Summary
There are estimated to be more than a hundred million hepatitis C virus (HCV) carriers worldwide. About 30% of carriers develop serious liver diseases, such as liver cirrhosis and hepatocellular carcinoma. HCV Genotype 1 is the most common genotype worldwide and the most difficult to treat with interferon-based therapy. Therapy for patients with chronic HCV infection is complicated by poor tolerability and inadequate rates of sustained virological response (SVR). Although the addition of a protease inhibitor in combination with peg-interferon alpha plus ribavirin improved SVR rates and shortened the treatment period, many patients could not tolerate this therapy because of advanced age and clinical conditions such as anemia and low platelet count. Interferon-free therapies that combine two or more direct-acting antiviral (DAA) agents can improve both efficacy and tolerability. Phase III trials of daclatasvir plus asunaprevir, ombitasvir plus parataprevir/r, and sofosbuvir plus ledipasvir all showed high overall SVR rates with few adverse events. However, development of antiviral resistance is a concern with DAA therapies, and it is important to avoid treating patients with existing NS5A Y93H mutations with daclatasvir plus asunaprevir or ombitasvir plus parataprevir/r therapy to prevent viral breakthrough. Fortunately, sofosbuvir plus ledipasvir therapy seems to be less affected by NS5A Y93H variants. An important goal of HCV therapy is to expand treatment to all patients. The current study aims to show the efficacy and safety of these therapies both in clinical trial and real world settings based on our own clinical experiences.
Keywords
Chronic hepatitis C ; Direct acting antiviral drugs ; Nonnucleoside polymerase inhibitors ; NS5A inhibitors ; Protease inhibitors
Introduction
Epidemiology
Hepatitis C virus (HCV) is a major public health challenge throughout the world, and there are estimated to be at least 185 million HCV carriers worldwide [1] ; [2] . In Japan the rate of chronic HCV infection is estimated to be up to 2% of the population, and Japanese patients are more likely to be older and female with prior treatment experience and more advanced liver disease than patients in western countries [3] . Although frequencies of the six HCV genotypes vary geographically, HCV Genotype 1 is the most common genotype worldwide and is among the most difficult to treat with interferon therapy. Failure to clear the virus increases the risk of severe liver disease, and up to 30% of carriers develop liver cirrhosis or hepatocellular carcinoma (HCC). The goal of HCV therapy is sustained virological response (SVR), defined as continuously undetectable HCV ribonucleic acid (RNA) levels 12 weeks (SVR12) or 24 weeks (SVR24) after the end of therapy. However, the risk of cirrhosis and HCC remains high even among patients who achieve SVR, although effective treatment is thought to reduce the risk of HCC [4] . Therefore, early identification and effective treatment of patients with chronic HCV is a high priority.
Until recently, peg-interferon plus ribavirin combination therapy was the standard of care for treatment of HCV Genotype 1. Under this demanding treatment, weekly injections with peg-interferon and daily dosing of ribavirin continued for 48 weeks, with possible extension to 72 weeks in slow responders. However, older patients or patients with cirrhosis or other contra-indications were ineligible, and even among eligible patients, expected SVR rates remained below 50%. While some patients responded transiently to treatment, other patients failed to respond and showed no change in HCV RNA levels. Due to poor tolerability and low rates of SVR with interferon therapy, a novel approach was urgently needed.
Direct acting antivirals
Despite its potent antiviral effects, interferon has only limited specificity against HCV, mediated through a large set of interferon-stimulated genes that establish a general antiviral state, and the virus has evolved mechanisms to disrupt interferon signaling in hepatocytes and impede the innate immune response. Patients with specific single nucleotide polymorphisms have also been shown to respond poorly to interferon therapy. The next advance in HCV therapy was the development of direct-acting antivirals (DAAs), which strongly inhibit replication of HCV by directly targeting essential viral proteins. High-throughput methods are used to screen candidate drugs, followed by lead optimization. Four main classes of DAAs have been developed so far that target three different viral proteins: NS3/4A protease inhibitors, NS5A inhibitors, and two types of NS5B polymerase inhibitors [5] . In 2011, the protease inhibitors telaprevir and boceprevir were the first DAAs to be approved for clinical use in the form of a triple therapy in combination with peg-interferon and ribavirin. Telaprevir triple therapy not only increased the SVR rate to 70–80%, but also shortened treatment duration [6] ; [7] . However, these improvements were not without cost, and severe adverse events including rash and anemia further restricted patient eligibility and led to frequent dose reductions and discontinuation. Fortunately, telaprevir and boceprevir have been largely superseded by second wave and second generation protease inhibitors with improved tolerability. Triple therapy with simeprevir [8] or vaniprevir [9] is currently used in Japan for treatment of Genotype 1 chronic HCV infection.
Interferon-free therapy
Interferon-free therapies that combine two or more DAAs promise improved efficacy and tolerability over interferon-based DAA therapies. The role played by interferon and ribavirin in triple therapy is partly to suppress selection for DAA resistant variants. In order to provide equivalent protection without interferon, DAAs belonging to two or more DAA classes with nonoverlapping resistance profiles are combined to increase the overall barrier to resistance. Phase III trials of daclatasvir plus asunaprevir, ombitasvir plus parataprevir/r, and sofosbuvir plus ledipasvir have shown high overall SVR rates with few adverse events. Several DAA combination therapies have recently been approved in Japan. Daclatasvir plus asunaprevir therapy [10] and sofosbuvir plus ledipasvir [11] have been approved for treatment of Genotype 1 infection, and sofosbuvir plus ribavirin has been approved for treatment of Genotype 2 infection [12] .
Antiviral resistance
The high rate of HCV replication combined with the low fidelity of HCV polymerase leads to rapid emergence of single and double point mutations throughout the HCV genome and the maintenance of multiple HCV quasispecies. The efficacy of a specific DAA combination therapy depends on the effectiveness of each coadministered DAA. Emergence of resistance to one class of DAA increases the selective pressure of the remaining DAAs and increases the probability of viral breakthrough. While emergence of resistance-associated variants (RAVs) during therapy in a subset of patients due to chance may be unavoidable, ineffective treatment of patients with preexisting variants can be avoided through screening. Genotype 1a has a lower genetic barrier to resistance to several protease inhibitors at R155 than Genotype 1b, which partly explains differences in approved treatments between the United States and Japan, where Genotype 1b is more prevalent [13] . The simeprevir-resistant Q80K mutation also occurs more frequently in patients with HCV Genotype 1a, suggesting that screening for the Q80 mutation should be considered for Genotype 1a patients prior to simeprevir therapy and an alternative therapy prepared if necessary. However, a number of DAA-treatment-naive Japanese patients have been found to harbor preexisting NS5A Y93H variants, which can confer resistance to NS5A inhibitors. Treatment of patients with existing NS5A Y93H variants can lead to viral breakthrough during daclatasvir plus asunaprevir or ombitasvir plus parataprevir/r therapy. In contrast to other treatment-emergent RAVs, which tend to have low fitness in competition with wild-type strains and are quickly lost after cessation of therapy, NS5A RAVs tend to persist even in the absence of the drug, which may confound future retreatment efforts with similar drugs. Fortunately, combination therapy with sofosbuvir and ledipasvir appears to be less affected by NS5A Y93H variants and may provide an alternative retreatment option for these patients. Nonetheless, these considerations underscore the need for increased screening and monitoring of resistance during the transition to interferon-free therapies.
Asunaprevir plus daclatasvir dual therapy
Asunaprevir plus daclatasvir dual therapy was the first approved interferon-free DAA therapy. The therapy combines asunaprevir, an NS3/4A protease inhibitor, with daclatasvir, an NS5A inhibitor [14] ; [15] . In a Phase II study in the United States, Genotype 1 patients were treated with daclatasvir and asunaprevir with or without peg-interferon and ribavirin for 24 weeks and achieved SVR12 rates of 78% for daclatasvir plus asunaprevir alone and 95% in four-way combination with peg-interferon and ribavirin [15] . In a Phase III study in Japan, 222 HCV Genotype 1 patients who were poor candidates for interferon therapy were treated for 24 weeks with daclatasvir and asunaprevir [10] . Eighty-seven percent of patients who were ineligible for interferon treatment achieved SVR24, and 81% of patients who had failed to respond to prior interferon therapy achieved SVR24. Ninety-one percent of patients with cirrhosis and 84% of patients without cirrhosis achieved SVR24. Thirteen percent of patients discontinued therapy due to poor response or adverse events such as alanine transaminase (ALT) or aspartate transaminase (AST) elevation, headache, or nasopharyngitis. This study demonstrated high SVR rates in distinct difficult-to-treat populations and showed that SVR24 rates were not affected by treatment history or baseline factors affecting response to interferon therapy, including sex, age, baseline HCV RNA levels, cirrhosis, and IFNL3 single nucleotide polymorphism genotype. A recent randomized Phase III clinical trial by Kumada et al [16] compared daclatasvir/asunaprevir dual therapy to telaprevir/peg-interferon/ribavirin triple therapy in treatment-naive Genotype 1b patients in Japan. Patients treated with daclatasvir plus asunaprevir achieved higher SVR12 rates (89%) than patients treated with telaprevir triple therapy (62%) and experienced markedly lower incidence of adverse events, especially with respect to drug discontinuation, rash, anemia and Grade 3 or 4 adverse events. A separate cohort of prior relapsers treated with asunaprevir and daclatasvir achieved a 95% SVR rate. The HALLMARK DUAL Phase III study showed similar results with SVR12 rates of 90% among treatment-naive patients and 82% among interferon-intolerant patients or prior null/partial responders [17] . While the Food and Drug Administration (FDA) initially approved daclatasvir for use in combination with sofosbuvir for treatment of Genotype 3 in July 2015, they expanded approval for daclatasvir plus sofosbuvir for treatment of Genotypes 1 and 3 in February 2016.
Asunaprevir plus daclatasvir plus beclabuvir triple therapy
An extension of dual DAA therapy is the addition of a third DAA to simultaneously target three viral proteins and increase the genetic barrier to resistance. Beclabuvir is a nonnucleoside inhibitor targeting the thumb 1 domain NS5B RNA dependent RNA polymerase. The drug has exhibited antiviral activity in vitro against Genotypes 1, 3, 4, 5, and 6 [18] , and patients treated for 12 or 24 weeks with beclabuvir in combination with daclatasvir and asunaprevir achieved a 92% SVR rate in a Phase II clinical trial [19] . In the open-label dual-arm UNITY-2 study, patients with cirrhosis were treated with asunaprevir, daclatasvir, and beclabuvir with or without ribavirin for 12 weeks [20] . In the ribavirin group, 98% of treatment-naive patients achieved SVR12, and 93% of treatment-experienced patients achieved SVR, whereas 93% and 87% of patients, respectively, achieved SVR12 in the nonribavirin group. Compared to nucleoside inhibitors like sofosbuvir, however, nonnucleoside inhibitors have a relatively lower barrier to resistance, and NS5B A421 and P495 variants have been observed to confer resistance to beclabuvir [21] ; [22] . However, in a replicon study in which no inhibitory effect was observed for daclatasvir and asunaprevir alone against NS5A L31M/Y93H double mutants, the addition of beclabuvir effectively suppressed HCV replication [23] , suggesting a potential role for beclabuvir in retreating daclatasvir and asunaprevir nonresponders. UNITY 3 (NCT02123654), a Phase III clinical trial examining the efficacy of daclatasvir plus asunaprevir plus beclabuvir therapy in Japan, is ongoing.
Sofosbuvir plus ledipasvir
Although triple DAA therapy is attractive in principle, the combination of three drugs increases the costs and limits opportunity for retreatment in the event of emergence of multidrug resistance. In another dual DAA approach, an NS5A inhibitor such as daclatasvir or ledipasvir is combined with an NS5B polymerase inhibitor such as sofosbuvir. In a Phase III open label study of treatment-naive Genotype 1 patients randomly assigned to ledipasvir and sofosbuvir combination therapy with or without ribavirin for 12 or 24 weeks, 96% of patients achieved SVR12 after 12 weeks of therapy and 99% achieved SVR12 after 24 weeks of therapy [24] ; [25] . In a related Phase III study, prior nonresponders (including nonresponders to prior telaprevir triple therapy) treated with sofosbuvir plus ledipasvir achieved SVR rates of 94% and 99% after 12 and 24 weeks of therapy, respectively [24] . In a Phase III study examining shorter treatment duration, 94% of noncirrhotic treatment-naive patients achieved SVR12 after 8 weeks and 95% after 12 weeks [26] . The FDA approved Gilead’s HARVONI (ledipasvir co-formulated with sofosbuvir) for Genotype 1 in October 2014 and granted approval for Genotypes 4, 5, and 6 in November 2015.
Although many of the competing DAAs reported in early stages of development have since been discontinued, the combination of paritaprevir with ritonavir (ABT-450/r, a protease inhibitor), ombitasvir (ABT-267, an NS5A inhibitor), and dasabuvir (ABT-333, a non-nucleoside NS5B polymerase inhibitor), with or without ribavirin, has emerged as an alternative to sofosbuvir/ledipasvir and asunaprevir/daclatasvir therapies in both the United States and Japan. AbbVies Viekira Pak (coformulated ombitasvir/paritaprevir/ritonavir and dasabuvir tablets) was approved in the United States in December 2014, and VIEKIRAX (paritaprevir/ritonavir coformulated with ombitasvir) was approved in Japan in September 2015. In a Phase III clinical trial, treatment-naive patients treated with paritaprevir/r, ombitasvir, dasabuvir, and ribavirin for 12 weeks achieved an SVR12 rate of 98% in Genotype 1b patients and 95% in Genotype 1a patients [27] . In another Phase III trial, 99.5% of Genotype 1b patients and 97% of Genotype 1a patients achieved SVR12 [28] . In the Phase III GIFT-1 clinical trial in Japan, 94% of noncirrhotic patients and 91% of cirrhotic patients achieved SVR12 [29] . In a Phase III trial examining prior treatment history, 96% of prior nonresponders achieved SVR after 12 weeks of paritaprevir/r, ombitasvir, dasabuvir, and ribavirin therapy. In a Phase III clinical trial examining treatment duration in patients with compensated cirrhosis, 91% of patients achieved SVR after 12 weeks of therapy, and 96% of patients achieved SVR after 24 weeks of therapy. However, the FDA recently issued a warning concerning treatment of cirrhotic patients after reports of serious liver injury or death, indicating that treatment of cirrhotic patients is not without risk.
Grazoprevir plus elbasvir
Despite issues with toxicity, genotype specificity, and high cross-resistance associated with early protease inhibitors, development of second generation protease inhibitors such as grazoprevir is expected to provide pan-genotypic activity as well as improved resistance profiles. Grazoprevir exhibits SVR rates ranging from 89 to 100% and is insensitive to most RAVs affecting first generation protease inhibitors [30] . In the Phase II C-WORTHY clinical trial, treatment-naive HCV Genotype 1 patients treated with grazoprevir plus elbasvir (an NS5A inhibitor) with or without ribavirin for 12 weeks achieved a 93% SVR rate [31] . Another C-WORTHY study examining treatment duration in prior null responders demonstrated SVR12 rates up to 100% after 18 weeks of grazoprevir, elbasvir, and ribavirin treatment [32] .
Clinical trials and real world DAA treatment outcomes at Hiroshima University Hospital
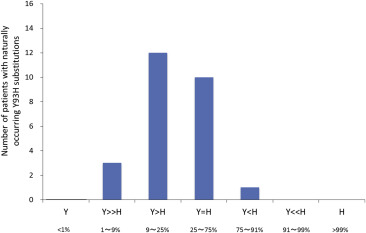
Since approval of the treatment in 2014, we have treated over 200 patients with asunaprevir plus daclatasvir therapy at Hiroshima University Hospital (Table 1 ). Fifty-seven percent of patients have been female, and patients have ranged in age from 36 to 88 years old with a mean age of 74.4. Thirty-four percent of patients were treatment-naive, while 54% had received prior interferon therapy. Another 12 patients had previously been treated with telaprevir triple therapy, and two patients had been treated with simeprevir triple therapy. Thirty-three percent of patients have cirrhosis, and three patients have undergone hemodialysis. Seven patients had received liver transplantation prior to asunaprevir plus daclatasvir therapy. All patients were wild-type for HCV NS3 D168, but 17.5% of patients had varying mixtures of mutant and wild-type amino acids for HCV NS5A L31 and Y93 (Figure 1 ). The presence of baseline NS3 and NS5A variants was determined either by nested PCR followed by the Invader assay [33] or by direct sequencing or deep sequencing.
| Male/female | 86/114 |
| Age, y (range) | 74.4 (36–88) |
| Treatment history | |
|---|---|
| Treatment-naïve | 68 |
| Interferon therapy | 109 |
| Telaprevir + PegIFN/RBV | 12 |
| Simeprevir + PegIFN/RBV | 2 |
| Liver cirrhosis | 66 |
| Baseline resistance-associated variants | |
| HCV NS3 D168 (wild/mutant)a | 198/0 |
| HCV NS5A L31, Y93 (wild/mutant)a | 163/35 |
| Post liver transplantation | 7 |
| Hemodialysis patients | 3 |
HCV = hepatitis C virus; PegIFN/RBV = peg-interferon plus ribavirin.
a. Detected by either direct sequencing or PCR-Invader assay.
|
|
|
Figure 1. Frequency of naturally occurring (preexisting) HCV NS5A Y93H substitutions in patients treated with daclatasvir plus asunaprevir therapy. Patients with Y93H substitutions >91% were not treated with daclatasvir plus asunaprevir. HCV = hepatitis C virus. |
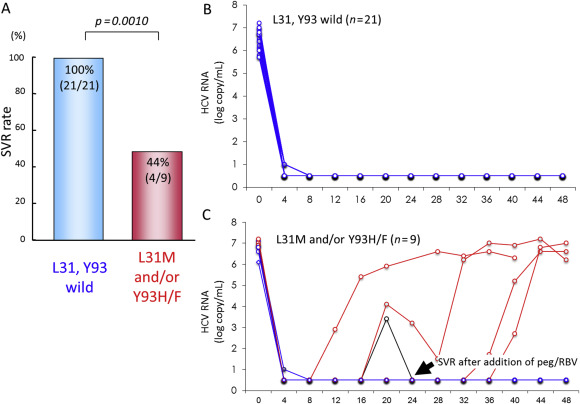
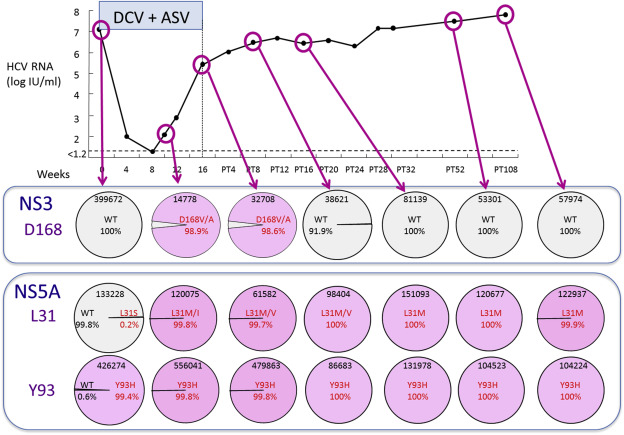
Despite the variability of this patient population, 90% of patients achieved SVR following 24 weeks of asunaprevir plus daclatasvir therapy, while six patients experienced viral breakthrough or relapse (Table 2 ). The SVR rate was low among patients with preexisting NS5A Y93H and/or NS5A L31V/A RAVs, and on-treatment emergence of NS5A L31/Y93 and NS3 D168 variants was associated with treatment failure [4] . In a population sequencing study of 30 patients in a Phase III clinical trial at Hiroshima University, patients with wild type NS5A L31 and Y93 were significantly more likely to achieve SVR. While all patients with wild type NS5A achieved SVR, only 44% of patients with NS5A L31M or Y93H/F substitutions achieved SVR (Figure 2 A). After declining sharply and remaining undetectable during the first weeks of therapy, HCV RNA levels rebounded rapidly to pretreatment levels in some patients with NS5A L31M or Y93H/F substitutions (Figures 2 B and 2C). Figure 3 shows changes in the frequencies of HCV NS3 D168 and NS5A L31/Y93 during treatment and follow-up in a patient who experienced viral breakthrough during asunaprevir plus daclatasvir therapy during a Phase III clinical trial at Hiroshima University. While HCV NS3 D168V/A variants emerged during viral breakthrough, they were replaced by wild type after treatment discontinuation. On the other hand, in addition to the patient’s preexisting Y93H substitution, an L31M/I substitution emerged at the time of viral breakthrough, and both variants persisted stably at >99% throughout the follow-up period.
| Age | Sex | Previous DAA treatment | NS3 D168 | NS5A L31 | NS5A Y93 | Outcome |
|---|---|---|---|---|---|---|
| 72 | M | — | Wildb | L31Mb | Wildb | Breakthrough |
| 74 | M | SMV/IFN/RBV | Wildc | Wild | Wild | Breakthrough |
| 66 | F | SMV/IFN/RBV | Wild | Wild | Wild | Breakthrough |
| 66 | F | — | Wild | Wild | Wild | Breakthrough |
| 65 | F | — | Wild | Wild | Wild | Breakthrough |
| 65 | M | — | Wild | Wild | Wild | Relapse |
DAA = direct-acting antiviral; SMV/IFN/RBV = simeprevir plus peg-interferon plus ribavirin triple therapy; Wild = wild-type amino acid.
a. Presence of preexisting NS3 and NS5A resistance-associated variants was determined by PCR-Invader assay unless otherwise noted.
b. Direct sequencing.
c. Deep sequencing.
|
|
|
Figure 2. Outcome of daclatasvir plus asunaprevir therapy stratified by presence of baseline NS5A substitutions during a Phase III clinical trial at Hiroshima University. Amino acid sequences of L31 and Y93 before daclatasvir plus asunaprevir therapy were determined by population sequencing. (A) SVR rate following daclatasvir plus asunaprevir therapy with respect to baseline NS5A substitutions; (B) time course of HCV RNA levels in patients with wild type HCV NS5A L31 and Y93 amino acids; and (C) time course of HCV RNA levels in patients with preexisting NS5A L31M and/or Y93H/F substitutions. HCV = hepatitis C virus; RNA = ribonucleic acid; SVR = sustained virological response. |
|
|
|
Figure 3. Time course of changes in HCV, NS3, D168, and NS5A L31 and Y93 substitutions in a 75 year old female who experienced viral breakthrough during daclatasvir (DCV) plus asunaprevir (ASV) therapy in a Phase III clinical trial at Hiroshima University. HCV variants were determined by deep sequencing. HCV = hepatitis C virus. |
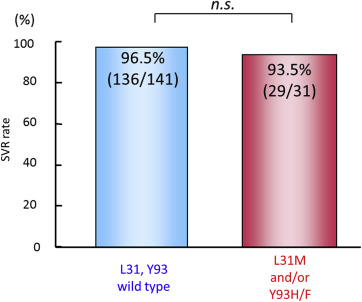
While asunaprevir plus daclatasvir therapy is contraindicated for patients with high pretreatment frequencies of RAVs, patients with relatively low frequencies of naturally occurring drug resistance mutations are nonetheless candidates for real world asunaprevir plus daclatasvir therapy. High SVR rates were achieved both for patients with wild type NS5A (96.5%) as well as those with NS5A L31M or Y93H/F substitutions (93.5%) in our real world experiences with asunaprevir plus daclatasvir therapy so far (Figure 4 ).
|
|
|
Figure 4. Real world SVR24 rates at Hiroshima University following daclatasvir plus asunaprevir therapy in treatment-naïve patients with wild type or L31M and/or Y93H/F NS5A substitutions. Patients with relatively low frequencies of NS5A resistance associated variants were candidates for real world asunaprevir plus daclatasvir therapy. High SVR rates were achieved for both patients with wild type NS5A as well as those with NS5A L31M or Y93H/F substitutions. HCV variants were determined by population sequencing. HCV = hepatitis C virus; SVR = sustained virological response. |
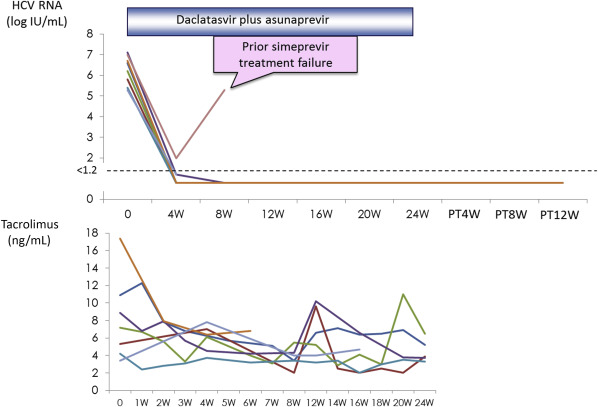
Eight patients with HCV infection after liver transplantation were treated with daclatasvir plus asunaprevir therapy. Figure 5 showed the changes of serum HCV RNA levels of the eight patients. Although one patient with prior simeprevir treatment failure developed viral breakthrough, the remaining seven patients achieved SVR. All patients received tacrolimus for immunosuppression. During the combination treatment, the changes of trough blood concentration of tacrolimus were minimal. No particular adverse events were observed during the therapy.
|
|
|
Figure 5. Changes in HCV RNA levels in eight patients treated with daclatasvir plus asunaprevir following liver transplantation. Although viral breakthrough occurred in one patient who had previously experienced simeprevir treatment failure, the other seven patients achieved SVR (upper panel). No adverse events were observed during therapy, and changes in trough blood concentrations were minimal (lower panel). HCV = hepatitis C virus; RNA = ribonucleic acid; SVR = sustained virological response. |
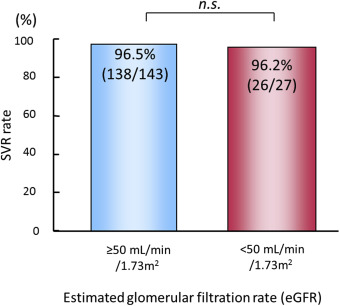
Daclatasvir and asunaprevir are metabolized in the liver and excreted through the bile ducts. Thus, combination therapy with these drugs does not seem to adversely affect renal function. The SVR rate of daclatasvir and asunaprevir for patients with renal dysfunction (estimated glomerular filtration rate [eGFR] < 50 mL/min/1.73m2 ]) was similar to that of patients with normal renal function (eGFR ≥ 50 mL/min/1.73m2 ) (Figure 6 ). The frequency of adverse events such as ALT elevation was also similar between the two groups.
|
|
|
Figure 6. SVR rates in patients with respect to renal function. The SVR rate did not differ significantly between patients with renal dysfunction (right; eGFR < 50 mL/min/1.73m2 ) compared with patients with normal renal function (left; eGFR ≥ 50 mL/min/1.73m2 ). eGFR = estimated glomerular filtration rate; ns = not significant; SVR = sustained virological response. |
Based on response to asunaprevir and daclatasvir therapy to date, we conclude that SVR rates approaching 90% can be achieved and that liver transplant recipients and patients with renal failure can be safely treated. However, SVR rates were low among patients with baseline or treatment-emergent Y93H or L31V/A substitutions, and patients with preexisting NS5A substitutions or who have experienced prior simeprevir plus peg-interferon plus ribavirin treatment failure should avoid treatment with asunaprevir plus daclatasvir. Care should be taken to monitor ALT elevation, although elevated ALT levels may decline rapidly after cessation of therapy. Ledipasvir plus sofosbuvir combination therapy may be effective for retreatment of patients who experience asunaprevir plus daclatasvir treatment failure.
Conclusion
Although the pace of entry of new DAAs into clinical testing may have peaked, a diverse set of DAAs have now been approved in the United States, Japan, and elsewhere, and a number of promising dual and triple DAA therapies are being evaluated in Phase III clinical trials. Antiviral resistance remains a potentially worrying but manageable problem, although the long-term implications of DAA resistance are difficult to predict. Pretreatment screening of NS3/4A or NS5A RAVs is advisable prior to simeprevir or daclatasvir therapy, respectively, and on-treatment monitoring should be used to detect treatment-emergent RAVs, although effective monitoring might await wide-spread adoption of simple and cost-effective RAV detection methods [33] . In principle, nearly all patients should now be eligible for HCV therapy and have excellent prospects for achieving SVR, but in practice some patients with unmet needs await further advances in the treatment of chronic HCV.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowedgments
This work was partially supported by research funding from the Research Program on Hepatitis from the Japan Agency for Medical Research and Development, AMED .
References
- [1] K. Mohd Hanafiah, J. Groeger, A.D. Flaxman, S.T. Wiersma; Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence; Hepatology, 57 (2013), pp. 1333–1342
- [2] D. Lavanchy; The global burden of hepatitis C; Liver Int, 29 (2009), pp. 74–81
- [3] K. Chayama, C.N. Hayes, K. Yoshioka, H. Moriwaki, T. Okanoue, S. Sakisaka, et al.; Accumulation of refractory factors for pegylated interferon plus ribavirin therapy in older female patients with chronic hepatitis C; Hepatol Res, 40 (2010), pp. 1155–1167
- [4] S. Yoshimi, M. Imamura, E. Murakami, N. Hiraga, M. Tsuge, Y. Kawakami, et al.; Long term persistence of NS5A inhibitor-resistant hepatitis C virus in patients who failed daclatasvir and asunaprevir therapy; J Med Virol (2015) http://doi.org/10.1002/jmv.24255 PubMed PMID: 25954851
- [5] A. Aghemo, R. De Francesco; New horizons in hepatitis C antiviral therapy with direct-acting antivirals; Hepatology, 58 (2013), pp. 428–438
- [6] I.M. Jacobson, J.G. McHutchison, G. Dusheiko, A.M. Di Bisceglie, K.R. Reddy, N.H. Bzowej, et al.; Telaprevir for previously untreated chronic hepatitis C virus infection; N Engl J Med, 364 (2011), pp. 2405–2416
- [7] S. Zeuzem, P. Andreone, S. Pol, E. Lawitz, M. Diago, S. Roberts, et al.; Telaprevir for retreatment of HCV infection; N Engl J Med, 364 (2011), pp. 2417–2428
- [8] N. Hayashi, N. Izumi, H. Kumada, T. Okanoue, H. Tsubouchi, H. Yatsuhashi, et al.; Simeprevir with peginterferon/ribavirin for treatment-naive hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial; J Hepatol, 61 (2014), pp. 219–227
- [9] N. Hayashi, N. Mobashery, N. Izumi; Vaniprevir plus peginterferon alfa-2a and ribavirin in treatment-experienced Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase II study; J Gastroenterol, 50 (2015), pp. 238–248
- [10] H. Kumada, Y. Suzuki, K. Ikeda, J. Toyota, Y. Karino, K. Chayama, et al.; Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection; Hepatology, 59 (2014), pp. 2083–2089
- [11] M. Mizokami, O. Yokosuka, T. Takehara, N. Sakamoto, M. Korenaga, H. Mochizuki, et al.; Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial; Lancet Infect Dis, 15 (2015), pp. 645–653
- [12] M. Omata, S. Nishiguchi, Y. Ueno, H. Mochizuki, N. Izumi, F. Ikeda, et al.; Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial; J Viral Hepatitis, 21 (2014), pp. 762–768
- [13] A.S. Lok, D.F. Gardiner, E. Lawitz, C. Martorell, G.T. Everson, R. Ghalib, et al.; Preliminary study of two antiviral agents for hepatitis C genotype 1; N Engl J Med, 366 (2012), pp. 216–224
- [14] K. Chayama, S. Takahashi, J. Toyota, Y. Karino, K. Ikeda, H. Ishikawa, et al.; Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders; Hepatology, 55 (2012), pp. 742–748
- [15] A.S. Lok, D.F. Gardiner, C. Hezode, E.J. Lawitz, M. Bourliere, G.T. Everson, et al.; Randomized trial of daclatasvir and asunaprevir with or without PegIFN/RBV for hepatitis C virus genotype 1 null responders; J Hepatol, 60 (2014), pp. 490–499
- [16] H. Kumada, F. Suzuki, Y. Suzuki, J. Toyota, Y. Karino, K. Chayama, et al.; Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese hepatitis C virus patients; J Gastroenterol Hepatol, 31 (2016), pp. 14–22
- [17] M. Manns, S. Pol, I.M. Jacobson, P. Marcellin, S.C. Gordon, C.Y. Peng, et al.; All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study; Lancet, 384 (2014), pp. 1597–1605
- [18] M. Liu, M. Tuttle, M. Gao, J.A. Lemm; Potency and resistance analysis of hepatitis C virus NS5B polymerase inhibitor BMS-791325 on all major genotypes; Antimicrob Agents Chemother, 58 (2014), pp. 7416–7423
- [19] G.T. Everson, K.D. Sims, M. Rodriguez-Torres, C. Hezode, E. Lawitz, M. Bourliere, et al.; Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection; Gastroenterology, 146 (2014), pp. 420–429
- [20] A.J. Muir, F. Poordad, J. Lalezari, G. Everson, G.J. Dore, R. Herring, et al.; Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis; JAMA, 313 (2015), pp. 1736–1744
- [21] N. Shi, N. Hiraga, M. Imamura, C.N. Hayes, Y. Zhang, K. Kosaka, et al.; Combination therapies with NS5A, NS3 and NS5B inhibitors on different genotypes of hepatitis C virus in human hepatocyte chimeric mice; Gut, 62 (2013), pp. 1055–1061
- [22] H. Abe, C.N. Hayes, N. Hiraga, M. Imamura, M. Tsuge, D. Miki, et al.; A translational study of resistance emergence using sequential direct-acting antiviral agents for hepatitis C using ultra-deep sequencing; Am J Gastroenterol, 108 (2013), pp. 1464–1472
- [23] J. Friborg, N. Zhou, Z. Han, X. Yang, P. Falk, P. Mendez, et al.; In vitro assessment of re-treatment options for patients with hepatitis C virus genotype 1b infection resistant to daclatasvir plus asunaprevir; Infect Dis Ther (2014 Dec 17) [Epub ahead of print]
- [24] N. Afdhal, K.R. Reddy, D.R. Nelson, E. Lawitz, S.C. Gordon, E. Schiff, et al.; Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection; N Engl J Med, 370 (2014), pp. 1483–1493
- [25] N. Afdhal, S. Zeuzem, P. Kwo, M. Chojkier, N. Gitlin, M. Puoti, et al.; Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection; N Engl J Med, 370 (2014), pp. 1889–1898
- [26] K.V. Kowdley, S.C. Gordon, K.R. Reddy, L. Rossaro, D.E. Bernstein, E. Lawitz, et al.; Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis; N Engl J Med, 370 (2014), pp. 1879–1888
- [27] J.J. Feld, K.V. Kowdley, E. Coakley, S. Sigal, D.R. Nelson, D. Crawford, et al.; Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin; N Engl J Med, 370 (2014), pp. 1594–1603
- [28] P. Ferenci, D. Bernstein, J. Lalezari, D. Cohen, Y. Luo, C. Cooper, et al.; ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV; N Engl J Med, 370 (2014), pp. 1983–1992
- [29] H. Kumada, K. Chayama, L. Rodrigues Jr., F. Suzuki, K. Ikeda, H. Toyoda, et al.; Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis; Hepatology, 62 (2015), pp. 1037–1046
- [30] I. Gentile, A.R. Buonomo, F. Borgia, E. Zappulo, G. Castaldo, G. Borgia; MK-5172: a second-generation protease inhibitor for the treatment of hepatitis C virus infection; Expert Opinion Investigat Drug, 23 (2014), pp. 719–728
- [31] M. Sulkowski, C. Hezode, J. Gerstoft, J.M. Vierling, J. Mallolas, S. Pol, et al.; Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial; Lancet, 385 (2015), pp. 1087–1097
- [32] E. Lawitz, E. Gane, B. Pearlman, E. Tam, W. Ghesquiere, D. Guyader, et al.; Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial; Lancet, 385 (2015), pp. 1075–1086
- [33] S. Yoshimi, H. Ochi, E. Murakami, T. Uchida, H. Kan, S. Akamatsu, et al.; Rapid, sensitive, and accurate evaluation of drug resistant mutant (NS5A-Y93H) strain frequency in genotype 1b HCV by invader assay; PLoS One, 10 (2015), p. e0130022
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?