Highlights
- We measured native and MDA-collagen type IV IgM and IgG in MI patients and controls.
- Post-infarction patients had increased levels of IgM against native collagen type IV.
- Presence of IgG against native collagen type IV was associated with MI.
- In contrast, IgG against MDA-collagen type IV was decreased in MI patients.
Abstract
Background
Collagen type IV is the major constituent of basement membranes underlying endothelial cells and is important for endothelial cell attachment and function. Autoantibodies against native collagen type IV have been found in various autoimmune diseases. Oxidation of LDL in the vascular wall results in the formation of reactive aldehydes, which could modify surrounding matrix proteins. Like oxidized LDL, these modified matrix proteins are likely to induce immune responses. We examined whether autoantibodies against native or aldehyde-modified collagen type IV are associated with myocardial infarction.
Methods
IgM and IgG against native and aldehyde-modified collagen type IV were measured by ELISA in serum from 387 survivors of a first myocardial infarction and 387 age- and sex-matched controls.
Results
Post-infarction patients had significantly increased levels of IgM against native collagen type IV, and IgG against native collagen type IV was present at detectable level in 17% of patients as opposed to 7% of controls (p < 0.001). Controlling for major cardiovascular risk factors demonstrated that the presence of IgG against native collagen type IV was associated with myocardial infarction (OR 2.9 (1.6–5.4), p = 0.001). Similarly, subjects in the highest quartile of IgM against native collagen type IV had increased risk of having suffered myocardial infarction (OR 3.11 (1.8–5.4), p < 0.001) after adjusting for cardiovascular risk factors. In contrast, IgG against aldehyde-modified collagen type IV was decreased in myocardial infarction patients, but this association was not independent of established cardiovascular risk factors.
Conclusion
Autoantibodies against collagen type IV are associated with myocardial infarction independently of traditional cardiovascular risk factors.
Keywords
Autoantibodies;Collagen type IV;Myocardial infarction
1. Introduction
Acute coronary events mainly arise from plaque rupture or plaque erosion [1]. Plaques prone to rupture are characterized by a large lipid-rich necrotic core with an overlying thin fibrous cap [1], which is believed to rupture due to increased inflammation resulting in the degradation of extracellular matrix components. The mechanisms for plaque erosion are not known, but lesions are characterized by the absence of endothelium [1]. The endothelium regulates the vascular tone, controls blood coagulation and regulates inflammatory processes [2]. Endothelial dysfunction predicts clinical events caused by atherothrombosis, and case–control studies indicate an association between endothelial dysfunction and acute coronary syndromes [2] ; [3]. Although endothelial dysfunction clinically is measured by parameters of vasodilation, it appears that this condition is equated with a loss of atheroprotection and promotion of atherothrombosis.
Endothelial cells are lying on and adhere to the basement membrane, a thin sheet of extracellular matrix. The main component of the basement membrane is the network forming collagen type IV, which comprises 50% of the basement membrane. Collagen type IV binds to cells via integrins on the cell surface or via discoidin domain receptors [4]; [5]; [6] ; [7]. As expected, the collagen type IV interaction with endothelial cells is important for maintaining endothelial cell function [8]. Interestingly, autoantibodies against collagen type IV are present in various inflammatory and autoimmune diseases [9]; [10]; [11]; [12] ; [13]. Increased levels of autoantibodies against collagen type IV have been associated with diabetes and development of microangiopathy [14] and have also been reported in patients with systemic vasculitis and Crohns disease [13] ; [15]. Moreover, autoantibodies against collagen type IV have been detected in children with type 1 diabetes and hypertension [16] ; [17].
Accumulation and oxidation of LDL in the vessel wall are considered as key events in the development of atherosclerotic lesions [18]. Thus, efforts so far have almost exclusively been focused on characterizing modifications of the LDL particles. Oxidation of fatty acids in LDL leads to the formation of reactive aldehydes, such as malondialdehyde (MDA) [19]. These aldehydes react with the LDL protein apoB100, and MDA-modified apoB100 becomes a target for the immune system. Accordingly, autoantibodies against oxidized LDL are associated with cardiovascular disease [19]. We have previously shown that during oxidation of LDL, reactive aldehydes leak out of the LDL particle and modify surrounding extracellular matrix proteins [20]. These modifications on extracellular matrix may impair structural and cell-binding properties of the proteins, resulting in decreased plaque stability. It is also possible that modification of basement membrane proteins enhances inflammation, as shown by increased monocyte attachment and intracellular adhesion molecule (ICAM)-1 expression on endothelial cells attached to oxidized laminin [21]. Similar to oxidized LDL, modified matrix proteins are likely to result in immune responses against the extracellular matrix in the plaque. Indeed, we have identified autoantibodies against several aldehyde-modified matrix proteins, including MDA-modified laminin present in basement membranes. Interestingly, these autoantibodies were associated with less cardiovascular disease in a prospective cohort, which may imply a protective effect in humans [22].
In the present work, we investigated the presence of autoantibodies against native and aldehyde-modified collagen type IV in patients with a recent myocardial infarction (MI) and population-based matched controls.
2. Methods
2.1. Cohort
The Stockholm Coronary Atherosclerosis Risk Factor (SCARF) study database and biobank were used for the present study. As reported elsewhere [23], 387 survivors of a first MI aged less than 60 years were recruited along with 387 age- and sex-matched controls from the general population of the same county. In both groups 82% of the subjects were male. All participants were interviewed, underwent a brief medical examination and donated fasting blood samples at an examination which took place 3 months after the cardiac event for the patients. Details of recruitment, representativeness and basic characteristics of the groups have been published earlier [23]. The study was approved by the ethics committee of the Karolinska University Hospital, and conducted in agreement with the Declaration of Helsinki. All patients and control persons gave their informed consent to participation.
2.2. Coronary angiography
Routine coronary angiography was performed in a subset of 243 patients during the initial hospital stay (n = 35) or 3 months after the cardiac event (n = 208) [23]. Angiograms were analyzed by quantitative computer-based evaluation (QCA; Medis QCA-CMS system, Leiden). Minimal lumen diameter, reference diameter, percentage diameter stenosis, mean segment diameter, segment length, plaque area, segment area and number of significant stenoses (> 50%) were registered in each of the 15 coronary segments.
2.3. Biochemical analysis
Analyses of C-reactive protein (CRP) [24], plasminogen activator inhibitor-1 (PAI-1) [23], matrix metalloproteinase-3 (MMP-3) [23] and MMP-9 [25] have been the subject of previous reports.
2.4. ELISA for autoantibodies against native and MDA-modified collagen type IV
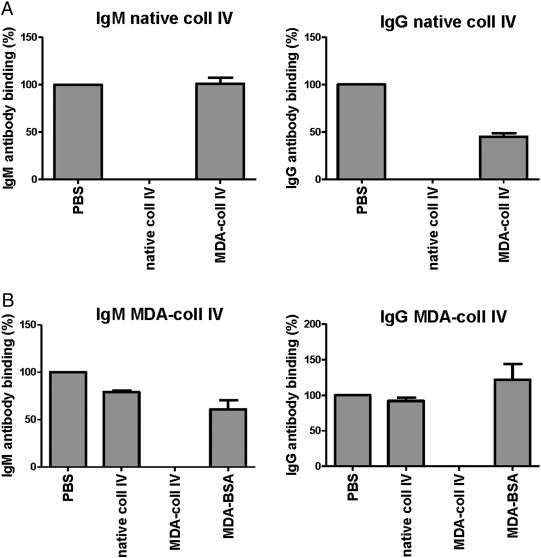
Proteins were MDA-modified at 37 °C during 3 h in a solution using 0.05 mol/L MDA in PBS, pH 7.4. MDA modifications were assayed using thiobarbituric acid reactive substances assay (TBARS) and Western blot using anti MDA antibody. Antibodies in plasma against native and MDA-modified collagen IV were detected by ELISA, essentially as described [26]. Briefly, 10 μg/mL of native or MDA-modified collagen IV was coated on microtiter plates (Nunc MaxiSorp, Roskilde, Denmark) and blocked with Superblock in Tris buffered saline (Pierce, Rockford, IL, USA). Plasma (dilution 1:100) was added to the wells and detection of bound antibodies was done with biotinylated rabbit anti-human IgG (Abcam) or IgM (Dako, Stockholm, Sweden), followed by alkaline phosphatase-conjugated streptavidin (Sigma), and alkaline phosphatase substrate kit (Pierce). Absorbance from wells coated with native collagen type IV was subtracted with absorbance from PBS-coated wells. Absorbance from wells coated with MDA-collagen type IV was subtracted with absorbance from wells coated with native collagen type IV to exclude antibodies binding to native epitopes of MDA-modified collagen type IV. Absorbance values were normalized against a plasma pool present on each plate. Arbitrary units (AU) are defined as percentage of the absorbance to the plasma pool. Intra-assay (n = 10) and inter-assay (n = 10) coefficients of variation for the antibody ELISA directed against native collagen IV were 2.6 and 7.6% for the IgM and 4.5 and 6.1% for the IgG, respectively. Intra-assay (n = 10) and inter-assay (n = 10) coefficients of variation for the antibody ELISA directed against MDA-modified collagen IV were 7.0 and 5.5% for the IgM and 11.9 and 10.2% for the IgG, respectively. Absorbance for antibodies against collagen type IV was in general low, but detectable levels were present in 592 individuals for IgM against native collagen type IV, in 89 individuals for IgG to native collagen type IV, in 536 individuals for IgM against MDA-collagen type IV, and in 723 individuals for IgG to MDA-collagen type IV, out of a total of 747 individuals. The specificities of ELISAs were determined by competition assay. Binding of both IgM and IgG in plasma to native collagen type IV was completely inhibited by the addition of 100 μg/mL native collagen type IV, whereas addition of MDA-collagen type IV did not compete (IgM) or only partly competed out the interaction (IgG) (Supplementary Fig. 1A). Binding of IgM and IgG in plasma to MDA-collagen type was completely inhibited by the addition of 100 μg/mL MDA-collagen type IV, whereas native collagen type IV or MDA-modified albumin did not compete or competed to a lesser extent (Supplementary Fig. 1A).
2.5. Statistical methods
Owing to missing samples in 19 patients and 8 controls, 368 patients and 379 controls were available for subsequent statistical analysis. For all types of antibodies (except IgG against native collagen type IV) values under the detection limit were replaced with 0 values. Variables are summarized as number (percentage) or medians (interquartile range). Differences in group comparisons were assessed by using Mann–Whitney test for continuous traits, and by Pearsons chi-squared test for binary. Detectable levels of IgG against native collagen type IV for patients and control individuals are summarized as group percentages and are compared using chi-square test. In univariable analysis, skewed data were transformed prior to analysis: anti-collagen IV IgM was divided into 5 groups (values below the detection limit, and 4 quartiles of positive values), anti-MDA-collagen IV IgM and IgG underwent square-root transformation. Anti-collagen IV IgG was used as a binary variable, depending on whether the values were above or below the limit of detection. The Jonckheere–Terpstra test for ordered alternatives was used to assess trends by quartiles of specific antibodies against collagen type IV. The associations between autoantibodies against native and MDA-modified collagen type IV and MI were determined by logistic regression analysis and calculation of odds ratios with 95% confidence intervals. Risk-adjusted models included established cardiovascular risk factors. Despite that the entire study population was controlled by age and sex, these two parameters were always adjusted for while analyzing subsets of cases and controls. Covariates with skewed distribution were log-transformed before being entered in regression analysis. Correlations were performed using Spearman rank test. Smoking habits are presented as current smokers vs former or never smokers. A two-sided p-value < 0.05 was considered significant for all analyses. Statistical analyses were performed using STATA 11.1 (Stata Corporation, USA).
3. Results
3.1. Increased levels of autoantibodies against native collagen type IV, but lower levels of autoantibodies against MDA-collagen type IV, in patients with MI
To determine whether auto-antibodies against collagen type IV in plasma are associated with MI, we measured IgM and IgG against native and MDA-modified collagen type IV in cases and controls. Basic characteristics of the groups are shown in Table 1. IgM levels against native collagen type IV were increased in subjects who had suffered MI (71 (31–125) AU) compared with healthy controls (42 (2.4–98) AU; p < 0.001) (Table 1). IgG antibodies recognizing native collagen type IV were not detected in most samples. However, among individuals with detectable levels of IgG against native collagen type IV, patients predominated over controls (17% of patients vs 6.7% of controls; p < 0.001, Table 1). In contrast, analysis of auto-antibodies against MDA-modified collagen type IV revealed that IgG antibodies were lower in patients (75 (55–104) AU) than in controls (88 (60–123) AU; p = 0.002, Table 1). IgM against MDA-modified collagen type IV, on the other hand, did not differ significantly between patients and controls (Table 1). Antibody levels were not associated with any coronary angiography measurements.
| Patients n = 368 | Controls n = 379 | p-Valuea | |
|---|---|---|---|
| Age (year) | 54 (49–57) | 54 (50–57) | 0.35 |
| Gender (% male) | 83.7 | 83.6 | 0.98 |
| Current smoking (% smokers) | 20.3 | 22.6 | 0.2674 |
| Lipid-lowering medication (%) | 33.3 | 0 | < 0.001 |
| BMI (kg/m2) | 27 (25–30) | 26 (24–28) | < 0.001 |
| SBP (mm Hg) | 130 (118–140) | 128 (118–140) | 0.73 |
| DBP (mm Hg) | 80 (75–87) | 80 (78–88) | 0.093 |
| Triacylglycerols (mmol/L) | 1.6 (1.2–2.3) | 1.2 (0.8–1.6) | < 0.001 |
| LDL cholesterol (mmol/L) | 3.2 (2.5–3.9) | 3.9 (3.3–4.5) | 0.0001 |
| HDL cholesterol, men (mmol/L) | 0.8 (0.7–1.0) | 1.1 (0.9–1.3) | < 0.001 |
| HDL cholesterol, women (mmol/L) | 1.15 (0.95–1.4) | 1.5 (1.25–1.75) | < 0.001 |
| Glucose (mmol/L) | 5.0 (5.3–5.9) | 4.8 (4.6–5.2) | < 0.001 |
| Insulin (pmol/L) | 46.5 (32.0–69.0) | 36.0 (28.0–49.0) | < 0.001 |
| Proinsulin (pmol/L) | 5.1 (3.4–7.4) | 3.5 (2.6–5.4) | < 0.001 |
| C-reactive protein (mg/L) | 1.5 (0.7–3.4) | 1.0 (0.5–1.8) | < 0.001 |
| IL-6 (ng/L) | 0.8 (0.6–1.4) | 0.6 (0.5–1.0) | < 0.001 |
| Fibrinogen (g/L) | 3.8 (3.3–4.3) | 3.5 (3.1–4.0) | < 0.001 |
| PAI-1 (IU/ml) | 13.1 (4.8–22.6) | 7.5 (3.1–17.8) | < 0.001 |
| Anti-collagen IV IgM (AU) | 71 (31–125) | 42 (2.4–98) | < 0.001 |
| Anti-collagen IV IgGb | 17.2 | 6.7 | < 0.001 |
| Anti-MDA-collagen IV IgM (AU) | 36 (15–71) | 39 (17–78) | 0.14 |
| Anti-MDA-collagen IV IgG (AU) | 75 (55–104) | 88 (60–123) | < 0.01 |
Basic characteristics of patients and controls of the entire cohort were originally reported by Samnegård et al. [23]. Values are expressed as median (interquartile range) or percentage. Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoproteins; HDL, high-density lipoproteins; IL-6, interleukin 6; PAI-1, plasminogen activator inhibitor-1; AU, arbitrary units; MDA, malondialdehyde.
a. p-Values were calculated by Mann–Whitney U test for continuous traits and by Pearsons chi-squared test for binary variables.
b. Values are expressed as percentage of individuals with detectable levels of IgG against native collagen type IV, and differences were compared using Pearsons chi-square test.
3.2. Autoantibodies against native collagen type IV are independently associated with MI
In multivariable logistic regression analysis, both IgM and IgG against native collagen type IV were associated with MI, and these associations were independent of established cardiovascular risk factors (p ≤ 0.001 for both IgM and IgG). Detectable levels of IgG against native collagen type IV were associated with a considerably higher risk of having suffered MI (OR 2.9 (1.6–5.4), p = 0.001 after adjusting for cardiovascular risk factors). Dividing IgM against native collagen type IV into quartiles revealed that the highest quartile of IgM against native collagen type IV was associated with a substantially increased risk of having contracted MI (OR 3.11 (1.8–5.4) for 4th vs 1st quartile, p < 0.001 after adjusting for risk factors; Table 2).
| Myocardial infarction | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| Odds ratio (95% CI) | |||||
| IgM native coll IV | |||||
| Unadjusted | 1 | 1.45 (0.96–2.2) | 2.28 (1.5–3.4)⁎⁎ | 2.46 (1.6–3.7)⁎⁎ | 0.001 |
| Risk factor adjusteda | 1 | 1.72 (0.99–3.0) | 2.92 (1.7–5.1)⁎⁎ | 3.11 (1.8–5.4)⁎⁎ | 0.002 |
| IgG MDA coll IV | |||||
| Unadjusted | 1 | 1.09 (0.73–1.6) | 0.65 (0.43–0.98)⁎ | 0.61 (0.40–0.91)⁎ | 0.002 |
| Risk factor adjusteda | 1 | 1.35 (0.79–2.3) | 0.70 (0.41–1.2) | 0.61 (0.36–1.0) | 0.09 |
Values are odds ratios (95% confidence intervals) in quartiles (Q1–Q4) of IgM against native collagen type IV or IgG against MDA-collagen type IV, respectively. Two-sided p-values calculated by Jonckheere–Terpstra trend test. The stars represent significant differences between the corresponding quartile and a reference Q1 (Mann–Whitney test). Coll IV, collagen type IV; CRP; C-reactive protein; HDL, high-density lipoproteins; LDL, low density lipoproteins; MDA, malondialdehyde.
⁎. p < 0.05.
⁎⁎. p < 0.001.
a. Adjusted to age, sex, height, smoking, diastolic blood pressure, hypertension, glucose, LDL-cholesterol, HDL-cholesterol, triglycerides and CRP.
In univariable logistic regression analysis, IgM and IgG against MDA-modified collagen type IV were associated with MI (p = 0.046 for IgM and p = 0.008 for IgG), however these associations did not remain after adjusting for established risk factors. Dividing IgG against MDA-collagen type IV into quartiles showed that a high level of IgG against MDA-collagen type IV was associated with decreased risk of MI (OR 0.61 (0.40–0.91) for 4th vs 1st quartile, p = 0.002; Table 2). Again, this association did not remain statistically significant after adjusting for cardiovascular risk factors (p = 0.09, Table 2).
3.3. Association of autoantibodies to cardiovascular risk factors with inflammatory and thrombotic markers
Autoantibodies against native or MDA-collagen type IV did not correlate significantly with smoking, body-mass index, systolic blood pressure, diastolic blood pressure, plasma glucose, insulin, LDL- or HDL-cholesterol, or CRP, except for an inverse association between IgG against native collagen type IV and HDL-cholesterol (r = − 0.13, p < 0.001).
When autoantibody levels were divided into quartiles, there were associations of PAI-1 with IgM against both native and MDA-collagen type IV. The lower quartile of IgM against native collagen type IV was associated with decreased levels of PAI-1 (quartile 1 vs quartiles 2–4, p = 0.004) in the entire cohort. In contrast, the upper quartile of IgM against MDA-modified collagen type IV was associated with decreased levels of PAI-1 (quartiles 1–3 vs quartile 4, p = 0.027). This difference remained statistically significant in controls (p = 0.025), but not in patients.
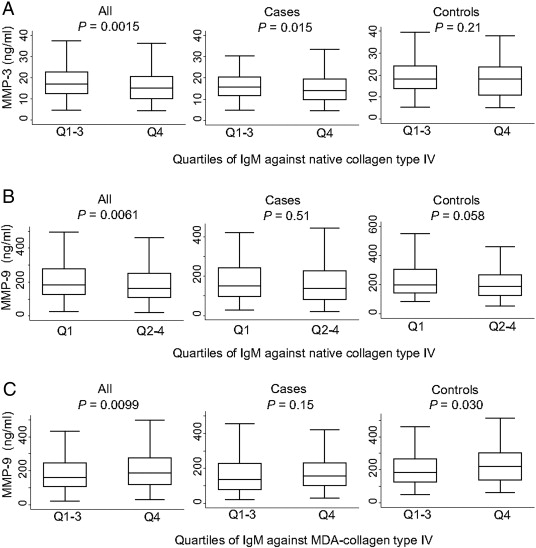
In addition, there was a significant negative trend between quartiles of IgM against native collagen type IV and MMP-3-levels (p = 0.003). The highest quartile of IgM against native collagen type IV displayed decreased levels of MMP-3 in the entire cohort (p = 0.0015) and in cases (p = 0.015), whereas no significant association was observed in controls (p = 0.21) (Fig. 1A). The highest quartile of IgM against native collagen type IV was also associated with decreased of levels of MMP-9 in the entire cohort (p = 0.0061). This association was stronger in controls (p = 0.058) than in cases (p = 0.51) (Fig. 1B). In contrast to antibodies against the native protein, the upper quartile of IgM against MDA-collagen type IV displayed increased levels of MMP-9 in the entire cohort (p = 0.0099) and in controls (p = 0.030), whereas there was no significant difference in cases (p = 0.15) (Fig. 1C).
|
|
|
Fig. 1. IgM antibodies against native collagen type IV are associated with decreased levels of circulating MMP-3 and MMP-9, whereas IgM against MDA-collagen type IV are associated with increased levels of MMP-9. IgM against native (A, B) and MDA-collagen type IV (C) were divided in quartiles and differences in MMP-3 (A) and MMP-9 (B, C) including all individuals, cases or controls were calculated by Mann–Whitney test. |
4. Discussion
Collagen type IV is the most abundant collagen in basement membranes and is considered to be responsible for their mechanical stability [27]. Collagen type IV is also essential for cell adhesion and cell-matrix communication and plays an important role in maintaining endothelial cell function. In this communication, we report that circulating IgM and IgG autoantibodies against native collagen type IV are increased in patients with recent MI and that these associations are independent of major cardiovascular risk factors. Moreover, autoantibodies against native collagen type IV were associated with a substantially increased risk of having contracted MI.
Autoantibodies against collagen type IV have been found in autoimmune diseases such as Goodpasture syndrome and Alport post-transplant nephritis. In both examples, type IV collagen is affected either by mutations or immune response leading to kidney and/or lung failure. In Goodpasture syndrome autoantibodies against collagen type IV are directed against epitopes in the non-collagenous-1 (NC1)-domain, which are exposed due to dissociation (or failure of association) of collagen type IV hexamers [10] ; [11]. Interestingly, local environmental factors such as endogenous oxidants and tobacco smoke are presumed to play a role in the dissociation of collagen type IV hexamers. Rheumatic fever is characterized by inflammatory changes in subendothelial and perivascular collagen tissue. According to a recent hypothesis, the carditis of rheumatic fever results from an immune response against collagen type IV, affecting the overlying endothelium [12]. It has previously been shown that streptococcal protein, causing rheumatic fever, binds to collagen type IV. Moreover, serum from patients with acute rheumatic fever contained increased titers of anti-collagen type IV antibodies [9]. In our study, IgM collagen type IV antibodies were present in 80% of the study population, whereas detectable levels of IgG were present in about 10% of the 747 individuals. Why autoantibodies against native collagen type IV were present in a significant proportion of subjects in our study remains to be determined. A small study using ELISA technique identified increased levels of IgG antibodies against collagen type IV in idiopathic dilated cardiomyopathy, but not in patients with acute MI [28]. IgG antibodies against collagen type IV were present in 18% of the MI patients one day after hospitalization, which decreased to 14% after two weeks. These numbers are in agreement with the presence of IgG against native collagen type IV in 17% of the MI patients in our cohort. The lack of difference between IgG against collagen type IV in MI patients versus controls in the previous study, may be due to the small number of subjects analyzed (20 MI patients and 20 controls).
Previous studies in an experimental rat MI model showed that collagen type IV together with laminin and collagen type III are present relatively early after MI [29]; [30]; [31] ; [32]. Moreover, collagen type IV was present in the infarct zone, not restricted to basement membranes, indicating that collagen type IV contributes to the healing process. Whether antibodies against native collagen type IV influence or may be markers of infarct healing remains to be studied.
Modifications of collagen type IV are likely to disturb its interactions with endothelial cells and basement membrane components, resulting in an impaired basement membrane structure and endothelial cell dysfunction. For example, glycosylation of collagen type IV affects assembly of collagen type IV networks [33] and causes endothelial cell detachment and decreases endothelial cell adhesion and spreading [34] ; [35]. Of note, it has been shown that modification of laminin, the other major basement membrane component, with reactive oxygen species induced increased monocyte attachment and expression of ICAM-1 on endothelial cells [21]. Thus, autoantibodies against MDA-modified collagen type IV may serve a protective function by facilitating removal of modified collagen type IV or simply by blocking modified sites. In agreement with this, autoantibodies against MDA-collagen type IV were found to be increased in controls compared with patients, although this difference was not independent of cardiovascular risk factors.
High levels of IgM against native collagen type IV were associated with lower levels of MMP-3 and MMP-9. Expression of MMPs in the fibrous cap is likely to result in increased risk of plaque rupture, which would argue against negative association to autoantibodies recognizing native collagen type IV. On the other hand, MMPs could contribute to plaque stability by facilitating smooth muscle cell migration. Accordingly, deletion of MMP-3 and MMP-9 in apoE−/− mice resulted in larger lesions and a more unstable plaque phenotype with less smooth muscle cells [36]. Interestingly, MMPs also play a role in thrombosis; e.g. MMP-9 has been shown to inhibit platelet aggregation in response to thrombin or collagen and to degrade fibrin [37] ; [38]. Clinical studies of plasma MMP-3 and MMP-9 identify MMP-3 as a biomarker negatively associated with coronary events, whereas MMP-9 instead is positively associated with coronary events. In the present cohort, both MMP-3 and MMP-9 were found to be lower in patients than in controls [23] ; [25], which is in agreement with the inverse relationship of autoantibodies against native collagen type IV and MMP-3 and -9.
A limitation of the study is that is does not address the question of whether the antibodies are markers of disease or indeed play a functional role. It is possible that autoantibodies against collagen type IV are induced when plaque components are exposed to the circulating immune system upon rupture of the lesions. This question needs to be addressed in further studies including additional cohorts.
5. Conclusion
In conclusion, we show that autoantibodies against native collagen type IV are associated with myocardial infarction independently of traditional cardiovascular risk factors, suggesting a break of tolerance.
Conflict of interest
There are no conflicts of interest.
The following are the supplementary data related to this article.
|
|
|
Supplementary Fig. 1. Specificity of the ELISAs for IgM and IgG against native and MDA-modified collagen type IV. The specificities of IgM and IgG autoantibodies against native (A) and MDA-modified collagen type IV (B) were analyzed by competition assay using native and MDA-modified collagen type IV as well as MDA-modified albumin (MDA-BSA). |
Acknowledgment of grant support
This work was supported by the Swedish Research Council (08311, 20113892, K26000523), the Swedish Heart–Lung Foundation (20110284, 20130554, 20130363, K26000493), the Swedish Strategic Research Foundation, the Strategic Cardiovascular and Diabetes Programmes of Karolinska Institutet and the Stockholm County Council, the Stockholm County Council (560183), the Skåne University Hospital funds, the Åke Wiberg Foundation, the Tore Nilsson foundation, the Magnus Bergvall foundation, the Albert Påhlsson foundation, Diabetesfonden, AFA Insurance Foundation, the Torsten and Ragnar Söderberg Foundation, King Gustaf V and Queen Victoria foundation, King Gustaf V 80th birthday foundation, Serafimer foundation and AstraZeneca.
References
- [1] F.D. Kolodgie, J. Narula, A.P. Burke, et al.; Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death; Am J Pathol, 157 (4) (2000), pp. 1259–1268
- [2] M.E. Widlansky, N. Gokce, J.F. Keaney Jr., J.A. Vita; The clinical implications of endothelial dysfunction; J Am Coll Cardiol, 42 (7) (2003), pp. 1149–1160
- [3] J.A. Vita; Endothelial function; Circulation, 124 (25) (2011), pp. e906–e912
- [4] J.A. Eble, R. Golbik, K. Mann, K. Kuhn; The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV); EMBO J, 12 (12) (1993), pp. 4795–4802
- [5] J. Emsley, C.G. Knight, R.W. Farndale, M.J. Barnes, R.C. Liddington; Structural basis of collagen recognition by integrin alpha2beta1; Cell, 101 (1) (2000), pp. 47–56
- [6] C.G. Knight, L.F. Morton, A.R. Peachey, D.S. Tuckwell, R.W. Farndale, M.J. Barnes; The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens; J Biol Chem, 275 (1) (2000), pp. 35–40
- [7] H. Xu, N. Raynal, S. Stathopoulos, J. Myllyharju, R.W. Farndale, B. Leitinger; Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1; Matrix Biol, 30 (1) (2011), pp. 16–26
- [8] L. Gonzalez-Santiago, S. Lopez-Ongil, M. Rodriguez-Puyol, D. Rodriguez-Puyol; Decreased nitric oxide synthesis in human endothelial cells cultured on type I collagen; Circ Res, 90 (5) (2002), pp. 539–545
- [9] K. Dinkla, M. Rohde, W.T. Jansen, E.L. Kaplan, G.S. Chhatwal, S.R. Talay; Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen; J Clin Invest, 111 (12) (2003), pp. 1905–1912
- [10] B.G. Hudson, K. Tryggvason, M. Sundaramoorthy, E.G. Neilson; Alports syndrome, Goodpastures syndrome, and type IV collagen; N Engl J Med, 348 (25) (2003), pp. 2543–2556
- [11] V. Pedchenko, O. Bondar, A.B. Fogo, et al.; Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis; N Engl J Med, 363 (4) (2010), pp. 343–354
- [12] R. Tandon, M. Sharma, Y. Chandrashekhar, M. Kotb, M.H. Yacoub, J. Narula; Revisiting the pathogenesis of rheumatic fever and carditis; Nat Rev Cardiol, 10 (3) (2013), pp. 171–177
- [13] H. Tiwana, R.S. Natt, R. Benitez-Brito, et al.; Correlation between the immune responses to collagens type I, III, IV and V and Klebsiella pneumoniae in patients with Crohns disease and ankylosing spondylitis; Rheumatology (Oxford), 40 (1) (2001), pp. 15–23
- [14] A. Nikolov, G. Nicoloff, I. Tsinlikov, I. Tsilnikova; Anti-collagen type IV antibodies and the development of microvascular complications in diabetic patients with arterial hypertension; J IMAB, 18 (3) (2012), pp. 315–322
- [15] H. Direskeneli, D. D'Cruz, M.A. Khamashta, G.R. Hughes; Autoantibodies against endothelial cells, extracellular matrix, and human collagen type IV in patients with systemic vasculitis; Clin Immunol Immunopathol, 70 (3) (1994), pp. 206–210
- [16] G. Nicoloff, S. Baydanoff, C. Petrova, P. Christova; Serum antibodies to collagen type IV and development of diabetic vascular complications in children with type 1 (insulin-dependent) diabetes mellitus. A longitudinal study; Vascul Pharmacol, 38 (3) (2002), pp. 143–147
- [17] G. Nicoloff, S. Baydanoff, N. Stanimirova, C. Petrova, P. Christova; Detection of serum collagen type IV in children with type 1 (insulin-dependent) diabetes mellitus—a longitudinal study; Pediatr Diabetes, 2 (4) (2001), pp. 184–190
- [18] G.K. Hansson; Inflammation, atherosclerosis, and coronary artery disease; N Engl J Med, 352 (16) (2005), pp. 1685–1695
- [19] W. Palinski, J.L. Witztum; Immune responses to oxidative neoepitopes on LDL and phospholipids modulate the development of atherosclerosis; J Intern Med, 247 (3) (2000), pp. 371–380
- [20] P. Duner, F. To, R. Alm, et al.; Immune responses against fibronectin modified by lipoprotein oxidation and their association with cardiovascular disease; J Intern Med, 265 (5) (2009), pp. 593–603
- [21] E. Kostidou, K. Topouridou, A. Daniilidis, M. Kaloyianni, G. Koliakos; Oxidized laminin-1 induces increased monocyte attachment and expression of ICAM-1 in endothelial cells; Int J Exp Pathol, 90 (6) (2009), pp. 630–637
- [22] P. Duner, F. To, K. Berg, et al.; Immune responses against aldehyde-modified laminin accelerate atherosclerosis in Apoe−/− mice; Atherosclerosis, 212 (2) (2010), pp. 457–465
- [23] A. Samnegard, A. Silveira, P. Lundman, et al.; Serum matrix metalloproteinase-3 concentration is influenced by MMP-3–1612 5A/6A promoter genotype and associated with myocardial infarction; J Intern Med, 258 (5) (2005), pp. 411–419
- [24] J. Hulthe, W. McPheat, A. Samnegard, P. Tornvall, A. Hamsten, P. Eriksson; Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6; Atherosclerosis, 188 (2) (2006), pp. 450–454
- [25] A. Samnegard, J. Hulthe, A. Silveira, C.G. Ericsson, A. Hamsten, P. Eriksson; Gender specific associations between matrix metalloproteinases and inflammatory markers in post myocardial infarction patients; Atherosclerosis, 202 (2) (2009), pp. 550–556
- [26] G.N. Fredrikson, G. Berglund, R. Alm, J.A. Nilsson, P.K. Shah, J. Nilsson; Identification of autoantibodies in human plasma recognizing an apoB-100 LDL receptor binding site peptide; J Lipid Res, 47 (9) (2006), pp. 2049–2054
- [27] R. Timpl, J.C. Brown; Supramolecular assembly of basement membranes; Bioessays, 18 (2) (1996), pp. 123–132
- [28] C. Matache, M. Stefanescu, D. Ivanov, G. Szegli, P. Popescu, Z. Filip; Presence and significance of some antibodies/autoantibodies in patients with acute myocardial infarction and idiopathic dilated cardiomyopathy; Roum Arch Microbiol Immunol, 51 (4) (1992), pp. 197–203
- [29] K. Inoue, S. Kusachi, K. Niiya, Y. Kajikawa, T. Tsuji; Sequential changes in the distribution of type I and III collagens in the infarct zone: immunohistochemical study of experimental myocardial infarction in the rat; Coron Artery Dis, 6 (2) (1995), pp. 153–158
- [30] M. Murakami, S. Kusachi, M. Nakahama, et al.; Expression of the alpha 1 and alpha 2 chains of type IV collagen in the infarct zone of rat myocardial infarction; J Mol Cell Cardiol, 30 (6) (1998), pp. 1191–1202
- [31] N. Morishita, S. Kusachi, S. Yamasaki, J. Kondo, T. Tsuji; Sequential changes in laminin and type IV collagen in the infarct zone—immunohistochemical study in rat myocardial infarction; Jpn Circ J, 60 (2) (1996), pp. 108–114
- [32] A. Yamanishi, S. Kusachi, M. Nakahama, et al.; Sequential changes in the localization of the type IV collagen alpha chain in the infarct zone: immunohistochemical study of experimental myocardial infarction in the rat; Pathol Res Pract, 194 (6) (1998), pp. 413–422 [Comparative Study]
- [33] E.C. Tsilibary, A.S. Charonis, L.A. Reger, R.M. Wohlhueter, L.T. Furcht; The effect of nonenzymatic glucosylation on the binding of the main noncollagenous NC1 domain to type IV collagen; J Biol Chem, 263 (9) (1988), pp. 4302–4308
- [34] D. Dobler, N. Ahmed, L. Song, K.E. Eboigbodin, P.J. Thornalley; Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification; Diabetes, 55 (7) (2006), pp. 1961–1969
- [35] C.S. Haitoglou, E.C. Tsilibary, M. Brownlee, A.S. Charonis; Altered cellular interactions between endothelial cells and nonenzymatically glucosylated laminin/type IV collagen; J Biol Chem, 267 (18) (1992), pp. 12404–12407
- [36] J.L. Johnson, S.J. George, A.C. Newby, C.L. Jackson; Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries; Proc Natl Acad Sci USA, 102 (43) (2005), pp. 15575–15580
- [37] C. Fernandez-Patron, M.A. Martinez-Cuesta, E. Salas, et al.; Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2; Thromb Haemost, 82 (6) (1999), pp. 1730–1735
- [38] B. Lelongt, S. Bengatta, M. Delauche, L.R. Lund, Z. Werb, P.M. Ronco; Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity; J Exp Med, 193 (7) (2001), pp. 793–802
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?