(Created page with "==Abstract== ====Background==== Categorization as a cytochrome P450 (CYP) 2C19 poor metabolizer (PM) is reported to be an independent risk factor for cardiovascular disease....") |
m (Scipediacontent moved page Draft Content 403813801 to Akasaka et al 2017a) |
(No difference)
| |
Latest revision as of 11:21, 19 May 2017
Abstract
Background
Categorization as a cytochrome P450 (CYP) 2C19 poor metabolizer (PM) is reported to be an independent risk factor for cardiovascular disease. Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid by CYP2C19 epoxygenases and anti-inflammatory properties, especially in microvascular tissues. We examined the impact of CYP2C19 polymorphisms and EETs on the patients with microvascular angina (MVA) caused by coronary microvascular dysfunction.
Methods and results
We examined CYP2C19 genotypes in patients with MVA (n = 81). MVA was defined as absence of coronary artery stenosis and epicardial spasms, and the presence of inversion of lactic acid levels between intracoronary and coronary sinuses in acetylcholine-provocation test or the adenosine-triphosphate-induced coronary flow reserve ratio was below 2.5. CYP2C19 PM have two loss-of-functon alleles (*2, *3). We measured serum dihydroxyeicosatrienoic acid (DHET) as representative EET metabolite.
In MVA, the patients with CYP2C19 PM were 34.6% and high sense C-reactive protein (hs-CRP) levels in CYP2C19 PM were significantly higher than that of non-PM group (0.165 ± 0.116 vs. 0.097 ± 0.113 mg/dL, P = 0.026). Moreover, DHET levels in CYP2C19 PM were significantly lower than that of non-PM (10.4 ± 4.58 vs. 15.6 ± 11.1 ng/mL, P = 0.003 (11,12-DHET); 12.1 ± 3.79 vs. 17.3 ± 6.49 ng/mL, P = 0.019 (14,15-DHET)).
Conclusions
The decline of EET owing to CYP2C19 variants may affects coronary microvascular dysfunction via chronic inflammation.
Keywords
CYP2C19;Genetic polymorphism;Microvascular angina;Epoxyeicosatrienoic acid;Chronic inflammation
1. Introduction
Microvascular angina (MVA) is caused by coronary microvascular dysfunction, which can arise from chronic inflammation [1]; [2] ; [3]. Epoxyeicosatrienoic acids (EETs) have been suggested to be anti-inflammatory [4] and potent vasodilators in the canine coronary microcirculation [5], raising the possibility that low levels of EETs may cause microvascular dysfunction in humans.
EETs are endothelium-derived hyperpolarizing factors (EDHFs) generated from metabolism of arachidonic acid by cytochrome P450 (CYP) 2C19 epoxygenases [6] ; [7].
Endothelial hyperpolarization mediated by the opening of the potassium and calcium (KCa) channels is originally, recognized as the important initiating step for EDH mediated vasodilation [8]. Node et al. reported that EETs prevented leukocyte adhesion to the vascular wall by a mechanism involving inhibition of transcription factor, nuclear factor-κB and IκB kinase. The inhibitory effects of EETs were independent of their membrane-hyperpolarizing effects, suggesting that these molecules play an important non-vasodilatory role in vascular inflammation. Thus, EETs have been suggested to be anti-inflammatory properties [4]. Moreover, EETs act as potent vasodilators in canine coronary microcirculation [5]. It is associated that low levels of EETs may cause microvascular dysfunction via inflammation in humans.
Previous evidence has suggested that CYP enzymes not only play an important role in vascular tone, but are also associated with a higher risk of cardiovascular disease [9]; [10]; [11]; [12] ; [13]. CYP2C19 is expressed in human endothelial cells, and enzymatic activity varies according to the number of CYP2C19 loss-of-function (LOF) alleles (*2, *3). CYP2C19 poor metabolizers (PM) have two LOF alleles (*2/*2, *2/*3, or *3/*3), and non-PM have none or one LOF allele (*1/*1, *1/*2, or *1/*3) [8] ; [14].
Bertrand-Thiebault et al. reported that the blood levels of inflammatory markers (interleuikin-6 and hs-CRP) were significantly increased in CYP2C19 PM [15]. Furthermore, CYP2C19 variants are an independent risk factor for diabetic retinopathy [16] and coronary artery disease (CAD) [17] in humans. It is associated that the low levels of EETs with CYP2C19 variants cause increases in inflammatory markers, diabetic retinopathy, and CAD.
Thus, we examined the incidence and impact of CYP2C19 variants in MVA by measuring serum hs-CRP and dihydroxyeicosatrienoic acid (DHET) as representative EET metabolite in patients with MVA.
2. Methods
2.1. Study population
From April 2009 to January 2015, 1489 consecutive, hemodynamically and symptomatically stable patients with suspected angina were registered and underwent angiography in Kumamoto University Hospital. We excluded patients with possible heart failure (left ventricular ejection fraction < 50%), previous diagnoses of CAD, left ventricular hypertrophy (defined as > 12-mm left ventricular wall thickness in echocardiography), severe hypertension (HT) (> 160/110 mm Hg), valvular heart disease, and malignant diseases. In addition, patients with elevated white blood cell counts (> 9000) and/or serum hs-CRP (> 0.5 mg/dL) were excluded to avoid the potentially confounding effects of occult infection or other systemic inflammatory diseases on hs-CRP levels.
After filtering, 1356 patients were enrolled in this study. Vasoactive drugs, including calcium-antagonists, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and nitrates were withdrawn at least 5 days before patients entered this study; sublingual nitroglycerin was withdrawn within 24-h before entering the study. Patients with HT were defined as > 140/90 mm Hg. Patients with diabetes mellitus (DM) were defined as those with a 2-h glucose tolerance test ≥ 200 mg/dL, a fasting glucose level ≥ 126 mg/dL, an HbA1c score ≥ 6.5%, physician-diagnosed diabetes and/or the use of diabetic medication, and chronic kidney disease (CKD) testing with an estimated glomerular filtration rate < 60 mL/(min · 1.73 m2). Dyslipidemia (DLP) was defined as low-density lipoprotein > 140 mg/dL, high-density lipoprotein < 40 mg/dL, or triglyceride > 150 mg/dL.
All procedures will be conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol has been approved by the human ethics committee of each institution and written informed consent will be obtained from each patient or from the family of the patient.
2.2. Definition of MVA
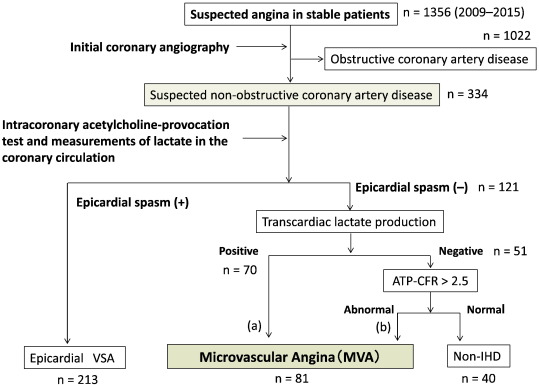
All MVA cases experiencing chest pain and ischemic changes in the electrocardiogram (ECG) in the exercise stress test underwent cardiac catheterization. We defined the MVA was not only without epicardial spasm but also lactic acid levels between intracoronary and coronary sinuses in ACh-provocation test were increase, or adenosine triphosphate-induced coronary flow reserve ratio (ATP-CFR) was below 2.5 if lactic acid levels were decrease (Fig. 1) [2]; [18] ; [19].
|
|
|
Fig. 1. Diagnostic flowchart. The definition of the patients with MVA is shown. ATP-CFR, adenosine triphosphate–induced coronary flow reserve; VSA, vasospastic angina; IHD, ischemic heart disease. |
Acetylcholine-provocation test and measurement of the ATP-CFR ratio were conducted according to the Japanese Circulation Society guidelines [20]. Measuring ATP-CFR scores was also used to diagnose microvascular coronary dysfunction in non-obstructive CAD [21] ; [22].
2.3. Intracoronary acetylcholine-provocation test
The method of intracoronary ACh-provocation test was described in detail previously [23]; [24]; [25] ; [26]. In brief, incremental doses (20, 50, and 100 μg) of ACh chloride were injected into the left coronary artery over a period of 30 s each, and CAG was performed 1 min after the start of each provocation. The doses of ACh were administered at 5-min intervals.
Subsequently, 50 μg of ACh was injected into the right coronary artery without the FloWire™, followed by CAG. After the administration of intracoronary isosorbide mononitrate (ISDN) and CAG at an interval of 10 min, adenosine triphosphate (ATP; 150 μg/kg per minute) was administered via the central vein until maximal hyperemia was achieved for the calculation of ATP-CFR. ATP-CFR was calculated with the following formula: Hyperemia average peak velocity (APV)/Post-ISDN APV [27].
2.4. Angiographical identification of epicardial spasm
A positive finding for coronary spasm on CAG in the ACh test is defined as “transient, total, or sub-total occlusion (> 90% stenosis) of a coronary artery.” In addition, a definite diagnosis of vasospastic angina requires simultaneous signs or symptoms of myocardial ischemia (angina pain and ischemic ECG change).
2.5. Lactate measurement in ACh-provocation test
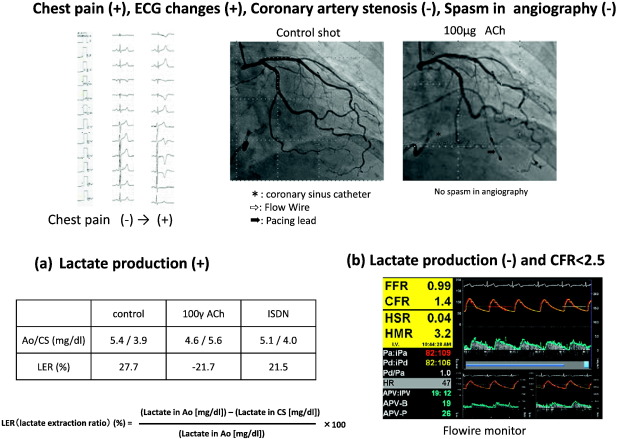
To assess myocardial ischemia on the basis of the detection of increased lactate production in the coronary circulation, paired blood samples were collected simultaneously from the aortic root (LAR) and the coronary sinus (LCS) by using a coronary sinus catheter at 3 time points: baseline, 1 min after delivery of 100 μg of ACh via the left coronary artery, and after administration of ISDN. The lactate production ratio was calculated with the following formula: (LAR–LCS)/LAR × 100 (%).The ratio is positive in healthy subjects while a negative ratio is a definite marker of myocardial ischemia [25].
2.6. Genotyping
Genomic DNA was extracted from whole blood using the DNA Extractor WB kit (Wako Pure Chemical Industries, Ltd., Japan), using a modified version of the protocol described by Richards et al. [28] CYP2C19*2 (681G > A) and *3 (636G > A) are thought to account for > 99% of LOF alleles generating null-activity enzymes in the Japanese population [29] ; [30]. Therefore, subjects were divided according to their CYP2C19 genotypes into two groups: PM with two LOF alleles (*2/*2, *2/*3, or *3/*3) and non-PM, consisting of EM (extensive metabolizer) lacking CYP2C19*2 and CYP2C19*3 (CYP2C19*1/*1) and IM (intermediate metabolizer) with one LOF allele (*1/*2 or *1/*3).
2.7. 11,12 and 14,15-DHET measurement
We measured serum 11,12 and 14,15- DHET in patients with MVA (n = 42) with agreement. Blood samples were collected simultaneously from the coronary sinus by using a coronary sinus catheter. DHET is a representative metabolite of soluble epoxide hydrolase-mediated metabolism of EETs, and reflects the levels of EET. Serum 11,12 and 14,15-DHET levels were measured using an ELISA kit (Detroit R&D, Inc.,USA).
Mixture of 1.0 mL serum and 1.0 mL ethyl acetate was centrifuged at 2000 rpm for 10 min. Upper organic phases were collected and evaporated to dryness with nitrogen gas. Dried sample was dissolved in 2 mL 20% KOH and incubated for 1 h at 50 °C. Formic acid was added to adjust pH to 5.5, then equal amount of ethyl acetate was applied and centrifuged at 2000 rpm for 10 min at room temperature. The upper phase was collected and dried up again. We reconstituted collected sample with 20 μL, the proceeded to the ELISA reaction.
2.8. Statistical analyses
Continuous variables are expressed as mean ± SD and compared using an unpaired t-test or Mann-Whitneys U test, as appropriate. Categorical variables were expressed as numbers or percentages and compared using the chi-squared test or Fishers exact test. P-values < 0.05 were regarded as statistically significant. SPSS software, Version 21.0 (IBM Institute Inc., USA) was used for all statistical analyses.
3. Results
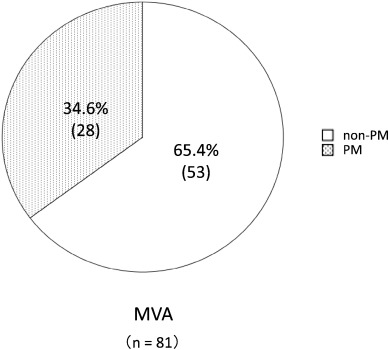
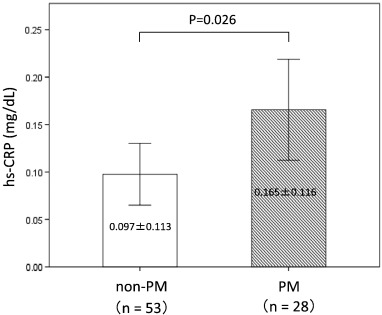
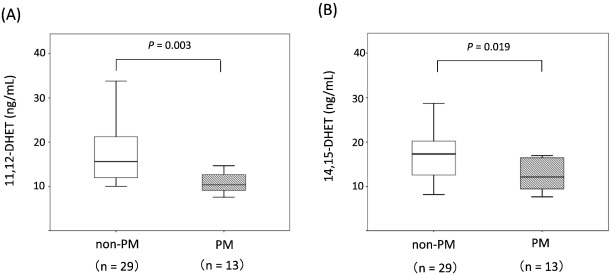
A total of 1356 patients were enrolled in this study, and 334 patients were defined as non-obstructive coronary artery disease after initial coronary angiography. Finally, 81 patients were defined as having MVA after an acetylcholine-provocation test, the measurement of lactate in the coronary circulation, and ATP-provocation (Fig. 1 ; Fig. 2). In the clinical characteristics, there is no significant differences between CYP2C19 PM group and non-PM group (Table 1). Fig. 3 shows that the incidence rates of CYP2C19 genotypes (PM and non-PM) were 34.6% and 65.4%, respectively, in the MVA patients. The levels of hs-CRP were significantly higher in CYP2C19 PM group than that of non-PM group (0.165 ± 0.116 vs. 0.097 ± 0.113 mg/dL, P = 0.026) (Fig. 4). In the levels of serum 11,12 and 14,15-DHET, the levels of serum 11,12 and 14,15-DHET in CYP2C19 PM were significantly lower than that of non-PM (10.4 ± 4.58 vs. 15.6 ± 11.1 ng/mL, P = 0.003 (11,12-DHET); 12.1 ± 3.79 vs. 17.3 ± 6.49 ng/mL, P = 0.019 (14,15-DHET) (Fig. 5A,B).
|
|
|
Fig. 2. Cases of MVA. (a) No coronary artery stenosis in angiography, no epicardial spasms and have inversion of lactic acid levels between intracoronary and coronary sinuses in ACh-provocation test. (b) No coronary artery stenosis in angiography, no epicardial spasms and without inversion of lactic acid levels between intracoronary and coronary sinuses (no transcardiac lactate production) in ACh-provocation test and adenosine triphosphate-induced coronary flow reserve ratio (ATP-CFR) was below 2.5. The transcardiac lactate production ratio then becomes positive during coronary circulation. LER, lactate extraction ratio; Ao, aorta; CS, coronary sinus. |
| Non-PM n = 53 | PM n = 28 | P-value | |
|---|---|---|---|
| Age (years) | 64.1 ± 11.5 | 66.1 ± 17.9 | 0.573 |
| Male (%) | 29 (53.7) | 9 (32.1) | 0.063 |
| BMI (kg/m2) | 24.2 ± 4.27 | 22.8 ± 3.35 | 0.207 |
| Body weight | 62.4 ± 11.92 | 59.9 ± 13.58 | 0.293 |
| Current smoker (%) | 6 (12.0) | 4 (14.3) | 0.792 |
| Hypertension (%) | 32 (59.3) | 19 (67.9) | 0.446 |
| Dyslipidemia (%) | 33 (61.1) | 13 (46.4) | 0.204 |
| Diabetes mellitus (%) | 14 (25.9) | 4 (14.3) | 0.227 |
| CKD (%) | 13 (24.1) | 6 (21.4) | 0.788 |
Data are means ± SDs.
BMI, Body mass index; CKD, Chronic kidney disease.
|
|
|
Fig. 3. Prevalence of CYP2C19 genotypes in MVA patients. |
|
|
|
Fig. 4. Comparison of hs-CRP levels between the CYP2C19 PM and non-PM. In the MVA group, the mean hs-CRP level for CYP2C19 PM is significantly higher than that of non-PM. |
|
|
|
Fig. 5. Comparison of serum 11,12 and 14,15-DHET levels. Comparison of non-PM and PM in the MVA group. The level of 11,12 and 14,15-DHET in CYP2C19 PM is significantly lower than that of non-PM. The box plots show the minimum, 25% quartile, median, 75% quartile, and maximum for each group. |
4. Discussions
Our results show that the decline of EET owing to CYP2C19 PM is one possible cause of MVA via chronic inflammation.
In this study, the incidence rates of CYP2C19 PM were 34.6% in patients with MVA. In general, in among Caucasians, the group with CYP2C19 PM was 2–3%, and in Asians, including Japanese subjects, the rate was approximately 15–20% [9]; [10]; [12]; [13] ; [31]. That is possible that CYP2C19 PM impacts with the pathogenesis of MVA.
The hs-CRP level was significantly higher in CYP2C19 PM group than that of the non-PM group. The decreased enzyme activity of CYP2C19 PM, which decreases the level of EETs, appears to lead to increased inflammation and impaired microcirculation. Measurement of 11,12- and 14,15-DHET levels revealed that EETs were significantly lower in CYP2C19 PM group than that of non-PM group. These results are consistent with a link between the low EET increase associated with decreased enzyme activity and the expansion of inflammation and impaired microcirculation in the CYP2C19 PM group. Spiecker et al. reported low EET levels in a subgroup with decreased enzymatic activity by CYP2J2 genetic mutations compared with another subgroup with normal enzymatic activity by wild CYP2J2 genotype among the CAD patients [32]. CAD is defined as the presence of epicardial organic stenosis in above studies, the definition of MVA, which was different from the definition of MVA in this study, but the lower EET levels in groups with LOF alleles (CYP2C19*2 and *3, CYP2J2*7) for patients with MVA and CAD suggest that EET has some effect in cases of epicardial or microcirculation.
Although there are several DHET isomers (8,9-DHET, 11,12-DHET, and 14,15-DHET) from EETs [33], we measured the levels of serum 11,12 and 14,15-DHET in this study. 11,12-DHET constitutes 24% of DHETs and14,15-DHET are 39% (largest percentage) [34]. The same result were shown in not only 11,12-DHET but also 14,15-DHET, as reported previously [32].
Microvascular dysfunction was reportedly observed in approximately 20% of patients who complained of chest pain but who had no significant organic stenosis in the epicardial coronary artery [1]. In the present study, to define MVA, not only did we perform catheterization examinations in all the subjects but we also performed acetylcholine provocation testing, measurement of lactic acid and ATP stress tests. Based on these results, we rigorously diagnosed the condition of the patients and obtained accurate results, we rigorously diagnosed the condition of the patients and obtained accurate results with no contradictions. From the group of patients in the present study who complained of chest pain and did not present with epicardial stenosis, MVA was diagnosed in approximately 24.2% of the patients, which was consistent with the rate reported by previous studies [1] ; [4].
5. Limitations
This study has a small sample size. Because we performed both cardiac catheterization and ACh and ATP provocation tests for all enrolled subjects to define MVA, the definition of MVA in this study is more accurate than that in studies in which subjects are not subjected to ACh and ATP provocation tests. Second, CYP2C19 PM are present in 2–3% of Caucasians, whereas they are found in < 20% of Asians, including Japanese subjects [9]; [10]; [12]; [13] ; [31]. The subjects in the present study were limited to the Japanese patients because there is a racial difference in CYP2C19 genotype and thus the results of this study may not be necessarily applicable to other populations. Thirds, we have no discuss about medical therapy, especially in clopidogrel. However, there is no patients with clopidogrel therapy in MVA group. Therefore, there is no influence on microvascular dysfunction of clopidogrel therapy in this study. Forth, we consider that it is desirable to measure EET, EET + DHET, EET/DHET ratio. In the present study, we used ELISA kit, it was difficult to measure EET itself concentration levels because preserved blood was hydrolyzed. We consider and discuss this study under the consideration that DHET is equal to EET, this consideration was also a study limitation.
6. Clinical implications
The variant of CYP2C19 genotype was associated with MVA in the Japanese. This study thus identified reduced DHET which reflects EET levels and hence insufficient defensive mechanism as a risk factor to be targeted and intervened for the treatment and prevention of MVA. Recently, John D. Iming et al. showed that a novel molecule inhibitor of epoxide hydrolase (EH) inhibited the activity of EH and effectively restored the EET concentration in animal models [35]. Accordingly, it is expected that this class of drug may serve as a new therapeutic for MVA in the future.
7. Conclusions
Our results indicate that the decline of EET owing to CYP2C19 variants may affects coronary microvascular dysfunction via chronic inflammation.
Funding sources
This study was supported in part by grants-in-aid for the Japan Heart Foundation, Tokyo, and the Japan Vascular Disease Research Foundation, Kyoto, Japan.
Disclosures
None.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
We wish to thank medical secretaries C. Yamamoto and R. Usui (Kumamoto University) for data collection.
References
- [1] M.A. Marinescu, A.I. Löffler, M. Ouellette, L. Smith, C.M. Kramer, J.M. Bourque; Coronary microvascular dysfunction, microvascular angina, and treatment strategies; JACC Cardiovasc. Imaging, 8 (2015), pp. 210–220
- [2] F. Crea, P.G. Camici, C.N.B. Merz; Coronary microvascular dysfunction: an update; Eur. Heart J. (2013), p. eht513 http://doi.org/10.1093/eurheartj/eht513
- [3] A. Recio-Mayoral, O.E. Rimoldi, P.G. Camici, J.C. Kaski; Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease; JACC Cardiovasc. Imaging, 6 (2013), pp. 660–667
- [4] K. Node, Y. Huo, X. Ruan, B. Yang, M. Spiecker, K. Ley, et al.; Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids; Science, 285 (1999), pp. 1276–1279
- [5] C.L. Oltman, N.L. Weintraub, M. VanRollins, K.C. Dellsperger; Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation; Circ. Res., 83 (1998), pp. 932–939
- [6] W.B. Campbell, J.R. Falck; Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors; Hypertension, 49 (2007), pp. 590–596
- [7] B.N. Zordoky, A.O. El-Kadi; Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases; Pharmacol. Res., 125 (2010), pp. 446–463
- [8] B. Fisslthaler, R. Popp, L. Kiss, M. Potente, D.R. Harder, I. Fleming, et al.; Cytochrome P450 2C is an EDHF synthase in coronary arteries; Nature, 401 (1999), pp. 493–497
- [9] S. Hokimoto, M. Mizobe, T. Akasaka, Y. Arima, K. Kaikita, K. Nakagawa, et al.; Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation; Thromb. Res., 133 (2014), pp. 599–605
- [10] M. Mizobe, S. Hokimoto, T. Akasaka, Y. Arima, K. Kaikita, K. Morita, et al.; Impact of CYP2C19 polymorphism on clinical outcome following coronary stenting is more important in non-diabetic than diabetic patients; Thromb. Res., 134 (2014), pp. 72–77
- [11] D. Sueta, S. Hokimoto, K. Enomoto, T. Ono, T. Tabata, I. Kajiwara, et al.; A case of repetitive and simultaneous stent thromboses; Int. J. Cardiol., 186 (2015), p. 210
- [12] N. Tabata, S. Hokimoto, T. Akasaka, Y. Arima, K. Kaikita, N. Kumagae, et al.; Chronic kidney disease status modifies the association of CYP2C19 polymorphism in predicting clinical outcomes following coronary stent implantation; Thromb. Res., 134 (2014), pp. 939–944
- [13] K. Yamamoto, S. Hokimoto, T. Chitose, K. Morita, T. Ono, K. Kaikita, et al.; Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy; J. Cardiol., 57 (2011), pp. 194–201
- [14] P. Shahabi, G. Siest, U.A. Meyer, S. Visvikis-Siest; Human cytochrome P450 epoxygenases: Variability in expression and role in inflammation-related disorders; Pharmacol. Ther., 144 (2014), pp. 134–161
- [15] C. Bertrand-Thiébault, H. Berrahmoune, A. Thompson, B. Marie, S. Droesch, G. Siest, et al.; Genetic polymorphism of CYP2C19 gene in the Stanislas cohort. A link with inflammation; Ann. Hum. Genet., 72 (2008), pp. 178–183
- [16] A. Kajiwara, J. Saruwatari, A. Kita, R. Kamihashi, H. Miyagawa, M. Sakata, et al.; Sex differences in the effect of cytochrome P450 2C19 polymorphisms on the risk of diabetic retinopathy: a retrospective longitudinal study in Japanese patients with type 2 diabetes; Pharmacogenet. Genomics, 23 (2013), pp. 717–720
- [17] S. Hokimoto, N. Tabata, T. Akasaka, Y. Arima, K. Kaikita, K. Morita, et al.; Gender differences in impact of CYP2C19 polymorphism on development of coronary artery disease; J. Cardiovasc. Pharmacol., 65 (2015), pp. 148–152
- [18] K. Ohba, S. Sugiyama, H. Sumida, T. Nozaki, J. Matsubara, Y. Matsuzawa, et al.; Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease; J. Am. Heart Assoc., 1 (2012), Article e002485
- [19] T. Akasaka, D. Sueta, Y. Arima, N. Tabata, S. Takashio, Y. Izumiya, et al.; Association of CYP2C19 variants and epoxyeicosatrienoic acids on patients with microvascular angina; Am. J. Physiol. Heart Circ. Physiol. (2016) http://doi.org/10.1152/ajpheart.00473.2016
- [20] Group JJW; Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013); Circ. J., 78 (2014), pp. 2779–2801
- [21] K. Kothawade, C.N.B. Merz; Microvascular coronary dysfunction in women—pathophysiology, diagnosis, and management; Curr. Probl. Cardiol., 36 (2011), pp. 291–318
- [22] V.L. Murthy, M. Naya, C.R. Foster, J. Hainer, M. Gaber, G. Di Carli, et al.; Improved cardiac risk assessment with noninvasive measures of coronary flow reserve; Circulation, 124 (2011), pp. 2215–2224
- [23] T. Akasaka, S. Hokimoto, D. Sueta, N. Tabata, K. Sakamoto, E. Yamamoto, et al.; Gender differences in the impact of CYP2C19 polymorphisms and low-grade inflammation on coronary microvascular disorder; Am. J. Physiol. Heart Circ. Physiol. (2016) (ajpheart. 00911.02015)
- [24] M. Ishii, K. Kaikita, K. Sato, T. Tanaka, K. Sugamura, K. Sakamoto, et al.; Acetylcholine-provoked coronary spasm at site of significant organic stenosis predicts poor prognosis in patients with coronary vasospastic angina; J. Am. Coll. Cardiol., 66 (2015), pp. 1105–1115
- [25] K. Kaikita, M. Ishii, K. Sato, M. Nakayama, Y. Arima, T. Tanaka, et al.; Determinants of myocardial lactate production during acetylcholine provocation test in patients with coronary spasm; J. Am. Heart Assoc., 4 (2015), Article e002387 http://doi.org/10.1161/JAHA.115.002387
- [26] S. Hokimoto, N. Tabata, K. Yamanaga, D. Sueta, T. Akasaka, K. Tsujita, et al.; Prevalence of coronary macro-and micro-vascular dysfunctions after drug-eluting stent implantation without in-stent restenosis; Int. J. Cardiol., 222 (2016), pp. 185–194
- [27] K. Yamanaga, K. Tsujita, N. Komura, K. Kaikita, K. Sakamoto, T. Miyazaki, et al.; Single-wire pressure and flow velocity measurement for quantifying microvascular dysfunction in patients with coronary vasospastic angina; Am. J. Physiol. Heart Circ. Physiol., 308 (2015), pp. H478–H484
- [28] B. Richards, J. Skoletsky, A.P. Shuber, R. Balfour, R.C. Stern, H.L. Dorkin, et al.; Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs; Hum. Mol. Genet., 2 (1993), pp. 159–163
- [29] T. Kubota, K. Chiba, T. Ishizaki; Genotyping of S-mephenytoin 4′-hydroxylation in an extended Japanese population*; Clin. Pharmacol. Ther., 60 (1996), pp. 661–666
- [30] S. De Morais, G.R. Wilkinson, J. Blaisdell, U.A. Meyer, K. Nakamura, J.A. Goldstein; Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese; Mol. Pharmacol., 46 (1994), pp. 594–598
- [31] M. Man, M. Farmen, C. Dumaual, C.H. Teng, B. Moser, S. Irie, et al.; Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans; J. Clin. Pharmacol., 50 (2010), pp. 929–940
- [32] M. Spiecker, H. Darius, T. Hankeln, M. Soufi, A.M. Sattler, J.R. Schaefer, et al.; Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2; Circulation, 110 (2004), pp. 2132–2136
- [33] D.C. Ellinsworth, S.L. Sandow, N. Shukla, Y. Liu, J.Y. Jeremy, D.D. Gutterman; Endothelium-derived hyperpolarization and coronary vasodilation: diverse and integrated roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap junctions; Microcirculation, 23 (2016), pp. 15–32
- [34] R. Kaspera, R.A. Totah; Epoxyeicosatrienoic acids: formation, metabolism and potential role in tissue physiology and pathophysiology; Expert Opin. Drug Metab. Toxicol., 5 (7) (2009), pp. 757–771 http://doi.org/10.1517/17425250902932923
- [35] J.D. Imig, X. Zhao, C.Z. Zaharis, J.J. Olearczyk, D.M. Pollock, J.W. Newman, et al.; An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension; Hypertension, 46 (2005), pp. 975–981
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?