(Created page with " ==Abstract== Sequestrating CO<sub>2</sub> in deep saline aquifers is maybe the most effective option to mitigate CO<sub>2</sub> emission. The storage capacity of CO<sub>2</...") |
m (Scipediacontent moved page Draft Content 370129456 to Jin et al 2017a) |
(No difference)
| |
Latest revision as of 14:14, 1 June 2017
Abstract
Sequestrating CO2 in deep saline aquifers is maybe the most effective option to mitigate CO2 emission. The storage capacity of CO2 is the key factor for site selection before a project is carried out. Most of the existing methods are for assessment of CO2 sequestered by stratigraphic and structural trapping, as well as residual trapping and solubility trapping. In this study, we used a new method that considers CO2 consumption through geochemical reactions with minerals of reservoir rocks, mainly sandstones. Contribution of storage capacity from carbonate mineral mainly refers to calcite is excluded. That is because lifetime of calcite (the whole time from reaction starting to calcite running out) is very short contrast with geological time-scale or it is a temporary trapping. The geochemical reactions between CO2 and feldspar minerals with thousand-year lifetime are regarded as the permanent methods for trapping CO2. CO2 consumptions by K-feldspar, albite, and anorthite are assessed with volume method based on corresponding geochemical reactions. Storage efficiency factor is also considered as one of the most important parameters in the reaction formula and it depends on specific surface area of minerals contacting with formation water and kinetics of precipitation and dissolution of minerals. We assessed the CO2 storage capacity of mineral trapping in Baokang sedimentary system, south Songliao Basin through the detailed analysis of geological data in this area. The calculated results show the total CO2 storage capacity of mineral trapping of the study area is 457.5–5114.5 Mt and the corresponding storage efficiency factor is 1%. The CO2 consumed by albite makes up more than 60% of the total storage capacity and it is about 30% for anorthite.
Introduction
CO2 capture and storage (CCS) technology is one of the best options to mitigate large-scale CO2 emissions of point sources [1, 2]. The captured CO2 is suggested to be inject into geological formations including depleted oil and gas fields, unmineable coal beds and deep saline aquifers, among which, deep saline aquifers hold the most potential that accounting for more than 90% of the total subsurface capacity [1, 3].
Estimation of CO2 storage capacity in deep saline aquifers is the first step before a storage project is located. The total CO2 storage capacity is composed of CO2 trapped in forms of free state, soluble state, and mineral state, which correspond with the stratigraphic-structural-residual trapping mechanism, solubility trapping mechanism and mineral trapping mechanism, respectively. Methods for CO2 capacity assessment of free state and soluble state are available from the studies of Carbon Sequestration Leadership Forum (CSLF), United Stated Department of Energy (USDOE), United States Geology Survey (USGS) and other authors from Europe and Japan [4-9].
The capacity of mineral trapping CO2 varies greatly from ~5% to ~40%, which depends on the composition of formation water and reservoir rocks [1, 10-12]. Now, CO2 mineral trapping per unit volume of rock is assessed through 2D transport reactive simulation and its contribution to the total storage capacity can be comparable to solubility trapping although it takes much longer time for geochemical reactions to have a significant impact [12-14].
The immediate and long-term changes of CO2 injected into sandstone reservoir were modeled by geochemical simulating methods. The results show the minerals which can trap CO2 are dawsonite, siderite, calcite or dolomite. The changes in sandstones porosity are insignificant in assessing capacity. The calculation methods of the capacity are based on the USDOEs method and the hypothesis that all the pores are filled with CO2 and all the CO2 are trapped [15, 16].
There is no mathematical method with detailed formula to calculate CO2 storage capacity in form of mineral state instead of numerical modeling yet. Though the contribution of mineral trapping to total storage capacity is variable and it works only on a timescale of a thousands of years, it is the safest way to sequester injected CO2.
A method is presented in this paper, in which mineral compositions of reservoir rocks and CO2 consumption by geochemical reactions were considered. The following methodology is proposed to establish the relationship between CO2 consumptions by silicate minerals and CO2 storage capacity by mineral trappings.
Reviews of the Assessment Methods of CO2 Storage Capacity
Existing calculation methods of CO2 storage capacity mainly deal with structural and stratigraphic trapping, residual trapping and solubility trapping. For structural, stratigraphic and residual trapping, the CO2 is thought to be stored in supercritical state and the total storage capacity is calculated based on effective pore volume of reservoir rocks. One of the important parameters of this method is the storage efficiency factor, which ranges from 1% to 4% for the confidence interval from 15% to 85% by Monte Carlo simulation, with the average of 2.4% for 50% confidence [6].
Another popular method is proposed by the CSLF Task Force, which includes theoretical, effective, practical and matched capacity depending on the nature and purpose of the assessment and the geological dataset [4, 5]. It is also can be divided into structural, stratigraphic, residual, solubility, and mineral trapping by their mechanisms. For structural and stratigraphic trapping, it is similar to storing CO2 in depleted oil and gas reservoirs. CO2 sequestrated by solubility trapping is assessed with volumetric method and the storage efficiency factor suggested by USDOE is adopted.
Assessing methods from Japan are based on geological structures, which include two main categories. Type A refers to oil-gas reservoirs, neighboring aquifers, and aquifers in anticlinal structures. Type B is aquifers in monoclinal structures on land or offshore [8, 9].
From the introductions above, almost all the methods deal with CO2 by structural and stratigraphic trapping, residual trapping and solubility trapping, while there is no method assessing CO2 stored by mineral trapping, even this method is the safest one. Since most of the reservoir rocks studied for CO2 sequestration are sandstones, the geochemical reactions between minerals of sandstones, such as feldspar minerals and formation water containing dissolved CO2 can be used to assess CO2 storage. This method can be defined as CO2 storage capacity of mineral trapping. The details of this method will be discussed in the following sections. The implication for CO2 storage capacity of mineral trapping in Baokang sedimentary system, south Songliao Basin will be carried out in this paper.
Methods
The reservoir rocks from Songliao Basin are smashed and panned, then the pure feldspar is obtained by hand pick. The density of the feldspar is measured in the Institute of Geology and Geophysics, Chinese Academy of Science, with the ZMD-4 Solid Density Meter produced by Shanghai Fangrui Instrument Ltd. based on the Archimedes principle. The porosity and permeability of the reservoir rocks are obtained by pressing mercury experiment. The instrument of this experiment is Pore Master 33 mercury injection apparatus produced by American Quantachrome Ins. The measuring range of this instrument is 1.5 KPa-231 MPa. The measuring pore diameter is 0.007–1000 μm. The balance time is 1–21,600 sec. The balance rate is 0–1000 μL/g/sec. The experiment is also performed in the Institute of Geology and Geophysics, Chinese Academy of Science, and the detailed process is followed Shangguans work [17].
Capacity Assessment Method of Mineral Trapping
Previous studies indicate that once being injected, the CO2 is stored in terms of structural and stratigraphic, residual, solubility and mineral trapping mechanisms, while the contributions of different mechanisms vary with time scale [1, 18, 19]. Among these four trapping mechanisms, mineral trapping is thought to be the safest way, because CO2 is transformed into secondary minerals by geochemical reactions. It is also defined as “permanent trapping”. The contribution of mineral trapping is indicated by the result of transport reactive modeling, with the help of plenty of detailed geological data. In the time scale of million years, the mineral trapping capacity differs in different regions. In the Gulf Coast, it is 2–5 kg CO2 per unit volume rock (1 m3) while it is 10 kg or even more in Songliao Basin [12, 20]. However, there is no exact mathematical method to assess CO2 storage capacity of mineral trapping now. In the paper, we proposed and discussed a calculation method.
The storage capacity of mineral trapping is constrained by mineral compositions of reservoir rocks and chemistry compositions of formation waters. Review of published papers and conference communication indicates that sandstone is the most typical CO2 trapping reservoirs for continental oil and gas fields [11, 12, 21-24]. Normally, sandstone is composed by quartz, feldspar, lithic fragment, clay minerals and sometimes carbonate cements, in particular calcite. Dominant geochemical reactions between acidic fluid caused by CO2 dissolution and feldspars and carbonate minerals are shown in the following equations (1)-(8) [25-27].
|
|
(1) |
|
|
(2) |
|
|
(3) |
|
|
(4) |
|
|
(5) |
|
|
(6) |
|
|
(7) |
|
|
(8) |
Average lifetimes of common minerals in sandstones are shown in Table 1 (Φ = 1 mm, T = 25°C, pH = 5), and they are important parameters of reaction rats [28]. Calcite is the most unstable mineral and it will firstly react with acidic formation water after CO2 is injected (eq. (1)). The CO2 would be trapped transitorily because bicarbonate is unstable and equation (1) is reversible. Therefore, the CO2 trapped by calcite is not included in the storage capacity of mineral trapping. The reaction between acidic fluid and feldspar minerals is permanent trapping.
| Mineral | Lifetime [year] | Mineral | Lifetime [year] |
|---|---|---|---|
| Calcite | 0.43 | Albite | 575,000 |
| Wollastonite | 79 | Microcline | 921,000 |
| Forsterite | 2300 | Epidote | 923,000 |
| Diopside | 6800 | Biotite | 2,600,000 |
| Enstatite | 10,100 | Baolinite | 6,000,000 |
| Sanidine | 291,000 | Quartz | 34,000,000 |
Different minerals will have different geochemical reactions with CO2 dissolution and form different reactants, including kaolinite, illite, dawsonite, and muscovite (eqs (3)-(8)). 1 mol of CO2 will be consumed for reaction with 1 mol of albite (eqs (5) and (6)), and all the equations listed above can be used for calculation of CO2 trapped by minerals. The physical properties and the CO2 consumptions of three typical feldspar minerals K-feldspar, albite, and anorthite are listed in Table 2.
| Mineral name | Chemical formula | Mfeldspar (molecular weight) | ρfeldspar [kg/m3] feldspar density | R (the ratio of feldspar mineral to CO2) |
|---|---|---|---|---|
| K-feldspar | KAlSi3O8 | 279.07 | 2.55–2.67 × 103 | 0.5 |
| Albite | NaAlSi3O8 | 262.96 | 2.55–2.60 × 103 | 1 |
| Anorthite | CaAl2Si2O8 | 278.94 | 2.75–2.76 × 103 | 1 |
The calculation method of mineral trapping is illustrated in equation (9).
|
|
(9) |
In equation (9), [Mt] is the total CO2 storage capacity of mineral trapping; ρfeldspar [kg/m3] is the average density of feldspar mineral, Vfeldspar [km3] is the total volume of feldspar mineral, [g/mol] and Mfeldspar [g/mol] are the molecular weight of CO2 and feldspar mineral; R is the ratio of feldspar mineral to consumed CO2. E is the storage efficiency factor, which is constrained by exposing the area of minerals to the formation water with dissolved CO2, especially the specific surface area of minerals, kinetics of precipitation, and dissolution of minerals. The value of E is 1% and 4% for the 15% and 85% confidence intervals for the time being, which is proposed by the USDOE Subgroup through Monte Carlo simulations in deep saline aquifer for conditions characteristic to North America.
Vfeldspar is the total volume of the feldspar that can be calculated by equation (10). V is the total volume of sandstone reservoir; ϕ is the effective porosity; Cfragments is the content of rock fragments; Cfeldspar is the content of feldspar minerals; The volumes of these three feldspar minerals can be calculated with equation (11). XK-feldsapr, Xalbite and Xanorthite are the proportions of K-feldspar, albite, and anorthite respectively.
|
|
(10) |
|
|
(11a) |
|
|
(11b) |
|
|
(11c) |
Combining the equations ((9)–11), the quantity of CO2 trapped by feldspar minerals can be calculated with equation (12).
|
|
(12a) |
|
|
(12b) |
|
|
(12c) |
|
|
(12d) |
Implications for the CO2 Storage Capacity Assessment of Mineral Trapping in South Songliao Basin
Location and geological setting
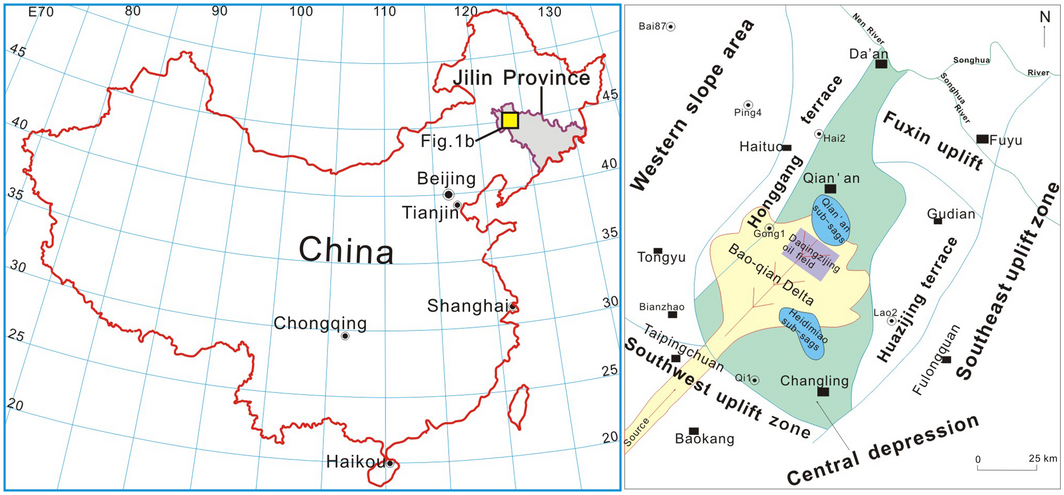
The southern part of Songliao Basin locates in the northeast of Jilin province and belongs to central part of Songliao Plain (Fig. 1). The south Songliao Basin is divided into four primary structural blocks, the western slope area, the central depression, the southeast uplift, and the southwest uplift [29, 30]. The Upper Cretaceous in the south Songliao Basin is divided into five formations, which are Qingshankou, Yaojia, Nenjiang, Sifangtai and Mingshui Formations from bottom upward.
|
|
|
Figure 1. The tectonic location of the study area (revised slightly from Wang et al., 2007) [30]. |
The Baokang sedimentary system studied in this paper is located in the central depression (Fig. 1) and it is a typical fluvial-delta sedimentation [29, 31]. The CO2 storage capacity of mineral trapping in the complex delta sedimentary system developed during the Qingshankou and Nenjiang period is assessed.
Reservoir properties
Normally, CO2 can be trapped in supercritical conditions with suitable temperature and pressure when reservoir depth is more than 800 m [32, 33]. When reservoir depth is more than 2500 m, though the temperature and pressure are satisfied to make CO2 in supercritical conditions, the facility for CO2 injection is much more expensive and the cost becomes major problem. The lithic columnar sections of 105 wells are analyzed and the reservoir depth information is listed in Table 3. We find the reservoirs of the Yaojia–Qingshankou Formation located in the depth interval suitable for CO2 storage.
| Formation | Minimum depth [m] | Maximum depth [m] | Average depth [m] |
|---|---|---|---|
| The top of Yaojia | 1287 | 2086 | 1809 |
| The bottom of Qingshankou | 1469 | 2583 | 2424 |
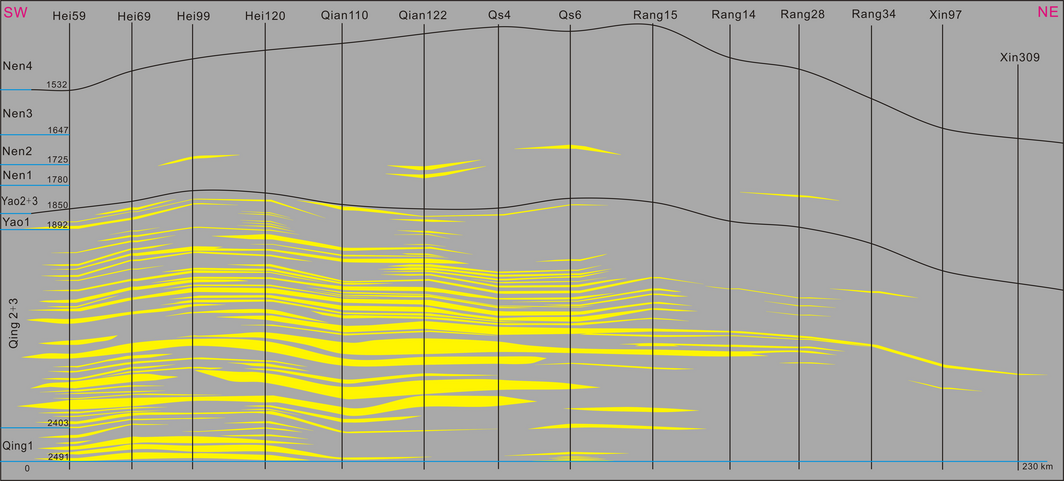
The mud-logging data and logging data of more than 150 wells of the study area are collected and analyzed. Based on the materials of 42 wells located at the delta front and the method of sequence stratigraphy, six connecting well profiles are drawn (Fig. 2). While here, due to the shortness of the paper, we present only one of them. In all these profiles, we have the maximum flooding surfaces (MFS) of the first section of Qingshankou Formation as their isochronous surface. We also made a connecting well profile along the stream direction of the Baokang Drainage, which is the first in the study history of this area. The lake in the Songliao Basin experienced an evolutionary process of “expansion-shrinkage-expansion- recession” when the Quantou Formation through Nenjiang Formation was deposited. This evolutionary process developed the progradational-type delta sand bodies of the First Section through the Third Section of the Qingshankou Formation and the retrogradation-type delta sand bodies of the Third Section of the Qingshankou Formation through the First Section of the Yaojia Formation. These two stacked sand bodies provide space for the storage of CO2, and they are the target reservoirs in the study area. After that the lake surface turned largely and developed dark mudstone with huge thickness of the Second-Third Member of the Yaojia Formation through the Third Member of the Nenjiang Formation, which is the effective cap rock in the study area. The two thick sand bodies and the thick dark mudstones form an excellent reservoir-seal assemblage, which is suitable for storage of CO2.
|
|
|
Figure 2. The connecting-well profile from Qingshankou to Nenjiang Formation. |
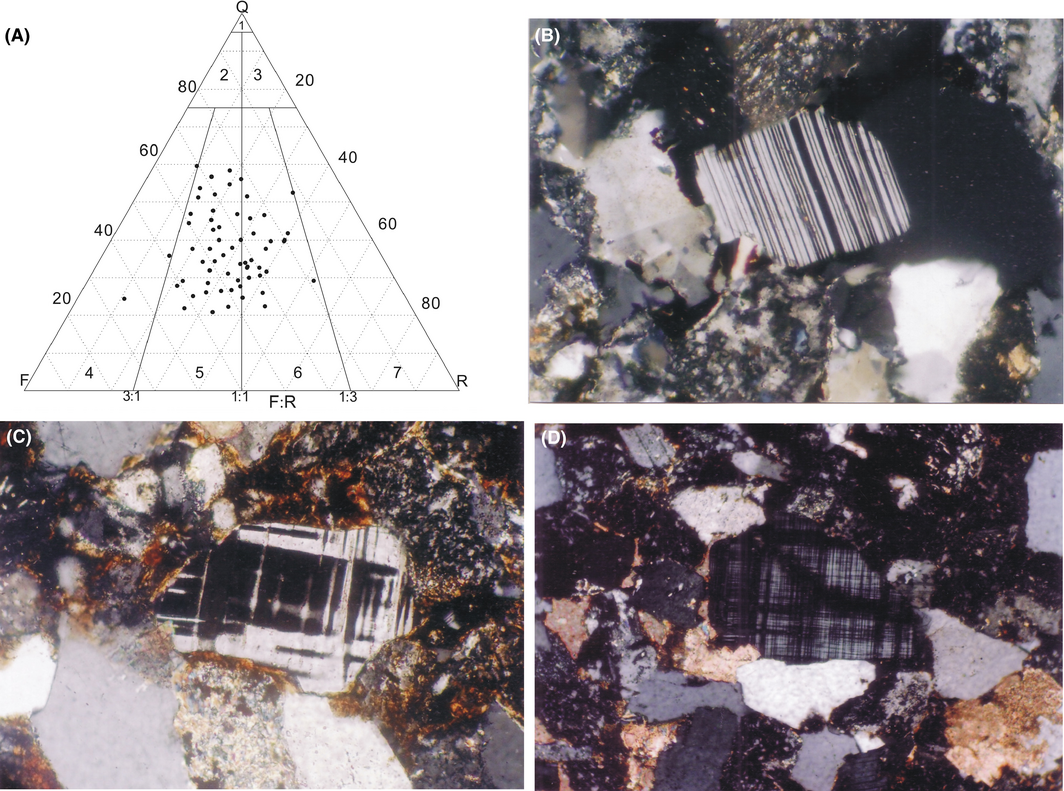
The average thickness of sandstone reservoirs is 200 m and lithic-feldspar sandstones and feldspar-lithic sandstones are the dominated rocks. The sandstones are composed of quartz, feldspar, rock fragments, and few heavy minerals. The contents of quartz, feldspar, and rock fragments are 23–60%, 15–52%, and 17–49%, respectively, while the content of mud is always <20% (Fig. 3). The main cementation types of the sandstones are porous and porous-regenerative. The main matrix and cement are clay, and the secondary ones are calcite with the content of 5.2–16.8%, 9.8% on average. The other main matrix and cement are authigenic quartz, authigenic clay minerals, and feldspar secondary enlargement.
|
|
|
Figure 3. The typical rocks and minerals of the sandstone reservoirs. (A) Sandstone types, 1- quartz arenite, 2-subarkose, 3-sublitharenite, 4- arkose, 5- lithicarkose, 6- feldspathic litharenite, 7- litharenite (Folks sandstone classification, 1974) [34]. (B) Hong75-9-1# plagioclase, cross-polarized light, ×50. (C) Dabei10-12# perthite, cross-polarized light, ×50. (D) Dabei10-12#-8-4, microcline, cross-polarized light, ×50. |
The porosity and permeability of the reservoir rocks in the study area vary greatly. The average effective porosity is 15.16% and the average permeability is 10 md according to the statistical data from 105 wells (Table 4).
| Formation | Porosity [%] | Main range [%] | Permeability [md] | Main range [md] |
|---|---|---|---|---|
| Yaojia | 3.40–23.9 | 10–20 | 0.045–42.50 | 1.9–20.0 |
| Qingshankou | 3.9–29.5 | 9–22 | 0.027–40.93 | 0.5–15 |
Cap rock properties
After analyzing the well columns, the thickness of the overlying cap rocks of mudstones from the Yaojia and Nenjiang Formation varies from 282 m to 486 m and the average is 358.4 m. The mudstones are composed of massive mudstones, silty mudstones, oil shales, and mud shales. The massive mudstones with an average thickness of 318 m amount to 89.6% of the total cap rocks. They are good completeness, high mechanical strength, and low permeability and are proved to be favorable cap rocks. The thickness of these mudstones and proportions are showed in Table 5.
| Cap rocks | Minimum thickness (m) | Maximum thickness (m) | Average thickness (m) | Minimum content (%) | Maximum content (%) | Average content (%) |
|---|---|---|---|---|---|---|
| Massive mudstones | 214.5 | 442.5 | 318.21 | 46.91 | 96.97 | 89.58 |
| Silty mudstones | 4 | 138 | 28.5 | 1.154 | 34.62 | 7.382 |
| Oil shales | 0 | 15 | 7.28 | 0 | 4.975 | 2.036 |
| Mud shales | 0 | 132.5 | 40.9 | 0 | 27.749 | 1.0 |
The total area of dark mudstone of the Songliao Basin is 22,600–35,100 km2 and it continuously existed in the horizontal direction making up the regional cap rocks. Tectonic fracture is the main type of fractures developing in the study area from the analysis of well logging and core samples. Regional geological investigations indicate that fractures are mainly developed in the reservoirs of the First and the Second Sections of the Qing Shankou Formation, while in the cap rocks of Yaojia Formation and Nenjiang Formation, there are few fractures [30, 35, 36].
CO2 storage capacity of mineral trapping in the study area
Mineral compositions
The Songliao Basin is a nonmarine sedimentary basin. The mineral composition of deposit in this basin is very complex. The content of unstable feldspars is high, amounting for 15–52% of the total clasts. By the analysis of SEM of LEO 1450vp, acidic plagioclases are the main feldspars; the content of K-feldspars is low and the content of anorthites is medium. The detailed mineral compositions of reservoir rocks are listed in Table 6. The contents of clasts in reservoir rocks, feldspars in clasts and K-feldspar, albite and anorthite in feldspars are analyzed, which are basic parameters for CO2 storage capacity calculation.
| Minerals | Content (%) |
|---|---|
| Rock fragments (Cfragments) | 40–85 |
| Feldspar in debris (Cfeldspar) | 15–52 |
| K-feldspar in feldspar (XK-feldsapr) | 5–10 |
| Albite in feldspar (Xalbite) | 50–70 |
| Anorthite in feldspar (Xanorthite) | 20–35 |
The total volume of sandstone reservoir of the study area
Four sections of sandstone reservoir are chosen to assess CO2 storage capacity, and they are the First Member of Yaojia Formation, the First to Third Member of Qingshankou Formation. The total net volume of the four sandstone reservoirs is 2459.2 km3 calculated from the sedimentary analysis of 105 wells of this area and regional geological survey report (Tab 7). The effective porosities of these four reservoirs vary from 9.2% to 18.7% (Table 7) and the net volumes of the sandstone reservoirs are 72.6–1220.1 km3 (Table 5). The sandstone reservoirs of the third Member of Qingshankou Formation are more favorable for CO2 storage than other reservoirs.
| Parameters | The 1st member of Qingshankou Fm. | The 2nd member of Qingshankou Fm. | The 3rd member of Qingshankou Fm. | The 1st member of Yaojia Fm |
|---|---|---|---|---|
| Total volume (V, km3) | 430 | 980 | 1500 | 80 |
| Effective porosity (ϕ, %) | 14.1 | 18.7 | 18.7 | 9.2 |
| Net volume (km3) | 369.3 | 797.1 | 1220.1 | 72.6 |
| Total net volume (km3) | 2459.2 | |||
CO2 storage capacity assessment of the study area
The storage efficiency factor E is assumed to be 1% with reference to the statistical values of USGS modeling. With the parameters discussed above, the minimum and maximum of CO2 storage capacity of minerals trapping are assessed and the results are showed in Table 8. Considering the minimum content of clasts and feldspars, the CO2 storage capacity of these four reservoirs is 457.5 Mt. Considering the maximum content of clasts and feldspars, it is 5114.4 Mt. The CO2 consumed by albite dissolution takes up more than 60% of the total storage capacity and the CO2 consumed by anorthite takes up about 30%.
| Trapping minerals | Parameters | CO2 capacity (Mt) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ (kg/m3) | Cfragments (%) | Cfeldspar (%) | X (%) | M (g/mol) | Min | Max | ||||
| Min | Max | Min | Max | Min | Max | |||||
| K-feldspar | 2550.0 | 40.0 | 85.0 | 15.0 | 52.0 | 5.0 | 10.0 | 279.1 | 14.8 | 218.5 |
| Albite | 2550.0 | 50.0 | 70.0 | 263.0 | 314.7 | 3246.0 | ||||
| Anorthite | 2750.0 | 20.0 | 35.0 | 279.0 | 128.0 | 1649.9 | ||||
| Minmum CO2 storage capacity (Mt) | 457.5 | |||||||||
| Maxmum CO2 storage capacity (Mt) | 5114.4 | |||||||||
Conclusions
In this paper, an assessing method of CO2 storage capacity by mineral trapping is used from the geochemical point of view. The reactions between the formations water with dissolved CO2 and the minerals of reservoir rocks, especially feldspar minerals are the basic for method establishment. All the minerals exposed to acidic fluid induced by CO2 injection in reservoirs are assumed to react sufficiently.
The CO2 storage capacity of mineral trapping in Baokang sedimentary system, south Songliao Basin is assessed with detailed analysis of the regional geological data. The most favorable reservoirs are the First to the Third Member of Qingshankou Formation, and the First Member of Yaojia Formation of the late Cretaceous. The corresponding cap rocks of Yaojia Formation and Nenjiang Formation have an average thickness of 358.4 m. The dominant feldspar minerals in the study area are albite, anorthite and K-feldspar. The total CO2 storage capacity of mineral trapping is 457.5–5114.5 Mt with the storage efficiency factor being 1%. The CO2 consumed by albite makes up more than 60% of the total storage capacity and anorthite makes up about 30%.
Acknowledgments
We thank Professor Li Dazhou and Professor Tian Xingyou of IGGCAS for their comments on this manuscript. This study was financially supported by a grant from Hebei Natural Science Foundation (No. D2015402125).
Conflict of Interest
None declared.
References
- IPCC. 2005. Pp. 207–224. Special report on carbon dioxide capture and storage: prepared by working group III of the intergovernmental panel on climate change. Cambridge University Press, New York, NY.
- IEA Greenhouse Gas RandD Program (IEA GHG). 2007. Role of Risk Assessment in Regulatory Framework for Geological Storage of CO2: Feedback from Regulators and Implementers, Report, 88.
- Burnol, A., I. Thinon, L. Ruffine and J. M. Herri. 2015. Influence of impurities (nitrogen and methane) on the CO2 storage capacity as sediment-hosted gas hydrates Application in the area of the Celtic Sea and the Bay of Biscay. Int. J. Greenh. Gas Control35:96–109.
- CSLF (Carbon Sequestration Leadership Forum). 2005. Phase I Final Report from the Task Force for Review and Identification of Standards for CO2 storage capacity Measurement, CSLF-T-2005-9. Available at http://www.cslforum.org/documents/Taskforce_Storage_Capacity_Estimation_Version_2.pdf
- CSLF (Carbon Sequestration Leadership Forum). 2007. Phase II Final Report from the Task Force for Review and Identification of Standards for CO2 storage capacity Measurement.
- USDOE. National Energy Technology Laboratory. 2008. Carbon sequestration altas of the United States and Canada (2d ed.: Atlas II): 142 p, Available at http://www.nelt.doe.gov/techonogies/carbon_seq/refshelf/atlasII/2008%20ATLAS_introduction_pdf (accessed 12 May 2010).
- Brennan, S., R. Burruss, M. Merrill, P. Freeman, and L. F. Ruppert. 2010. A probabilistic assessment methodology for the evaluation of geologic carbon dioxide storage. US Department of the Interior, US Geological Survey.
- Tanaka, S.1995. Possibility of underground CO2 storage in Japan. Energy Convers. Manag.36:527–530.
- Takahashi, T., T. Ohsumi, K. Nakayama, K. Koide, and H. Miida. 2009. Estimation of CO2 aquifer storage potential in Japan. Energy Procedia1:2631–2638.
- Stuart, M. V., B. S. L. Gilfillan, G. Holland, D. Blagburn, S. Stevens, M. Schoell et al. 2009. Solubility trapping in formation water as dominant CO2 sink in natural gas fields. Nature458:614–618.
- Audigane, P., I. Gaus, I. Czernichowski-Lauriol, K. Pruess, and T. Xu. 2007. Two-dimensional reactive transport modeling of CO2 injection in a saline aquifer at the Sleipnersite, North sea. Am. J. Sci.307:974–1008.
- Xu, T., J. A. Apps, and K. Pruess. 2003. Reactive geochemical transport simulation to study mineral trapping for CO2 disposal in deep Cretaceous formations. J. Geophys. Res.108:2071.
- (Maja) Buschkuehle, B. E., and E. H. Perkins. 2005. Mineralogy and geochemical trapping of acid gas in the Edmonton area of central Alberta, Canada. Greenhouse Gas Control Technol.1:459–467.
- Labus, K., and P. Bujok. 2011. CO2 mineral sequestration mechanisms and capacity of saline aquifers of the Upper Silesian Coal Basin (Central Europe)-Modeling and experimental verification. Energy36:4974–4982.
- Labus, K., R. Tarkowski, and M. Wdowin. 2010. Assessment of CO2 sequestration capacity based on hydrogeochemical model of Water-Rock interactions in the potential storage site within the Bełchatów area (Poland). Min. Resour. Manag.26:69–84.
- Labus, K., R. Tarkowski, and M. Wdowin. 2015. Modeling gas–rock–water interactions in carbon dioxide storage capacity assessment: a case study of Jurassic sandstones in Poland. Int. J. Environ. Sci. Technol.12:2493–2502.
- Shangguan, H. Y.2014. Research on oil shale pore characteristics based on mercury intrusion method. [Masters Thesis], Taiyuan University of Technology, Taiyuan.
- Torp, T. A., and J. Gale. 2004. Demonstrating storage of CO2 in geological reservoirs: the sleipner and SACS projects. Energy29:1361–1369.
- Metz, B., O. Davidson, H. D. Coninck et al. 2005. IPCC special report on carbon dioxide capture and storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, P. 442.
- Zhang, W., Y. Li, T. Xu, H. Cheng, and Y. Zheng. 2009. Long-term variations of CO2 trapped in different mechanisms in deep saline formations: a case study of the Songliao Basin, China. Int. J. Greenh. Gas Con.3:161–180.
- Pang, Z. H., Y. M. Li, F. T. Yang, and Z. F. Duan. 2012. Geochemistry of a continental saline aquifer for CO2 sequestration: the Guantao formation in the Bohai Bay Basin, North China. Appl. Geochem.27:1821–1828.
- Kharaka, Y. K., D. R. Cole, S. D. Hovorka, W. D. Gunter, K. G. Knauss, and B. M. Freifeld. 2006. Gas-water-rock interactions in Frio Formation following CO2 injection: implications for the storage of greenhouse gases in sedimentary basins. Geology34:577–580.
- Talman, S., E. Perkins, A. Wigston, D. Ryan, and S. Bachu. 2013. Geochemical effects of storing CO2 in the Basal Aquifer thatnderlies the Prairie Region in Canada. Energy Procedia37:5570–5579.
- Stalker, L., C. Boreham, J. Underschultz, B. Freifeld, E. Perkins, U. Schacht et al. 2015. Application of tracers to measure, monitor and verify breakthrough of sequestered CO2 at the CO2 CRC Otway Project, Victoria, Australia. Chem. Geol.399:2–19.
- Ryoji, S., and L. D. Thomas. 2000. Experiment al study on water-rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA. Appl. Geochem.15:265–279.
- Ryzhenko, B. N.2006. Genesis of dawsonite mineralization: thermo-dynamic analysis and alt ernative. Geochem. Int.44:835–840.
- Robert, J. R., K. Tamer, and L. P. James. 2005. Experiment al investigation of CO2- brine-rock interactions at elevated temperature and pressure: implications for CO2 sequestration in deep-saline aquifers. Fuel Process. Technol.86:1581–1597.
- Vaughan, D. J., and R. A. Wogelius. 2000. Environmental mineralogy. Eotvos University Press, Budapest.
- Petroleum Geology of Jinlin Oilfield Editorial Group. 1993. Pp. 20–282. Petroleum geology of China. Vol. 2 (Rudin). Jilin Oilfield. Petroleum Industry Press, Beijing (in Chinese).
- Wang, Y. C., W. L. Kang, and C. L. Mao. 2007. Pp. 4–135. Oil and gas exploration theory and practice of Jilin exploratory area. Petroleum Industry Press, Beijing (in Chinese).
- Guo, W., Z. J. Liu, Q. Liu, L. Ma, H. R. Ding, and S. L. Sun. 2010. Research on the hydrocarbon accumulation dynamics of gaotaizi reservoir in the southern Songliao basin. J. Jilin Univ.40:482–490 (in Chinese with English abstract).
- Holloway, S., and D. Savage. 1993. The potential for aquifer disposal of carbon dioxide in the UK. Energy Convers. Manage.34:925–932.
- Shafeen, A., E. Croiset, P. L. Douglas, and I. Chatzis. 2004. CO2 sequestration in Ontario, Canada. Part I: storage evaluation of potential reservoirs. Energy Convers. Manage.45:2645–2659.
- Folk, R. L.1974. Petrology of sedimentary rocks: Hemphill, Austin, Texas. P. 182.
- Li, Q., X. C. Li, and N. B. Mu. 2003. Sequence stratigraphy study and subtle oil and gas reservoir prediction in the Daqingzijing area of the south of Songliao basin. China Petrol. Explor.8:18–22 (in Chinese with English abstract).
- Xu, Z. G.2009. Geological storage framework and reservoir modeling of CO2 subsurface storage in the Daqingzijing block, southern Songliao Basin. [Doctoral Dissertation]. Institute of Geology and Geophysics, Chinese Academy of Sciences, Pp. 56-87 (in Chinese with English abstract).
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?