| Line 195: | Line 195: | ||
where <math display="inline">{\overline{TAC}}_{0}</math>, <math display="inline">{\overline{TAC}}_{1}</math>, <math display="inline">{\overline{TAC}}_{peak}</math>, <math display="inline">{\overline{TAC}}_{last}</math> is the baseline, the <math>1^{st}</math>, the peak, and the last HU value, respectively. | where <math display="inline">{\overline{TAC}}_{0}</math>, <math display="inline">{\overline{TAC}}_{1}</math>, <math display="inline">{\overline{TAC}}_{peak}</math>, <math display="inline">{\overline{TAC}}_{last}</math> is the baseline, the <math>1^{st}</math>, the peak, and the last HU value, respectively. | ||
| − | On average, the <math>E^{1}</math>, <math>E_{peak}</math> and ER for normal group were significantly larger (<math>p <0.05</math>) than abnormal group. There were weak negative correlations (<math>r = 0.32 – 0.36</math>, <math>p < 0.05</math>) between runoff scores and quantitative kinetic parameters. The ROC analysis between the two groups had area under the curve of 0.64 – 0.77. | + | On average, the <math>E^{1}</math>, <math>E_{peak}</math> and ER for normal group were significantly larger (<math>p <0.05</math>) than abnormal group. There were weak negative correlations (<math> r = 0.32 – 0.36</math>, <math> p < 0.05</math>) between runoff scores and quantitative kinetic parameters. The ROC analysis between the two groups had area under the curve of 0.64 – 0.77. |
'''(3) Clustering analysis''' | '''(3) Clustering analysis''' | ||
Revision as of 11:13, 28 January 2020
Abstract
The purpose of this study is to review the research which was mainly about the evaluation the vascular stenosis or muscle ischemia to assist in the diagnosis of peripheral arterial occlusive disease using dynamic CTA data. With the recent rapid evolution of multislice computed tomography scanners, runoff CTA of the peripheral artery has become a widely used diagnostic option in patients with PAOD. Furthermore the dynamic CTA of the lower extremity provides improved arterial contrast enhancement and a higher diagnostic confidence for assessment of stenosis and occlusion than runoff CTA. The method, the data characteristic and the conclusion of all the qualitative and quantitative research were covered in this study. Finally, the future research direction of the dynamic CTA analysis to assist diagnosis was given.
Keywords: Peripheral arterial occlusive disease, dynamic CTA, vascular stenosis, muscle ischemia, qualitative and quantitative research
1. Introduction
Lower extremity peripheral arterial occlusive disease (PAOD) prevalence and incidence are both sharply age-related, rising >10% among patients in their 60s and 70s [1]. The prevalence in high income country at age 85–89 years was 18.38% (11.16–28.76%) in women and 18.83% (12.03–28.25%) in men. Globally, 202 million people were living with peripheral artery disease in 2010 [2]. Usually, diffuse progressive accumulation of arterial wall plaque results in luminal narrowing of the peripheral arteries and ultimately leads to organ ischemia. Some research supported the hypothesis that lower extremity ischemia has a direct adverse effect on calf skeletal muscle area [3,4]. With severe degree of vascular stenosis, physical function was most affected. Besides that, patients with PAOD often have concomitant coronary and cerebral artery disease (Bhatt et al. 2006). These conditions are common causes of death.
Duplex ultrasound, computed tomographic angiography (CTA), magnetic resonance angiography (MRA) and digital subtraction angiography (DSA) usually are available for diagnosis of PAOD. This study review the research about the evaluation the vascular stenosis or muscle ischemia to assist in the diagnosis of peripheral arterial occlusive disease using dynamic CTA data.
2. Applicability of dynamic CTA data
The runoff CTA is often adopted in the treatment planning of patients because of its availability, short examination time, and relatively low cost. The diagnosis of lower extremity runoff CTA mainly depends on CT morphology [5]. Usually, a runoff CTA of the lower extremity artery from the diaphragm to the toes is often used for diagnosis of PAOD. A runoff score was given based on anatomical vessel assessment [6]. However, the evaluation of vascular lumen beneath knee is difficult for several reasons, such as its location is far from the heart, the proximal stenosis will change the blood flow status, and the lumen is thinner than vessels of thigh.
Dynamic vascular imaging of the lower extremity could offer a solution for the lower legs arteries diagnostic inaccuracies of runoff CTA [7]. Recently introduced examination techniques, such as continuous bidirectional table movements, achieve dynamic volume coverage in a field up to 45 cm long and enable complete dynamic imaging of the calves [8]. The feasibility studies provide promising results for dynamic imaging of the lower extremity vessels [9]. For the evaluation of below-the-knee arteries, dynamic CT could be an alternative approach. Sommer et al. reported a technique of dynamic CTA in patients with critical limb ischemia caused by peripheral artery occlusive disease (PAOD) [10]. The research indicated that dynamic CTA of the lower extremity provides improved arterial contrast enhancement and a higher diagnostic confidence for assessment of stenosis and occlusion than runoff CTA. However, there is a lack of quantitative analysis on the study. Buls et al. quantitatively assessed arterial enhancement by measuring the mean CT values (HU) in all arteries, and gave the conclusion that dynamic CT scan increased image quality and diagnostic confidence for the assessment of presence and degree of arterial stenosis in below-the-knee arteries, however, the study is lack of quantitative analysis of muscle ischemia [11], which is more relevant with patients’ symptoms and limb functions.
3. Material and Method
There are not many studies on vascular diseases of lower limbs based on dynamic CTA. Table 1 showed the parameter analysis of relevant research results. Only one paper reviews their experience with dynamic computed tomographic angiography (CTA) as an imaging modality in the evaluation of popliteal Artery Entrapment Syndrome (PAES) [12]. Most researches focused on the Peripheral Artery Occlusive Disease based on dynamic CTA data.
| Sommer 2010 | Sommer 2012 | Buls 2019 | Lin 2019 | |
| Patient No. | 59 | 29 | 20 | 35(1 arterial aneurysm) |
| Age (year) | 60 10 | 72 12 | 66.1 14.9 | 66.6 11.7 |
| Males percent | 45% | 72% | - | 69% |
| Fontaine stage I | 4 (7%) | - | 9 (45%) | 5(14.5%) |
| Fontaine stage II | 41 (69%) | - | 8 (40%) | 21(60%) |
| Fontaine stage III | 11 (19%) | 13 (45%) | 1(5%) | 3(9%) |
| Fontaine stage IV | 3 (5%) | 16 (55%) | 2(10%) | 5(14.5%) |
| Phases | 12 | 8 | 18 | 9 |
| Scan range | 27cm | 48 cm | - | 45 |
| Flow rate | 5.0 ml /s | 5.0 ml /s | 5.0 ml /s | 4.0 ml /s |
| Contrast medium | 50 ml | 50 ml | 35 ml | 30 ml |
| Tube voltage | 80 kV | 100 kV | 100 kV | 70 kV |
| Tube current | 120 mAs | 120 mAs | Auto mA | 80 mAs |
| Collimation(mm) | 1280.6 | 2640.6 | 2560.625 | 2640.6 |
| Scan time | 30 s | 28 s | 45s | 30s |
| Image thickness | 0.75 mm | 0.75 mm | - | 5 mm |
| increment | 0.5 mm | 0.5 mm | - | 3 mm |
| Saline flush | 50ml 5.0ml/s | 50ml 5.0ml/s | 40ml 3.0ml/s | 50ml 4.0ml/s |
As can be seen from the table, the age of PAOD patients is generally on the older, and only Lin [13] did the quantified analysis of muscle ischemia.
4. Dynamic CTA data Analysis
4.1 Qualitative Research
The dyn-CTA can improve the diagnostic confidence and diagnostic accuracy of PAOD. The first study evaluated the feasibility of a combined CTA protocol of the lower extremities and gave the conclusion that dyn-CTA leading to higher subjective diagnostic confidence in the assessment of significant stenosis or assessing vessel patency [8,10]. The MIP for signal phase of dyn-CTA was also used to assist diagnosis, and the increased enhancement in all three evaluated arteries can be seen with the dyn-CTA compared to standard run-off CTA [11]. But the max increased enhancement of all phases was not given, and which can provide a better image.
4.2 Quantitative Research
(1) Histogram Analysis
Histogram Analyses was performed using Matlab with in-house software in the research [14]. The analysis process tries to avoid the interference of human factors. Due to the large amount of data, 5mm thick data was used for analysis, with 150 slices in total. First, background, fat, and bone are automatically removed from the source data. Second, the data signals of 10 to 20 seconds after contrast agent injection were averaged for use. And then, for each lower extremity, the distribution analysis was performed on all muscle voxels from the 10th slice to the 80th slice (10HU < signal mean <100HU). Finally, voxel-based histogram data were generated for each lower extremity, and the following parameters were calculated: mean, median, mode, skewness, kurtosis, and CV.
On average, the mean, median, mode for normal group were significantly larger () than ischemia group, and CV was obviously lower (). There were weak correlations (, ) between runoff scores and histogram parameters. The ROC analysis between the two groups had area under the curve of 0.61 – 0.76.
(2) Muscle time attenuation curves analysis
Dyn-CTA muscle time attenuation curves (TACs) was analyzed between the to slices [14]. For each leg, average TAC () was calculated for all TACs with peak enhancement larger than 1.5 times muscle baseline HU. Three quantitative kinetic parameters (initial enhancement), (peak enhancement), and ER(enhancement ratio) were calculated for as follows:
|
|
|
where , , , is the baseline, the , the peak, and the last HU value, respectively.
On average, the , and ER for normal group were significantly larger () than abnormal group. There were weak negative correlations (Failed to parse (syntax error): r = 0.32 – 0.36 , ) between runoff scores and quantitative kinetic parameters. The ROC analysis between the two groups had area under the curve of 0.64 – 0.77.
(3) Clustering analysis
The curve-based fuzzy C-means (CBFCM) was used to segment the different enhancement classes on the given slices [13]. First, background is automatically removed from the source data. Second, the 20th, 30th and 40th slice will be automatic segmented by CBFCM. The segmentation of fat, muscle and bone was carried out by exploiting the differences in curve trend among the structures of interest using a CBFCM algorithm. Starting from a predefined number of classes (in our case, six: 3 class bone, 2 class muscle and 1 class fat and the background). For every slice, the MER can be obtained by dividing the number of enhanced muscle voxels by the total number of muscle voxels.
|
|
(4) |
|
|
(5) |
And the average ratio showed the muscle healthy of the lower legs. Third, the MER can be relative with the diagnosis. Finally, the whole muscle (lower muscle class and higher muscle class) tissue was compared with manual segmentation.
The muscle enhancement ratio (MER) was calculated for (i) higher enhanced area over total area; and (ii) corresponding average signal value at higher enhanced are over total area. Lower extremities were diagnosed as a normal group () with each vessel segment score and runoff score , and otherwise as an ischemia group (). The MER for the ischemia group was significantly different () than the normal group. There were weak correlations (, ) between runoff scores and the MER values. The receiver operating characteristics (ROC) analysis between the two groups had area under the curve of 0.71-0.73.
4.3 the ROC analysis results
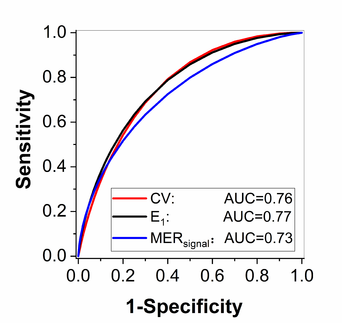
The Figure 1 shows the AUC value of the quantitative research, including histogram analysis, muscle time attenuation curves analysis and clustering analysis.
|
| Figure 1. The receiver operating characteristics (ROC) analysis results |
It can be seen that the quantitative analysis method based on dynamic CTA can assist the PAOD diagnosis, but there is still room for improvement.
5. Conclusion
The study reviewed the research using dyn-CTA data analysis to assist the diagnosis of PAOD. For the qualitative research, the dyn-CTA improved arterial contrast enhancement and at the same time a higher diagnostic confidence for assessment of stenosis and occlusion had been given than runoff CTA. For the quantitative research, the histogram analysis, muscle time attenuation curves analysis and clustering analysis had shown the feasibility of quantitatively evaluating lower leg muscle ischemia of the patients with PAOD. In all, the quantitative researches gave a more intuitive assistance and a new perspective, so the future research direction of the dynamic CTA analysis to assist diagnosis will be quantitative study.
Acknowledgements
This research was funded by the Natural Science Foundation of China under Grant (51875113), Natural Science Foundation of the Heilongjiang Province of China under Grant (F2016003), Harbin youth reserve talent project (2017RAQXJ102).
Reference
[1] Criqui, M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res., 116(9):1509-26, 2015.
[2] Fowkes F.G., Rudan D. et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet., 382(9901):1329-40, 2013.
[3] Tanaka R., Yoshioka K. et al. Novel developments in non-invasive imaging of peripheral arterial disease with CT: experience with state-of-the-art, ultra-high-resolution CT and subtraction imaging. Clin Radiol., 74(1):51-58, 2019.
[4] McDermott M.M., Hoff F. et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc., 55(3):400-6, 2007.
[5] Preuss A., Schaafs L.A. et al. Run-off computed tomography angiography (CTA) for discriminating the underlying causes of intermittent claudication. PLoS One., 11(4):e0152780, 2016.
[6] Stoner M.C., Calligaro K.D. et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg., 64(1):e1-e21, 2016.
[7] Werncke T., Ringe K.I. et al. Diagnostic confidence of run-off CT-angiography as the primary diagnostic imaging modality in patients presenting with acute or chronic peripheral arterial disease. PLoS One, 10(3) :e0119900, 2015.
[8] Sommer W.H., Helck A. et al. Diagnostic value of time-resolved CT angiography for the lower leg. European radiology, 20(12):2876-81, 2010.
[9] Kortman H.G., Smit E.J. et al. 4D-CTA in neurovascular disease: a review. AJNR Am J Neuroradiol., 36(6) :1026-33, 2015.
[10] Sommer W.H., Bamberg F. et al. Diagnostic accuracy of dynamic computed tomographic angiographic of the lower leg in patients with critical limb ischemia. Invest Radiol., 47(6):325-31, 2012.
[11] Buls N., de Brucker Y., et al. Improving the diagnosis of peripheral arterial disease in below-the-knee arteries by adding time-resolved CT scan series to conventional run-off CT angiography. First experience with a 256-slice CT scanner, Eur J Radiol., 110:136-141,2019.
[12] Anil G.T.K., Howe T.C., Tan B.S. Dynamic computed tomography angiography: role in the evaluation of popliteal artery entrapment syndrome. Cardiovasc Intervent Radiol., 34(2):259-70,2011.
[13] Lin Z.H., Xu H., Zhang D.M. Automated muscle segmentation from dynamic computed tomographic angiography images for diagnosis of peripheral arterial occlusive disease. IEEE ACCESS, 7:146505-146511, 2019.
[14] Zhou X.Y., Zhang D.M. et al. Quantitative analysis of lower leg muscle enhancement measured from dynamic computed tomographic angiography for diagnosis of peripheral arterial occlusive disease. Journal of Computer Assisted Tomography, 44(1):20-25, 2020.
Document information
Published on 30/01/20
Accepted on 27/01/20
Submitted on 24/12/19
Volume 36, Issue 1, 2020
DOI: 10.23967/j.rimni.2020.01.007
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?