(Created page with "==Summary== ====Background==== Hepatocellular carcinoma is a common cancer with an increasing incidence worldwide because of the dissemination of hepatitis B and hepatitis C...") |
m (Scipediacontent moved page Draft Content 137000281 to Chung et al 2014a) |
(No difference)
| |
Latest revision as of 11:58, 15 May 2017
Summary
Background
Hepatocellular carcinoma is a common cancer with an increasing incidence worldwide because of the dissemination of hepatitis B and hepatitis C virus infection. Surgical resection is the most important therapeutic option with a curative intent. Early tumor detection through screening and improvements in surgical techniques have significantly improved the outcome of patients with hepatocellular carcinoma. However, local recurrence after curative hepatic resection is common and is the most frequent cause of death in these patients.
Patients and Methods
In an attempt to identify the risk factors that predict tumor recurrence, we conducted this retrospective study in a single institution for a 6-year period. Of the 100 consecutive patients who underwent curative tumor resection, we analyzed age, sex, viral etiology (hepatitis B virus vs. hepatitis C virus), preoperative levels of aspartate aminotransferase and alanine aminotransferase, the α-fetoprotein level, underlying liver disease status (chronic hepatitis vs. cirrhosis), number and size of tumors, type of resection, and presence of microvascular invasion.
Results
In the median follow-up period of 36 months (range, 12–85 months), the 1-year, 3-year, and 5-year overall survival rates were 90%, 84%, and 73%, respectively; tumor recurrence occurred in 38 (38%) patients and was the leading cause of death among the patients who died (15 of 17 patients; 88%). On univariate analysis, the only factor significantly associated with a higher incidence of tumor recurrence was preoperative levels of aspartate aminotransferase greater than twice the upper normal value (p < 0.01) and this factor remained significant with multivariate analysis. Subgroup analysis of the risk factor of early tumor recurrence (≤2 years) and late tumor recurrence (>2 years) was conducted and a preoperative aspartate aminotransferase level greater than twice the upper normal value was still significant in both groups (p = 0.02 and p = 0.044, respectively).
Conclusion
Although this is a small-scale study, our findings could be easily applied clinically and used as readily available indicators to help the follow-up algorithm. We also suggest antiviral management as soon as possible for patients with hepatocellular carcinoma undergoing curative resection, especially those with a high preoperative aspartate aminotransferase level.
Keywords
Alanine aminotransferase ; Aspartate aminotransferase ; Curative resection ; Hepatocellular carcinoma ; Recurrence
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in Asia and Africa and its incidence is increasing worldwide because of the dissemination of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection [1] . It is prevalent in Taiwan with around 10,000 new cases each year and has been the leading cause of cancer death, accounting for approximately 8000 deaths annually [2] . Recent advances in screening, such as ultrasonography, dynamic computed tomography, and magnetic resonance imaging, have made early diagnosis possible and thus improved survival [3] ; [4] . Surgery is the most important therapeutic option for patients with HCC [5] ; [6] . With the progress in early diagnosis and operative techniques, the outcome of patients with HCC after tumor resection have significantly improved, with median survival rates of 80% (range, 63–97%) at 1 year and 50% (range, 17–69%) at 5 years, with a surgical mortality rate of less than 2% [5] ; [7] ; [8] ; [9] . The wide ranges of survival rates are attributed mainly to differences in the HCC stage among various studies, with an obvious survival advantage in early-stage tumors [10] . However, a significant proportion of patients cannot achieve a cure or a sustained tumor-free survival and the long-term outcome after initial treatment remains unsatisfactory because of a high recurrence rate, ranging from 43% to 100% at 5 years [6] ; [7] ; [11] ; [12] ; [13] ; [14] ; [15] .
HCC recurrence has been classified as early (within 2–3 years) or late. Early recurrence is usually due to metastasis from the primary tumor (dissemination from the primary tumor), whereas late recurrence is often due to de novo second primary tumors occurring in a cirrhotic or HBV-infected liver [16] . Many factors have been identified to be associated with tumor recurrence after surgical resection, including: the accompanying chronic viral hepatitis status (Ishak activity score); serum levels of albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), α-fetoprotein, and HBV DNA; the extent of hepatectomy; and various tumor factors [12] ; [13] ; [16] ; [17] ; [18] ; [19] ; [20] ; [21] . Among these, the most powerful predictors of recurrence are the presence of microvascular invasion or additional tumor sites besides the primary lesions, or both [12] . In this retrospective study we aimed to identify the risk factors for recurrence in patients undergoing curative surgical resection in a single institution.
Methods
We retrospectively searched the database of the Chi-Mei Medical Center in southern Taiwan for patients diagnosed with HCC who underwent curative liver resection from March 2002 to March 2008. The institutional review board approved this study. We used either ultrasonography and abdominal computed tomography (CT) scan or magnetic resonance imaging (MRI) to determine the anatomical location of HCC; the operative procedures were defined according to Couinauds classification of hepatic segments [22] . Curative resection referred to a complete resection of all macroscopic tumors with tumor clearance along the parenchymal transection line.
We reviewed the medical records of the patients for the underlying causes of hepatitis and coexisting liver diseases. Those patients with cirrhosis were classified according to the Child–Pugh scheme [23] . The clinicopathological variables evaluated included age at surgery, sex, etiology of hepatitis, α-fetoprotein level, microvascular invasion, number and size of tumors, type of resection, underlying liver disease status (chronic hepatitis vs. cirrhosis), and preoperative levels of AST and ALT. The Barcelona Clinic Liver Cancer (BCLC) classification was used for clinical staging. All patients were followed up regularly every 2–3 months after surgery. Tumor recurrence was suspected in the presence of progressive elevation of serum α-fetoprotein levels and detection of a new hepatic lesion by ultrasonography. Tumor recurrence was diagnosed by dynamic CT scan or MRI showing contrast enhancement during the arterial phase and washout in the venous phase, or hepatic angiography disclosing a high vascularity tumor. Once tumor recurrence was suspected, patients were admitted to hospital for the confirmation of diagnosis and appropriate management, including resection, local ablation treatment, transcatheter arterial chemoembolization, chemotherapy, radiotherapy, or supportive treatment.
Continuous data were expressed as mean ± standard deviation and compared with the unpaired t test. Dichotomous variables were evaluated with χ2 analysis or Fishers exact test to define various patient groups contributing significantly to the factors affecting disease-free survival and recurrence. Survival analysis was performed by the Kaplan–Meier method and the difference in the curves was tested with the log-rank test. To determine prognostic factors for disease-free survival during the follow-up period, the Cox proportional hazard model was used to estimate the risk of the following potential variables: age, sex, viral etiology (HBV or HCV), α-fetoprotein level, number and size of tumors, type of resection, underlying liver disease status (chronic hepatitis, cirrhosis), and preoperative levels of AST and ALT. After univariate analysis, only those variables that reached p < 0.05 were used in the multivariate analysis. The multivariable Cox proportional hazard model was built by stepwise variable with entry and removal exit criteria set at p = 0.05 and p = 0.1, respectively. Statistical significance was set at p < 0.05. SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
We identified a total of 113 patients receiving curative resection for HCC during this study period; 13 patients had data missing, but the remaining 100 patients were analyzed in this study. Table 1 summarizes the clinicopathological characteristics of the patients. There were 69 men and 31 women with a median age of 59.5 years (range, 52–66 years). Sixteen patients were 70 years or older. Twenty-one patients had chronic hepatitis; 79 had liver cirrhosis according to pathological findings, all Child–Pugh class A. The underlying causes of disease were HBV and HCV in 52% and 32% of patients, respectively, whereas coinfection of HBV and HCV occurred in 6%. The remaining cases included 7% of patients with alcoholic liver disease and 3% with other unidentified causes. The hepatic tumors were single in 91 (91%) patients and multiple in the remaining 9 (9%) patients. The median diameter of the largest tumor in each patient was 4.7 cm (range, 1–11 cm). Twenty-six patients were BCLC stage 0, 58 patients were stage A, and the remaining 16 patients were stage B. Seventy-six patients underwent segmentectomy and the remaining 24 underwent major hepatectomy. Microvascular invasion was identified by pathological findings in only two (2%) patients. The median follow-up time was 36 months (range, 12–85 months). The 1-year, 3-year, and 5-year overall survival rates were 90%, 84%, and 73%, respectively.
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 69 (69) |
| Female | 31 (31) |
| Etiology of liver disease | |
| Hepatitis B | 52 (52) |
| Hepatitis C | 32 (32) |
| Hepatitis B + C | 6 (6) |
| Alcohol | 7 (7) |
| Other | 3 (3) |
| Non-neoplastic liver disease | |
| Liver cirrhosis | 79 (79) |
| Chronic hepatitis | 21 (21) |
| Diameter of main nodule (cm) | |
| <3 | 51 (51) |
| ≥3 | 26 (26) |
| ≥5 | 23 (23) |
| Number of nodules | |
| Single | 91 (91) |
| Multiple | 9 (9) |
| BCLC stage | |
| Stage 0 | 25 (25) |
| Stage A | 59 (59) |
| Stage B | 16 (16) |
| Microvascular invasion | |
| Positive | 2 (2) |
| Negative | 98 (98) |
BCLC = Barcelona Clinic Liver Cancer classification.
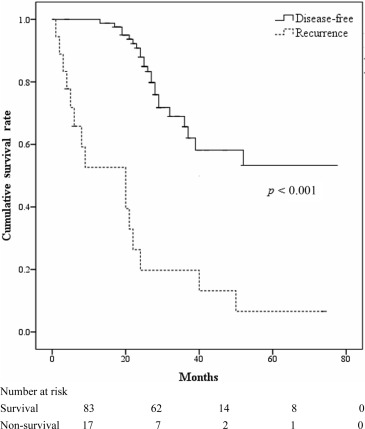
Tumor recurrence occurred in 38 (38%) patients. Seventeen patients died during the follow-up period and the causes of death included tumor recurrence (15 patients, 88%) and one patient (6%) each with intracranial hemorrhage and operative complications. The 1-year, 3-year, and 5-year disease-free survival rates were 77.5%, 61.1%, and 48.6%, respectively. The survival rate between the disease-free and tumor recurrence groups was significant (p < 0.001; Fig. 1 ).
|
|
|
Figure 1. Survival curves for patients with hepatocellular carcinoma who underwent curative surgical resection with and without recurrence. |
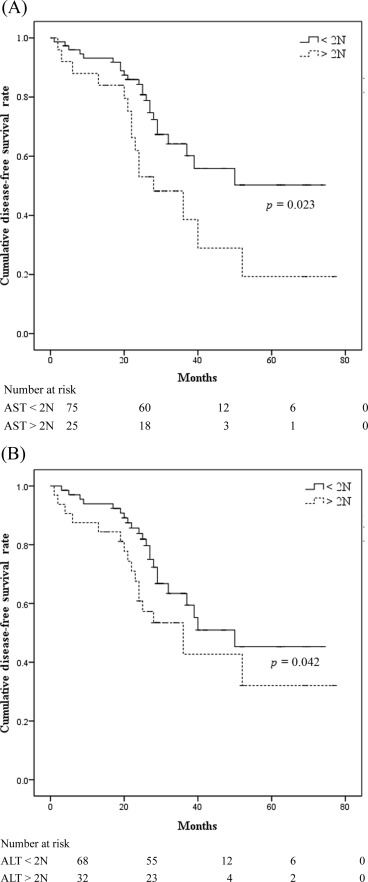
On univariate analysis, the only factor significantly associated with a higher incidence of tumor recurrence was preoperative levels of AST greater than twice the upper normal value (2N; p < 0.01). Tumor recurrence was not related to age at surgery, sex, or HBV or HCV infection, α-fetoprotein level, tumor size (<3 cm, 3–5 cm, or >5 cm), tumor multifocality (single vs. multiple), type of resection, underlying chronic hepatitis versus cirrhosis, or preoperative levels of ALT greater than twice the normal value ( Table 2 ). Preoperative AST level greater than twice the normal value remained significantly (p < 0.05) related to a higher incidence of tumor recurrence with multivariate analysis. Meanwhile, patients with preoperative levels of AST and ALT greater than twice the upper normal value had a higher cumulative proportion of local recurrence with statistical significance (2N; p = 0.023 and p = 0.042, respectively; Fig. 2 A and B). In addition, subgroup analysis was conducted to determine the risk factor of early tumor recurrence (≤2 years; 32 patients, 84%) and late tumor recurrence (>2 years; 6 patients, 16% ) in this study. A significant correlation of high AST level and tumor recurrence, in both early and late recurrence, was noted, as shown in Table 3 ; Table 4 .
| Variable | Recurrence of HCC (n = 38) | Disease-free survival (n = 62) | HR (95% CI) | p |
|---|---|---|---|---|
| Age (y) | 56.4 ± 10.6 | 59.3 ± 11.6 | 0.22 | |
| Male sex | 30 (78.9) | 39 (62.9) | 1.76 (0.88–3.52) | 0.092 |
| Etiology of liver disease | ||||
| Hepatitis B | 21 (55.3) | 31 (50.0) | 1.12 (0.73–1.72) | 0.609 |
| Hepatitis C | 13 (34.2) | 19 (30.6) | 1.05 (0.79–1.40) | 0.711 |
| Hepatitis B + C | 2 (5.3) | 4 (6.5) | 0.98 (0.89–1.10) | 0.808 |
| AST > 1N | 27 (71.1) | 35 (56.5) | 1.50 (0.85–2.67) | 0.144 |
| ALT > 1N | 30 (78.9) | 41 (66.1) | 1.61 (0.79–3.21) | 0.17 |
| AST > 2N | 15 (39.5) | 10 (16.1) | 1.39 (1.05–1.83) | 0.009 |
| ALT > 2N | 16 (42.1) | 16 (25.8) | 1.28 (0.94–1.74) | 0.09 |
| Liver cirrhosis | 33 (86.8) | 46 (74.2) | 1.97 (0.76–6.89) | 0.132 |
| Liver nodules number | 0.676 | |||
| Single | 34 (89.5) | 57 (91.9) | ||
| Multiple | 4 (10.5) | 5 (8.1) | ||
| Diameter of nodule (cm) | 0.541 | |||
| <3 | 18 (47.4) | 33 (53.2) | ||
| ≥3 | 9 (23.7) | 17 (27.4) | ||
| ≥5 | 11 (28.9) | 12 (19.4) | ||
| BCLC stage | 0.248 | |||
| Stage 0 | 8 (21.1) | 17 (27.4) | ||
| Stage A | 21 (55.3) | 38 (61.3) | ||
| Stage B | 9 (23.7) | 7 (11.3) | ||
| AFP > 20 | 18 (47.4) | 27 (43.5) | 0.92 (0.59–1.43) | 0.709 |
Data are presented as n (%) or mean ± SD.
1N = greater than the upper normal value; 2N = greater than twice the upper normal value; AFP = α-fetoprotein; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BCLC = Barcelona Clinic Liver Cancer classification; CI = confidence interval; HCC = hepatocellular carcinoma; HR = hazard ratio.
|
|
|
Figure 2. (A) Impact of preoperative aspartate aminotransferase (AST) levels greater than twice normal values on the risk of local recurrence. (B) Impact of preoperative alanine aminotransferase (ALT) levels greater than twice normal on the risk of local recurrence. 2N = greater than twice the upper normal value. |
| Variable | No recurrence (n = 62) | Recurrence ≤2 y (n = 32) | RR (95% CI) | p |
|---|---|---|---|---|
| Male sex | 39 (62.9) | 28 (87.5) | 1.98 (0.89–4.36) | 0.068 |
| Etiology of liver disease | ||||
| Hepatitis B | 31 (50.0) | 20 (62.5) | 0.80 (0.56–1.15) | 0.249 |
| Hepatitis C | 19 (30.6) | 8 (25.0) | 1.23 (0.60–2.49) | 0.567 |
| Hepatitis B + C | 4 (6.5) | 2 (6.3) | 1.03 (0.20–5.34) | 0.97 |
| AST > 1N | 35 (56.5) | 22 (68.8) | 0.82 (0.59–1.13) | 0.247 |
| ALT > 1N | 41 (66.1) | 25 (78.1) | 0.85 (0.66–1.10) | 0.228 |
| AST > 2N | 10 (16.1) | 12 (37.5) | 0.43 (0.21–0.89) | 0.02 |
| ALT > 2N | 16 (25.8) | 14 (43.8) | 0.60 (0.33–1.10) | 0.077 |
| Liver cirrhosis | 46 (74.2) | 27 (84.5) | 0.88 (0.71–1.08) | 0.261 |
| AFP > 20 | 27 (43.5) | 15 (46.9) | 0.93 (0.58–1.48) | 0.759 |
1N = greater than the upper normal value; 2N = greater than twice the upper normal value; AFP = α-fetoprotein; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence interval; RR = relative risk.
| Variable | No recurrence (n = 62) | Recurrence >2 y (n = 6) | RR (95% CI) | p |
|---|---|---|---|---|
| Male | 39 (62.9) | 2 (33.3) | 1.89 (0.60–5.95) | 0.158 |
| Etiology of liver disease | ||||
| Hepatitis B | 31 (50.0) | 1 (16.7) | 3.00 (0.49–18.27) | 0.118 |
| Hepatitis C | 19 (30.6) | 5 (83.3) | 1.84 (0.58–5.80) | 0.184 |
| Hepatitis B + C | 4 (6.5) | 0 (0.0) | 1.06 (0.99–1.14) | 0.521 |
| AST > 1N | 35 (56.5) | 5 (83.3) | 0.68 (0.45–1.03) | 0.201 |
| ALT > 1N | 41 (66.1) | 5 (83.3) | 0.79 (0.53–1.18) | 0.39 |
| AST > 2N | 10 (16.1) | 3 (50.0) | 0.32 (0.12–0.86) | 0.044 |
| ALT > 2N | 16 (25.8) | 2 (33.3) | 0.77 (0.23–2.60) | 0.69 |
| Liver cirrhosis | 46 (74.2) | 6 (100.0) | 0.74 (0.64–0.86) | 0.155 |
| AFP > 20 | 27 (43.5) | 3 (50.0) | 0.87 (0.37–2.03) | 0.761 |
1N = greater than the upper normal value; 2N = greater than twice the upper normal value; AFP = α-fetoprotein; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence interval; RR = relative risk.
Discussion
The overall survival after liver resection for patients with HCC has been prolonged because of improvements in the early detection of tumors and advances in surgical techniques and perioperative care [3] ; [4] ; [6] . However, the long-term results are not satisfactory as a result of tumor recurrence, which is the main cause of mortality [11] ; [16] ; [24] ; [25] . Previous studies have identified that various factors, such as tumor factors and α-fetoprotein levels, are related to a high incidence of tumor recurrence [11] ; [13] ; [17] ; [18] ; [24] ; [26] . In our study, tumor recurrence occurred in 38% of patients and it was the leading cause of death. Both univariate and multivariate analysis showed that preoperative levels of AST greater than twice the normal value was the only factor significantly associated with a higher rate of tumor recurrence after curative surgery.
Several Japanese groups have pointed out that chronic active hepatitis and cirrhosis were the most significant risk factors for intrahepatic tumor recurrence, which probably develops through multicentric hepatocarcinogenesis, the so-called “multicentric occurrence” after hepatectomy [17] ; [18] ; [27] . This can be explained by the fact that repeated inflammation and cellular necrosis in patients with chronic hepatitis or cirrhosis enhance proliferating activity and accelerate the development of new foci of HCC through an increased rate of random mutations and promotion due to gene instability [28] ; [29] . Adachi et al [13] considered active inflammation as a basic cause of recurrence. They found that in livers with inflammatory changes, some adhesion molecules that adhered to cancer cells in the remnant liver after resection appeared and caused postoperative recurrence [13] . In patients with chronic hepatitis or cirrhosis, the serum aminotransferase levels are usually considered an index of inflammatory activity that reflects the etiopathogenetic mechanism of hepatocytic necrosis [30] . In keeping with these findings, we found that an elevated preoperative AST level over twice upper normal values (>2N) was the only variable closely associated with tumor recurrence. In a cohort of 244 liver resections for HCC in cirrhosis, Ercolani et al [7] identified that single nodules and preoperative AST levels less than twice the upper normal value (<2N) were related to a better 5-year disease-free survival and lower tumor recurrence. In particular, among HCV-positive patients the recurrence rate was strongly affected by the preoperative AST level [7] .
Similar results on the significance of elevated serum aminotransferase levels predicting a higher chance of recurrence, including in patients with small HCC, have also been reported by other workers [9] ; [17] ; [31] . These data indicate an important pathogenetic role of hepatic inflammation and the patients with a higher risk of recurrence may benefit from adjuvant treatments with anti-inflammatory drugs to prevent tumor recurrence after liver resection. For these reasons, several workers have investigated the efficacy of interferon, an inflammation-modulating drug, for preventing the occurrence of HCC in patients with HBV- or HCV-related liver disease [26] ; [32] ; [33] .
Antiviral treatment with interferon has been recommended to prevent tumor recurrence after primary curative treatments in HCV-related HCC [5] . However, there was less evidence for antiviral treatment with nucleos(t)ide analogues to prevent tumor recurrence in HBV-related HCC [34] . The major reason for this phenomenon is that early recurrence is probably due to previously undiscovered metastasis from the primary tumor. The early recurrence accounts for most of the total number of recurrences [35] . However, a recent retrospective analysis showed a favorable result on the 1-year, 3-year, and 5-year disease-free survival rates in the early antiviral treatment group compared with those without treatment [36] . Su et al [37] also reported that antiviral treatment could decrease recurrence in patients with HBV-induced HCC after resection surgery. Our result of high preoperative ALT levels correlating with both early and late recurrences also supports the view that early antiviral treatment may contribute to the survival of patients with HCC after curative treatment, especially for those with hepatitis activity.
A limitation of our study is the small number of patients, especially when they are subdivided into three groups based on tumor size and the underlying viral etiologies or different age groups. Nonetheless, we identified a significant correlation of high AST level and tumor recurrence in both early and late recurrence. Our findings also suggest that early antiviral treatment may improve the prognosis of HBV-related HCC.
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- [1] European Association for the Study of the LiverEuropean Organisation for Research and Treatment of Cancer; EASL-EORTC clinical practice guideline: management of hepatocellular carcinoma; J Hepatol, 56 (2012), pp. 908–943
- [2] http://liver.org.tw/index.php?potion=com-content&review . Liver Disease Prevention & Treatment Research Foundation [In Chinese].
- [3] S.T. Fan, L.C. Mau, R.T. Poon, C. Yeung, L.C. Leung, W.K. Yuen, et al.; Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience; Ann Surg, 253 (2011), pp. 745–758
- [4] N.N. Rahbari, A. Mehrabi, N.M. Mollberg, S.A. Müller, M. Koch, M.W. Büchler, et al.; Hepatocellular carcinoma: current management and perspectives for the future; Ann Surg, 253 (2011), pp. 453–469
- [5] K. Takayasu, S. Arii, M. Kudo, T. Ichida, O. Matsui, N. Izumi; Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guideline; J Hepatol, 56 (2012), pp. 886–892
- [6] Y. Kishi, K. Hasegawa, Y. Sugawara, N. Kokudo; Hepatocellular carcinoma: current management and future development—improved outcomes with surgical resection; Int J Hepatol, 23 (2011), p. 728103 Epub
- [7] G. Ercolani, G.L. Grazi, M. Ravaioli, M. Del Gaudio, A. Gardini, M. Cescon, et al.; Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence; Ann Surg, 237 (2003), pp. 536–543
- [8] C. Hubert, C. Sempoux, J. Rahier, Y. Horsmans, A. Geubel, B.E. Van Beers, et al.; Prognostic risk factors of survival after resection of hepatocellular carcinoma; Hepatogastroenterology, 54 (2007), pp. 1791–1797
- [9] C.Y. Wu, Y.J. Chen, H.J. Ho, Y.C. Hsu, K.N. Kuo, M.S. Wu, et al.; Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection; JAMA, 308 (2012), pp. 1906–1913
- [10] K.C. Lim, P.K. Chow, J.C. Allen, F.J. Siddiqui, E.S. Chan, S.B. Tan; Systemic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria; Br J Surg, 99 (2012), pp. 1622–1629
- [11] J. Belghiti, Y. Panis, O. Farges, J.P. Benhamou, F. Fekete; Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis; Ann Surg, 214 (1991), pp. 114–117
- [12] J. Bruix, M. Sherman; Management of hepatocellular carcinoma; Hepatology, 42 (2005), pp. 1208–1236
- [13] E. Adachi, T. Maeda, T. Matsumata, K. Shirabe, N. Kinukawa, K. Sugimachi, et al.; Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma; Gastroenterology, 108 (1995), pp. 768–775
- [14] C.H. Chen, G.T. Huang, P.M. Yang, P.J. Chen, M.Y. Lai, D.S. Chen, et al.; Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis; Eur J Cancer, 42 (2006), pp. 2524–2529
- [15] G.L. Grazi, G. Ercolani, F. Pierangeli, M. Del Gaudio, M. Cescon, A. Cavallari, et al.; Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value; Ann Surg, 234 (2001), pp. 71–78
- [16] O. Morimoto, H. Nagano, M. Sakon, Y. Fujiwara, T. Yamada, H. Nakagawa, et al.; Diagnosis of intrahepatic metastasis and multi-centric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas; J Hepatol, 39 (2003), pp. 215–221
- [17] K. Shirabe, T. Kanematsu, T. Matsumata, E. Adachi, K. Akazawa, K. Sugimachi, et al.; Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses; Hepatology, 14 (1991), pp. 802–805
- [18] S. Ko, Y. Nakajima, H. Kanehiro, M. Hisanaga, Y. Aomatsu, T. Kin, et al.; Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy. Result of multivariate analysis; Ann Surg, 224 (1996), pp. 591–595
- [19] K. Shirabe, K. Takenaka, A. Taketomi, N. Kawahara, K. Yamamoto, M. Shimada, et al.; Postoperative hepatitis status as a significant risk factor for recurrence in cirrhotic patients with small hepatocellular carcinoma; Cancer, 77 (1996), pp. 1050–1055
- [20] J. Yamamoto, T. Kosuge, T. Takayama, K. Shimada, S. Yamasaki, H. Ozaki, et al.; Recurrence of hepatocellular carcinoma after surgery; Br J Surg, 83 (1996), pp. 1219–1222
- [21] J.C. Wu, Y.H. Huang, G.Y. Chau, C.W. Su, C.R. Lai, P.C. Lee, et al.; Risk factors for early and late recurrence of hepatitis B-related hepatocellular carcinoma; J Hepatol, 51 (2009), pp. 890–897
- [22] H. Strunk, G. Stuckmann, J. Textor, W. Willinek; Limitations and pitfalls of Couinauds segmentation of the liver in transaxial imaging; Eur Radiol, 13 (2003), pp. 2472–2482
- [23] R.N. Pugh, I.M. Murray-Lyon, J.L. Dawson, M.C. Pietroni, R. Williams; Transection of the oesophagus for bleeding oesophageal varices; Br J Surg, 60 (1973), pp. 646–649
- [24] N. Nagasue, M. Uchida, Y. Makino, Y. Takemoto, A. Yamanoi, T. Hayashi, et al.; Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma; Gastroenterology, 105 (1993), pp. 488–494
- [25] N. Yamanaka, E. Okamoto, A. Toyosaka, M. Mitunobu, S. Fujihara, T. Kato, et al.; Prognostic factors after hepatectomy for hepatocellular carcinomas. A univariate and multivariate analysis; Cancer, 65 (1990), pp. 1104–1110
- [26] T. Nagao, S. Inoue, F. Yoshimi, M. Sodeyama, Y. Omori, T. Mizuta, et al.; Postoperative recurrence of hepatocellular carcinoma; Ann Surg, 11 (1990), pp. 28–33
- [27] K. Ikeda, S. Saitoh, Y. Arase, K. Chayama, Y. Suzuki, M. Kobayashi, et al.; Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis; Hepatology, 29 (1999), pp. 1124–1130
- [28] F. Marks, S. Bertsch, W. Grimm, J. Schweizer; Hyperplastic transformation and tumor promotion in mouse epidermis: possible consequence of disturbances of endogenous mechanisms controlling proliferation and differentiation; T.J. Slaga, A. Sivak, R.K. Boutwell (Eds.), Carcinogenesis, vol. 2, Raven Press, New York (1978), pp. 97–116
- [29] K. Tarao, S. Ohkawa, A. Shimizu, M. Harada, Y. Nakamura, Y. Ito, et al.; Significance of hepatocellular proliferation in the development of hepatocellular carcinoma from anti-hepatitis C virus-positive cirrhotic patients; Cancer, 73 (1994), pp. 1149–1154
- [30] K. Tarao, S. Takemiya, S. Tamai, Y. Sugimasa, S. Ohkawa, M. Akaike, et al.; Relationship between the recurrence of hepatocellular carcinoma (HCC) and serum alanine aminotransferase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC; Cancer, 79 (1997), pp. 688–694
- [31] M. Kaibori, M. Ishizaki, T. Saito, K. Matsui, A.H. Kwon, Y. Kamiyama; Risk factors and outcome of early recurrence after resection of small hepatocellular carcinomas; Am J Surg, 198 (2009), pp. 39–45
- [32] W.Y. Kao, C.W. Su, G.Y. Chau, W.Y. Lui, C.W. Wu, J.C. Wu; A compression of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery; World J Surg, 35 (2011), pp. 858–867
- [33] Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma; Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements; J Gastroenterol Hepatol, 25 (2010), pp. 657–663
- [34] M. Omata, L.A. Lesmana, R. Tateishi, P.J. Chen, S.M. Lin, H. Yoshida, et al.; Asian Pacific Association for the Study of the Liver consensus recommendation on hepatocellular carcinoma; Hepatol Int, 4 (2010), pp. 439–474
- [35] A.S.F. Lock; Does antiviral therapy prevent recurrence of hepatitis B virus-related hepatocellular carcinoma after curative liver resection?; JAMA, 308 (2012), pp. 1922–1924
- [36] A.C. Chan, K.S. Chok, W.K. Yuen, S.C. Chan, R.T. Poon, C.M. Lo, et al.; Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma; Arch Surg, 146 (2011), pp. 675–681
- [37] C.W. Su, Y.W. Chiou, R.D. Teng, G.Y. Chau, H.J. Lei, et al.; The influence of hepatitis B viral load and pre-S deletion mutations on post-operative recurrence of hepatocellular carcinoma and the tertiary preventive effects by anti-viral therapy; PLoS One, 8 (2013), p. e66457
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?