin resistance, decreased slightly. However, there was no statistical significance.
|
|
|
Figure 2. Glucose, insulin, and C-peptide concentrations during oral glucose tolerance tests (OGTTs). Fasting glucose levels did not change, while 120-minute glucose levels decreased significantly. Peak insulin levels and C-peptide levels tended to be more enhanced than those at baseline. Data are presented as mean ± SD. GBP = gastric bypass. ∗p < 0.05, significant difference between values at the same time into the OGTTs before and after surgery. |
3.6. GLP-1 and GIP levels during the OGTT
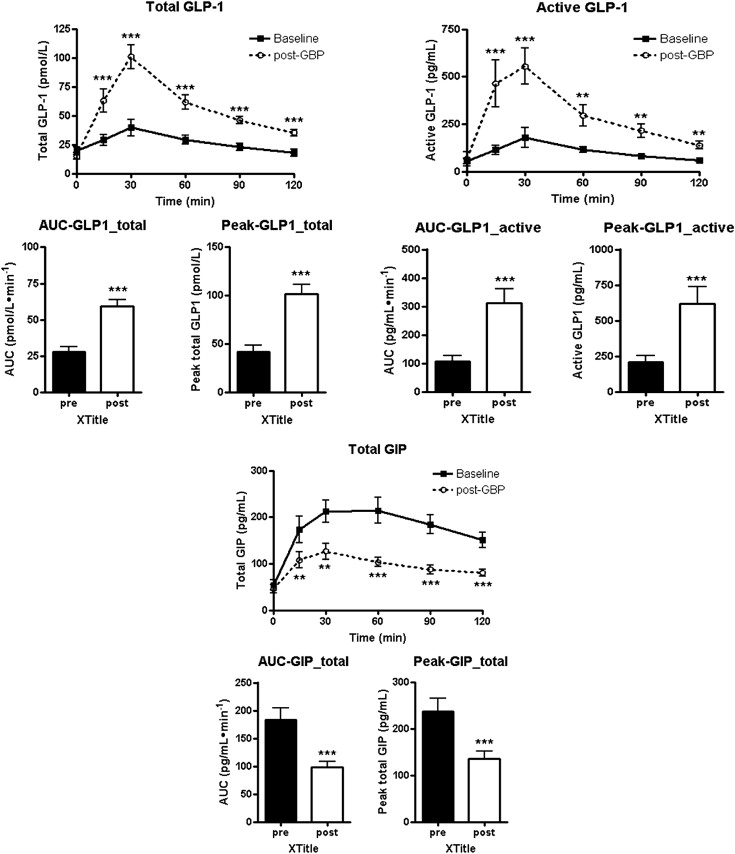
The levels and AUC of total GLP-1 and the levels and AUC of active GLP-1 increased significantly 1 month after surgery. The levels and AUC of GIP decreased significantly 1 month after surgery (Fig. 3). All changes occurred regardless of the duration of T2DM.
|
|
|
Figure 3. Time course for the changes in mean total glucagon-like peptide-1 (GLP-1), active GLP-1, and gastric inhibitory peptide (GIP) levels and the AUC during an oral glucose tolerance test (OGTT) before and 1 month after surgery. The levels and AUC of total glucagon-like peptide-1 (GLP-1) and the levels and AUC of active GLP-1 increased significantly 1 month after surgery. The levels and AUC of gastric inhibitory peptide (GIP) decreased significantly 1 month after surgery. Data are presented as mean ± SD. ∗p < 0.05, significant difference between values at the same time into the OGTTs before and after surgery. |
4. Discussion
Type 2 DM is a chronic metabolic disease which leads to a number of complications. Lifelong diabetes control is mandatory.21 ; 22 However, the current glycemic control with antidiabetic medications and intensive lifestyle intervention is unsatisfactory.
Studies in a diabetic rat model suggest that the exclusion of the upper gut, rather than weight loss, benefits glucose tolerance.23 This improvement in glucose tolerance was shown to be independent of weight and food intake. These rodent studies underline potential mechanisms by which diabetes can be improved after bariatric surgery and support a role of the gut incretins in glucose tolerance after RYGB.9
The achievement of glycemic control, along with significant excess weight loss, has been reported in diabetic patients with morbid obesity after bariatric surgery such as RYGB or BPD.24 ; 25 It was thought that the incretins could play a major role in the marked immediate improvements of diabetes control before weight loss is observed after bariatric surgery.26
Evidence is growing that gastrointestinal bypass operations involving the rerouting of the GI tract can induce T2DM remission, independent of any weight loss, in a proportion of patients, and this phenomenon can occur even in the nonmorbidly obese patient.27 ; 28 The GI tract plays an important role in energy regulation, and many gut hormones are involved in the regulation of glucose metabolism,29 ; 30 also known as the incretin effect. In addition, recent data suggest that high glycemic index foods may lead to a hormonally hyperactive proximal gut and a hypoactive distal gut, which are linked to metabolic syndrome.31 It has been shown that GIP is overproduced at the proximal gut and its response to food is downregulated in T2DM patients.32 By contrast, the production of GLP-1,33 mainly distal gut hormones, is deficient. It is suggested that an excessive proximal absorption due to dietary interventions, such as refining or liquefying, could be the cause for these enterohormonal disorders.31 GLP-1 is now being used in the clinic to enhance insulin secretion and improve glycemic control in T2DM. However, the GLP-1 analogs have several side effects, such as weight loss and GI discomfort.
We need to look at the change of GIP secretion after the surgery. GIP is released from intestinal K cells and the majority of these are located in the foregut-duodenum and proximal part of the jejunum. GIP is secreted in response to nutrients ingested, especially glucose or fat.34 We provided complete exclusion of the foregut, so there was minimal chance to stimulate K cells in the duodenum and proximal jejunum by ingested nutrients after the surgery.
A further important consideration is the significance of decreased GIP secretion. Many studies provide unequivocal support for the role of GIP as an incretin hormone and suggest a role of GIP in an early pathophysiologic step that could lead to impaired glucose tolerance and T2DM.35 However, in T2DM, plasma glucagon fails to decrease properly or, paradoxically, can even increase after oral glucose ingestion.36 This lack of glucagon suppression contributes significantly to postprandial hyperglycemia in T2DM patients.37 ; 38 Also, GIP is unable to further amplify the insulinotropic and glucose-lowering effects of GLP-1 in T2DM patients. Besides, the suppression of glucagon by GLP-1 is antagonized by GIP.39 Therefore, decreased postprandial GIP level is much more helpful to control hyperglycemia.
We can find another importance of decreased GIP from the BPD procedure. While RYGB does not affect insulin resistance, but increases insulin secretion via the stimulation of nutrient-mediated incretin secretion, BPD induces a full recovery of insulin resistance. Part of the results observed after BPD can be attained by the reduction of GIP secretion and the simultaneous increase of GLP-1.40
Interestingly, the action of incretin has a greater effect on lowering serum glucose in Asian diabetic patients with BMI < 30 kg/m2.41 Also, a recent gene study demonstrated a nonsynonymous polymorphism of the GIP gene, which is shown in East Asians more commonly compared to other ethnic groups.42 In other words, there could have been ethnical differences in secretion and action of incretin.
The relationship between the bypassed limb length and the T2DM remission is clear.43 No extreme weight loss occurred in nonobese cases, and the patients BMI should not be the only factor when considering surgery. Thus, nonobese diabetic patients should also be candidates for metabolic surgery.19; 44; 45 ; 46
This study was conducted in 12 Korean T2DM patients, whose range of BMI was from 23.1 kg/m2 to 30.0 kg/m2. This ranges from upper normal to overweight, based on the WHO definition of obesity. However, this ranges from overweight to mild obesity according to the Asian criteria of obesity. Most patients were able to advance to their usual diet without early satiety 2 weeks after the surgery. This could be achieved because of larger and longer gastric pouches relative to RYGB. The length of the bypassed limbs was 200 cm from the ligament of Treitz. There were no nutritional side effects, such as severe diarrhea, hypoproteinemia, or malnutrition. Also, we were able to achieve procedural safety and simplicity by performing single anastomosis. The achievement of reversibility was also possible. The small gastric pouch in the classic loop gastric bypass of Mason was high in the abdomen, close to the gastroesophageal junction. This could lead to esophagitis because of bile refluxes.47 It has been considered as ulcerogenic, or even potentially oncogenic. However, we placed the jejunal loop much lower around the stomach, just proximal to the pyloric ring. Also, we made the anchoring suture at the mid-portion of the gastric pouch with the jejunum, so that we were able to make parallel lines between the pouch and the efferent loop. No patients suffered from a gastric ulcer after the surgery. We reduced bile refluxes through more physiologic positions of the anastomosis and the direction of bile flow.
All 12 patients showed significantly increased GLP-1 levels and decreased GIP levels 1 month after the surgery, regardless of the duration of T2DM. Similar changes are seen after malabsorptive procedures such as BPD.
We need to discuss the possible reasons why the fasting blood glucose remained unchanged 1 month after the surgery. There are two major causes of increased fasting blood glucose in T2DM patients. One is impaired secretion of insulin and the other is increased endogenous glucose production. Pancreatic beta cell masses proliferate in response to GLP-1. It might take a few years for beta cells to proliferate in response to GLP-1.48 After improving secretion of insulin, the fasting plasma glucose would be reduced. One month after the surgery was a short period to show changes in serum glucose profiles in nonmorbid obese patients. However, what we mainly wanted to highlight in this study was that the changes of incretin levels in nonmorbid T2DM patients had occurred soon after the surgery. Also, human bodies react to physical stress such as surgery and trauma by increasing metabolism. That is, there are phenomena such as increased glycolysis, gluconeogenesis, proteolysis, or lipolysis in liver, muscle, and adipose tissue to react to physical stress and to retain homeostasis. Glucagon release also increases. As a result, blood glucose levels can be increased soon after the surgery, unless regulatory hormones act properly.
By contrast, postprandial serum glucose level has shown to be decreased soon after the surgery in nonmorbid T2DM patients. We think this is because a rapid decrease of GIP secretion occurs after the surgery. It has become known that decreased GIP secretion plays a part in glucose control through suppression of glucagon after meal.36 ; 37
Patients showed a slight decline in weight immediately after the surgery because of the decreased food intake and caloric restrictions. However, a long-term evaluation of weight change is needed, since the patients recovered the amount of intake about 1–2 weeks after the surgery without early satiety.
This study has several limitations. The sample size was small and there was no control group. The control group could have been another surgical group or a group treated with other medical interventions, such as a low caloric diet, GLP-1 agonists, or DPP-IV inhibitors. Also, small changes in body weight owing to the decreased intake and caloric restrictions could affect the change of incretin levels. In addition, other possible mechanisms due to the anatomical rearrangement of the procedure, e.g., alteration of bile flow, rerouting of the gastrointestinal tract, etc., could affect the results. A diet-controlled, matched long term study with a larger series needs to be conducted in the future.
These data clearly showed significant increased insulin secretion and changes in incretin levels in nonmorbid obese patients. The results tended to be independent of the duration of diabetes. There is a positive relation between the duration of disease and the changes in total GLP-1 levels (r = 0.428, p = 0.166) and GIP (r = 0.145, p = 0.653), but there is no significance. However, there is a positive relation between the duration of disease and the change in active GLP-1 (r = 0.622, p = 0.031). The single anastomosis gastric bypass (SAGB) surgery might be able to induce early beneficial hormonal changes and to be a very efficient surgical therapy for rapid glycemic control in nonmorbidly obese patients with T2DM.
Our major findings are a significant increase of GLP-1 and a decrease of GIP, along with improved diabetes control soon after surgery in nonmorbidly obese T2DM patients. We are going to identify long-term changes of these data excluding the effects of diet and weight loss. We are also going to follow up for a longer period, to reveal the durability of these changes.
5. Conclusion
Soon after the single anastomosis gastric bypass in nonobese T2DM patients, control of T2DM could be achieved. The enteroinsular impairment of T2DM is markedly improved. Early diabetes control could be a consequence of changes in the enteroinsular axis, particularly in the incretins.
Acknowledgments
This work was supported in part by the Soonchunhyang University Research Fund. This work was presented at the International Federation for the Surgery of Obesity and Metabolic disorders (IFSO) 2012 XVII World Congress India.
References
- 1 IDF Diabetes Atlas (5th ed.), International Diabetes Federation, Brussels (2011) http://www.idf.org/diabetesatlas
- 2 D.P. Guh, W. Zhang, N. Bansback, Z. Amarsi, C.L. Birmingham, A.H. Anis; The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis; BMC Public Health, 9 (2009), p. 88
- 3 Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey, the Ministry of Health and Welfare (2010) http://knhanes.cdc.go.kr
- 4 H. Buchwald, Y. Avidor, E. Braunwald, et al.; Bariatric surgery: a systematic review and meta-analysis; JAMA, 292 (2004), pp. 1724–1737
- 5 G. Mingrone, A. DeGaetano, A.V. Greco, et al.; Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids; Diabetologia, 40 (1997), pp. 599–605
- 6 W.J. Pories, R.J. Albrecht; Etiology of type 2 diabetes mellitus: role of the foregut; World J Surg, 25 (2001), pp. 527–531
- 7 F. Rubino, M. Gagner; Potential of surgery for curing type 2 diabetes mellitus; Ann Surg, 236 (2002), pp. 554–559
- 8 E.G. Scott, L.G. Frank, K. Stanley; Effects of obesity surgery on non-insulin-dependent diabetes mellitus; Arch Surg, 137 (2002), pp. 1109–1117
- 9 B. Laferrere, J. McGinty, S. Heshka, et al.; Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes; Diabetes Care, 30 (2007), pp. 1709–1716
- 10 H.U. Roger, M.E. Anna; Entero-insular axis; Arch Intern Med, 123 (1969), pp. 261–266
- 11 W. Creutzfeldt; The incretin concept today; Diabetologia, 16 (1979), pp. 75–85
- 12 M.J. Perley, D.M. Kipnis; Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects; J Clin Invest, 46 (1967), pp. 1954–1962
- 13 F. Preitner, M. Ibberson, I. Franglin, et al.; Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors; J Clin Invest, 113 (2004), pp. 635–645
- 14 J.K. Timothy, F.H. Joel; The glucagon-like peptides; Endocr Rev, 20 (1999), pp. 876–913
- 15 M.G. Fiona, W. Leanne, K.S. Anna, R. Frank; A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line; Diabetes, 52 (2003), pp. 1147–1154
- 16 J.M. Juris, A.N. Michael, E.S. Wolfgang, G. Baptist; Gastric inhibitory polypeptide: the neglected incretin revisited; Regul Pept, 107 (2002), pp. 1–13
- 17 W. Creutzfeldt; The entero-insular axis in type 2 diabetes – incretins as therapeutic agents; Exp Clin Endocrinol Diabetes, 109 (suppl 2) (2001), pp. S288–S303
- 18 R. Fetner, J. McGinty, C. Russell, X.P. Sunyer, B. Laferrere; Incretins, diabetes, and bariatric surgery: a review; Surg Obes Relat Dis, 1 (2005), pp. 589–598
- 19 W.J. Lee, W. Wang, Y.C. Lee, M.T. Huang, K.H. Ser, J.C. Chen; Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI > 35 kg/m2 and < 35 kg/m2; J Gastrointest Surg, 12 (2008), pp. 945–952
- 20 M. Garcia-Caballero, M. Valle, J.M. Martinez-Moreno, et al.; Resolution of diabetes mellitus and metabolic syndrome in normal weight 24–29 BMI patients with one anastomosis gastric bypass; Nutr Hosp, 27 (2012), pp. 623–631
- 21 E. Shafrir; Development and consequences of insulin resistance: lessons from animals with hyperinsulinemia; Diabete Metab, 22 (1997), pp. 131–148
- 22 B. Detournay, S. Cross, B. Charbonnel, et al.; Managing type 2 diabetes in France: the ECODIA survey; Diabete Metab, 26 (2000), pp. 363–369
- 23 F. Rubino, M. Gagner, P. Gentileschi, et al.; The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism; Ann Surg, 240 (2004), pp. 236–242
- 24 N. Scopinaro, G. Marinari, G.B. Camerini, et al.; Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study; Diabetes Care, 28 (2005), pp. 2406–2411
- 25 T. Tejirian, C. Jensen, E. Dutson; Bariatric surgery and type 2 diabetes mellitus: surgically induced remission; J Diab Sci Technol, 2 (2008), pp. 685–691
- 26 R. Fetner, F.X. Pi-Sunyer, C.D. Russell, B. Laferrère; Incretins, diabetes and bariatric surgery: a review; Term Med, 6 (2005), pp. 589–597
- 27 J.E. Varela; Bariatric surgery: a cure for diabetes?; Curr Opin Clin Nutr Metab Care, 14 (2011), pp. 396–401
- 28 C.K. Huang, A. Shabbir, C.H. Lo, C.M. Tai, Y.S. Chen, J.Y. Houng; Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25 kg/m2–35 kg/m2; Obes Surg, 29 (2011), pp. 1344–1349
- 29 F. Rubino, S.L. R'bibo, F. del Genio, M. Mazumdar, T.E. McGraw; Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus; Nat Rev Endocrinol, 6 (2010), pp. 102–109
- 30 F. Rubino, A. Forgione, D.E. Cummings, et al.; The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes; Ann Surg, 244 (2006), pp. 741–749
- 31 S. Santoro, L.C. Castro, M.C. Velhote, et al.; Sleeve gastrectomy with transit bipartition. A potent intervention for metabolic syndrome and obesity; Ann Surg, 256 (2012), pp. 104–110
- 32 T. Vilsboll, T. Krarup, J. Sonne, et al.; Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus; J Clin Endocrinol Metab, 88 (2003), pp. 2706–2713
- 33 T. Vilsboll, T. Krarup, C.F. Deacon, et al.; Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients; Diabetes, 50 (2001), pp. 609–613
- 34 L.L. Baggio, D.J. Drucker; Biology of incretins: GLP-1 and GIP; Gastroenterology, 132 (2007), pp. 2131–2157
- 35 Y.M. Cho, C.E. Merchant, T.J. Kieffer; Targeting the glucagon receptor family for diabetes and obesity therapy; Pharmacol Ther, 135 (2012), pp. 247–278
- 36 F.K. Knob, T. Vilsboll, S. Madsbad, J.J. Holst, T. Krarup; Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus; Diabetologia, 50 (2007), pp. 797–805
- 37 S. Dinneen, A. Alzaid, D. Turk, R. Rizza; Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM; Diabetologia, 38 (1995), pp. 337–343
- 38 P. Shah, A. Vella, A. Basu, R. Basu, W.F. Schwenk, R.A. Rizza; Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus; J Clin Endocrinol Metab, 85 (2000), pp. 4053–4059
- 39 N. Mentis, I. Vardarli, L.D. Kothe, et al.; GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes; Diabetes, 60 (2011), pp. 1270–1276
- 40 G. Mingrone; Role of the incretin system in the remission of type 2 diabetes following bariatric surgery; Nutr Metab Cardiovasc Dis, 18 (2008), pp. 574–579
- 41 Y.G. Kim, S. Hahn, T.J. Oh, S.H. Kwak, K.S. Park, Y.M. Cho; Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis; Diabetologia, 56 (2013), pp. 696–708
- 42 C.L. Chang, J.J. Cai, C. Lo, J. Amigo, J.I. Park, S.Y. Hsu; Adaptive selection of an incretin gene in Eurasian populations; Genome Res, 21 (2011), pp. 21–32
- 43 Y.H. Kao, C.H. Lo, C.K. Huang; Relationship of bypassed limb length and remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass; Surg Obes Relat Dis, 8 (6) (2012 Nov–Dec), pp. e82–e84 http://dx.doi.org/10.1016/j.soard.2011.10.011. [Epub 2011 Oct 30]
- 44 G. Mingrone, L. Castagneto-Gissey; Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery; Diabete Metab, 35 (2009), pp. 518–523
- 45 M. Fried, G. Ribaric, J.N. Buchwald, S. Svacina, K. Dolezalova, N. Scopinaro; Metabolic surgery for the treatment of type 2 diabetes in patients with BMI < 35 kg/m2: an integrative review of early studies; Obes Surg, 20 (2010), pp. 776–790
- 46 W.J. Lee, K. Chong, C.Y. Chen, et al.; Diabetes remission and insulin secretion after gastric bypass in patients with body mass index < 35 kg/m2; Obes Surg, 21 (2011), pp. 889–895
- 47 R. Rutledge; The mini-gastric bypass: experience with the first 1274 cases; Obes Surg, 11 (2011), pp. 276–280
- 48 M.C. Bunck, A. Corner, B. Eliasson, et al.; Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes; Diabetes Care, 34 (2011), pp. 2041–2047
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?