(Created page with "==Summary== ====Background==== Conventional monopolar radiofrequency ablation (RFA) bears the risks of incomplete ablation and tumor seeding. This study aimed to evaluate th...") |
(No difference)
|
Revision as of 11:54, 15 May 2017
Summary
Background
Conventional monopolar radiofrequency ablation (RFA) bears the risks of incomplete ablation and tumor seeding. This study aimed to evaluate the effectiveness and safety of multipolar RFA with non-touch technique for hepatocellular carcinoma (HCC) ≤ 3 cm.
Methods
Fifteen cirrhotic patients (9 men, 6 women; age 51–83 years, mean 64.4 years, Child-Pugh score: A = 10 and B = 5) with 17 HCCs of ≤ 3 cm (mean: 26 mm), which were diagnosed based on typical radiologic findings were enrolled. Two or three Celon Prosurge Bipolar electrodes with 3-cm active tip were deployed with non-touch technique via percutaneous approach under ultrasound guidance.
Results
Complete ablation was achieved in all 17 lesions. This is defined as no enhanced part around the ablated index tumors according to dynamic computed tomography or magnetic resonance imaging at least 1 month after ablation. No local tumor progression was detected at follow-up (range, 3–21.5 months; mean, 10 months). No track seeding was observed. There was one distant recurrence 15.4 months after ablation. One patient had procedure-related biliary stricture and died of pneumonia 3.5 months after tumor ablation.
Conclusion
Multipolar RFA with non-touch technique is an effective method to achieve complete tumor ablation and an adequate safety margin. This method has low complication rate and bears minimal risk of tumor seeding.
Keywords
Hepatocellular carcinoma ; Multipolar radiofrequency ablation ; Non-touch technique
Introduction
Radiofrequency ablation (RFA) is an effective and safe curative therapy used to treat small hepatocellular carcinoma (HCC) [1] ; [2] . However, a higher local recurrence rate than surgical resection and the risk of tumor seeding after ablation are major drawbacks of this procedure [3] . Conventionally, monopolar electrode is deployed into the tumor and the ablation process is executed from the center to the periphery. The difficulty in ensuring an adequate safety margin when using this method is the main reason for treatment failure and contributes to both local tumor growth and distant recurrence.
Bipolar electrodes are devices with particular physical characteristics. The radiofrequency (RF) current could flow between each pole of the electrodes if the interelectrode distance is ≤ 3 cm [4] ; [5] . This differs from the switch control monopolar devices, in which individual electrodes work independently rather than simultaneously, as in bipolar devices. In bipolar devices, six and 15 RF current directions are enabled by the deployment of two and three electrodes, respectively. This distinct characteristic allows the electrodes to be placed close to the outer margin of the tumor and ablate the tumor from the periphery to the center. Hence, ablation could be performed without touching the tumor itself. Using this method, complete tumor ablation in the interelectrode space can be ensured. In addition to performing ablation in the interelectrode space, ablation could be extended to a distance of 0.5–1 cm outside the interelectrode space. This characteristic allows the safety margin to be guaranteed.
Tumor seeding along the puncture route is another concern when using monopolar RFA [6] ; [7] ; [8] . Although the complication rate can be lowered by track ablation, the non-touch technique can completely avoid this treatment related complication by circumventing direct puncture of the tumor.
A French study, using the non-touch technique for HCC ablation, demonstrated excellent local tumor control, evidenced by pathological assessment of the explanted livers. By contrast, more than half of the explanted livers that had been previously ablated using a monopolar device remained positive for residual tumors [9] .
In this prospective study, we present the preliminary results of the novel ablation method for HCC ≤ 3 cm.
Materials and methods
Fifteen cirrhotic patients (Child–Pugh score: A or B) with 17 HCCs were enrolled in this study between October 2010 and March 2013. All 15 patients had chronic hepatitis B (CHB), chronic hepatitis C (CHC), or both. The diagnosis of cirrhosis was based on ultrasound and clinical findings such as esophageal varices, gastric varices, ascites, thrombocytopenia, and coagulopathy. The diagnosis of HCC was based on typical radiologic findings, which were determined according to AASLD guidelines [10] . None of the patients received liver biopsies because the aim of the non-touch technique was to prevent tumor seeding during the procedure.
All procedures were performed by one physician with 4 years' experience in performing ultrasound-guided liver tumor ablations. All procedures were executed in the operation room under general anesthesia. Airway protection was ensured by endotracheal tube placement. Toshiba Xario XG (Toshiba, Tokyo, Japan) ultrasound was used for ablation guidance.
Celon Prosurge Bipolar electrodes (Celon, Teltow, Germany) with 3 cm active tip were deployed. These electrodes are 1.8 mm in diameter and 15 cm or 20 cm in total needle length. The electrodes were used with coolant pump to have internal cooling. No grounding pads were needed with this device. Two or three electrodes were deployed according to the tumor size and location. Most ablation procedures were performed with the free-hand technique. The energy output was adjusted during the ablation process, according to the formation of air bubbles, as shown on the ultrasound monitor and by automated resistance and impedance calculation software. Ablation was considered complete based on 2 parameters. The first parameter was continuous air bubble formation in the interelectrode space and in the outer area of the electrodes. The second parameter was the formation of a ladder-like form on the panel indicating resistance profiles. The draw-back technique was applied to ensure adequate ablation depth and safety margin. Needle track ablation was done during every electrode withdrawal with 40 W energy output to prevent bleeding and unexpected tumor seeding.
Artificial ascites or pleural effusion or both were created by infusion of 5% dextrose water if the tumors were close to other organs such as the diaphragm, bowel, or kidney.
Four-phase computed tomography (CT) scan or dynamic magnetic resonance imaging (MRI) of the liver were conducted 1 month after tumor ablation. Complete tumor ablation was defined by the absence of an enhanced region within or adjacent to the ablated index tumor. Distant and local tumor recurrence were monitored using ultrasound examinations every 3 months and CT/MRI every 6 months.
Results
Patient characteristics
Nine men and six women were enrolled in this study. The patients' age ranged from 51 years to 83 years, with a mean of 64.4 years. Nine of the fifteen patients (60%) were diagnosed with CHC, four with CHB (26.7 %), and two with both CHB and CHC (13.3%). Five of the 15 patients had received antiviral therapy; the patients who had not received antiviral therapy all had CHC, and were precluded from treatment because of old age and potential intolerance of treatment-related adverse effects.
Two patients exhibited two lesions: a 60-year-old man with CHC exhibited a 20-mm lesion in Segment 4 and a 30-mm lesion in Segment 5; and a 51-year-old man with CHB exhibited two 16-mm lesions in Segments 5 and 8, respectively.
The 17 tumors ranged from 14 mm to 30 mm, and the mean diameter was 26 mm. Three were located in Segment 4, five in Segment 5, one in Segment 6, three in Segment 7, and five in Segment 8.
The demographic characteristics of the patients are listed in Table 1 .
| Characteristics | |
|---|---|
| Age (y) | 64.4 ± 11.8 |
| Sex, male | 9 (60%) |
| CHB/CHC/CHB and CHC | 9/4/2 |
| CTP score: A/B | 10 (67%)/5 (33%) (all < CTP: 8) |
| Tumor size (mm) | 26.0 ± 3.7 |
| Tumor location (Seg 4/5/6/7/8) | 3/5/1/3/5 |
| AFP (ng/mL) | 51.2 ± 39.2 |
AFP = α-fetoprotein; CHB = chronic hepatitis B; CHC = chronic hepatitis C; CTP score = Child–Turcotte–Pugh score.
Ablation process and technique
Five of the seventeen lesions were ablated using only two electrodes because of difficulty in placing three electrodes simultaneously. Artificial ascites or pleural effusion were produced for six tumors, of which five were located in the subphrenic area, and one was located close to the right kidney.
The ablation time ranged from 10.2 minutes to 33.3 minutes, and the mean ablation time was 18.5 minutes. The total energy deposition ranged from 25.0 kJ to 111.6 kJ, and the mean energy deposition was 52.1 kJ.
When all electrodes were withdrawn, track ablation was performed using a 40-W energy output to form a “comet-tail” appearance along the needle route on the ultrasound monitor.
Photographs of the non-touch technique and track ablation are presented in Figure 1 ; Figure 2 , respectively.
|
|
|
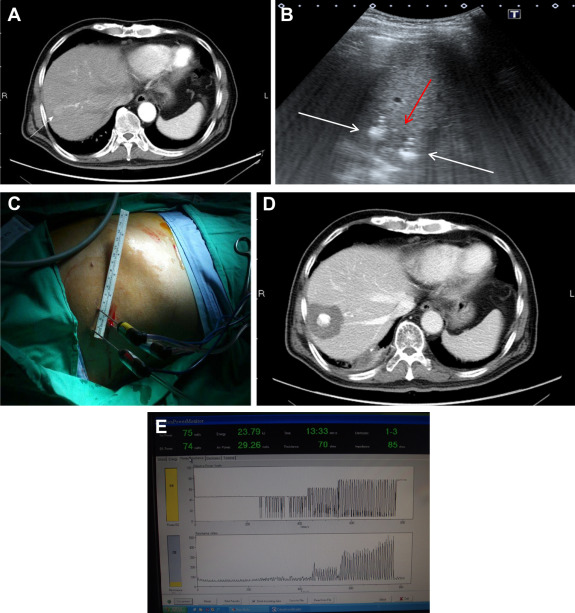
Figure 1. (A) One 2 cm HCC in the S7. (B) Three electrodes (white arrows) were deployed around the tumor (red arrow). The third electrode was not shown in this plane. (C) The deployment of three electrodes to encircle the tumor. (D) CT of liver, 6 months after ablation, showed complete ablation with 1 cm safety margin. (E) Ladder-like appearance of the resistance profile on monitor of energy generator indicating completion of ablation. |
|
|
|
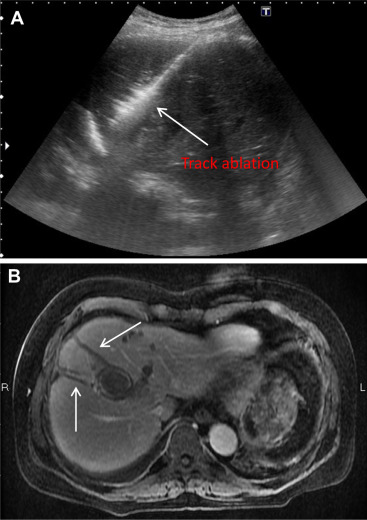
Figure 2. (A) Track ablation under 40 W energy output to form “Comet tail” appearance. (B) Evident track ablation shown in the following MRI. |
Local tumor control
Successful local tumor control was defined by the absence of an enhanced region within or around the ablated areas on CT or MRI images 1 month and every 6 months after the procedure. All of the 17 index tumors met the criteria, and a 0.5–1-cm safety margin was achieved around each tumor. The maximal, minimal, and mean follow-up periods were 21.5 months, 3 months, and 10 months, respectively.
Distant recurrence
There was only one instance of distant recurrence, 15.4 months after initial ablation in a 60-year-old man. The initial two ablated index tumors were located in Segments 4 and 5, and were 2 cm and 3 cm in diameter, respectively. The distant recurrence, which occurred in Segment 2, was 2 cm in diameter.
Complications
Most patients experienced mild puncture wound pain, which subsided gradually. One patient experienced profound jaundice 3 months after the procedure; a CT scan revealed left biliary tree dilation, and postprocedure fibrosis-related biliary stricture was assumed to be the cause.
Discussion
To our knowledge, this is the first report to use the nontouch technique to treat HCC in Taiwan. This new method overcomes the shortcomings of current ablation techniques, including inadequate safety margins, the difficulty of ablating larger tumors, the heat-sink effect, and track seeding. By addressing these shortcomings, our study demonstrated that multipolar RFA achieves excellent local tumor control, eliminates the risk of track seeding, and presents a minimal risk of distant recurrence.
RFA is an effective method to treat small HCC, particularly in patients with cirrhosis, because surgical resection might be precluded in certain patients because of comorbidities [11] . However, most studies have observed that RFA presents a higher risk of tumor recurrence compared with surgical resection; incomplete local tumor control and inadequate safety margins in studies using RFA might explain why RFA exhibits worse disease-free survival and overall survival rates than surgical resection does. Two reports from Korea have indicated that a 1-cm safety margin is crucial to ensure complete tumor ablation and to reduce the risk of local tumor progression [12] ; [13] . The basic physical characteristics of monopolar devices require that the tumor is punctured by the electrode, and that the ablation process is initiated from the tumor center to the periphery. As a consequence, a 0.5–1-cm safety margin is difficult to obtain using this device. By using two or three electrodes to puncture the area around the tumor, multipolar RFA extends the ablation zone to obtain a minimal 0.5-cm safety margin easily.
Most monopolar electrodes are able to obtain ablation diameters of 3 cm, or slightly larger. Sequential overlapping ablation to enable a larger ablation zone when using monopolar devices is difficult, because air bubbles created during ablation can hamper the visualization of subsequent electrode deployment. Using the non-touch technique and multipolar electrodes, complete ablation can be easily achieved once the electrodes are placed in the appropriate locations.
Another shortcoming of monopolar devices is the presence of the heat-sink effect if the index tumor is close to a large vessel (> 3 mm) [14] ; [15] . Using multipolar devices enables electrodes to be placed between the vessel and the index tumor, and complete ablation to be obtained in the interelectrode space. Therefore, the heat-sink effect can be overcome by using this method.
Track seeding is always a concern in percutaneous liver interventions such as liver biopsies or RFA. Tumor seeding after percutaneous RFA, with an occurrence rate as high as 12.5 % (4 of 32 cases), was reported by Llovet et al [16] in 2001. In another study from Taiwan, a 1.2 % track-seeding rate was reported [17] . These various seeding rates might be related to follow-up duration, the number of ablation sessions, whether tumor biopsies had been performed prior to RFA, and the completeness of the track ablation. In our study, no track seeding was observed during the follow-up period. Two reasons might account for this successful result. The most critical reason is the use of the non-touch approach: by avoiding contact with the tumor itself, the adherence of tumor cells to the electrodes was reduced, and track seeding during the advance and retraction of the electrodes was prevented. The second reason is that track ablation was strictly performed using a 40-W energy output. During each electrode retraction, we created a “comet-tail” image along the track on the ultrasound monitor. Hence, in the following spiral CT or MRI, the evident track ablation route can be clearly seen.
An increased number of RF probe punctures increases the risk of damage to adjacent organs, and increases the need for artificial ascites or pleural effusion. Artificial ascites or pleural effusion were created in six patients because the locations of their index tumors were difficult to operate on; five of these six tumors were in the subphrenic area, and one was located close to the right kidney. Five patients with subphrenic tumors received concomitant artificial ascites and pleural effusions. One patient with an S6 tumor received only artificial ascites. The mean artificial ascites volume was 1200 mL and the maximal volume was 3000 mL. The mean artificial pleural effusion volume was 500 mL. The introduction of artificial ascites and pleural effusion did not result in any procedure-related injuries to the adjacent diaphragm or right kidney. Most of the injected artificial ascites and pleural effusion were absorbed spontaneously the day after the procedure. Artificial ascites and pleural effusion also allowed the index tumor to be visualized more clearly.
Despite the use of artificial ascites and pleural effusion, three electrodes could not be simultaneously placed when treating one third of the tumors in our study (5/17) because of the problematic locations of the index tumors and the narrow echo-window. In these cases, only two electrodes were applied. All of these tumors were < 2 cm. However, it seems that two electrodes were adequate, because the rate of complete tumor ablation was determined to be 100% in the short-term follow-up period.
The only procedure-related complication in our study occurred 3 months after tumor ablation. A patient presented with profound jaundice and the spiral CT showed that the left intrahepatic ducts were substantially dilated, and the total bilirubin level was 30 mg/dL. The ablated index tumor was located in Segment 4 and was approximately 3 cm in diameter. The tumor was ablated using three electrodes, and the patient was uneventful during and immediately after the procedure. We assumed that postprocedure fibrosis led to the stricture of the left biliary tract. Direct thermal injury to the bile duct during the procedure was assumed to be unlikely, because the electrodes were 1.5 cm away from the left portal vein, considered a marker for the major left biliary tract; the bile duct was therefore beyond the ablation ability of the multipolar device. A biliary stent and external drainage was used to treat the obstructive jaundice. However, the patient died of pneumonia 2 weeks after the onset of profound jaundice. Therefore, we used prophylactic biliary stenting to treat patients with index tumors close to major bile ducts (n = 4) thereafter, and no further biliary complications occurred.
Certain points deserve mention. First, in Taiwan, most ultrasound-guided interventions are performed with the assistance of a puncture probe. However, it is relatively difficult to insert three electrodes in the narrow echo-window and the limited treatment area on the abdominal wall because the large size of the puncture probe might impede the operation of the electrodes. In this situation, the free-hand technique is required to place the three electrodes in parallel, to allow each electrode to interact with other electrodes and function effectively. Therefore, an aseptic ruler and surgical mark-pen are useful to deploy electrodes properly. Second, the non-touch technique and multiple electrodes deployment require a longer procedural time and operator learning curve. Third, the cost of the electrodes must be considered.
This study has certain limitations. First, the follow-up period was not adequate to assess disease-free survival or overall survival. Second, a larger sample is necessary to obtain a more solid conclusion regarding this technique.
In summary, multipolar RFA using the non-touch technique to treat HCCs ≤ 3 cm is an effective and safe method. This promising ablation method yielded excellent local tumor control with low complication rates. Track seeding was also eliminated using the new concept. However, assessing long-term results regarding disease-free survival and overall survival requires a longer follow-up time.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
Many thanks To Miss Po-Yao Chiang and Mr. I-Chen Chen for their assistance during every procedure.
References
- [1] S.M. Lin, C.J. Lin, C.C. Lin, C.W. Hsu, Y.C. Chen; Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma ≤4 cm; Gastroenterology, 127 (2004), pp. 1714–1723
- [2] S.M. Lin, C.J. Lin, C.C. Lin, C.W. Hsu, Y.C. Chen; Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less; Gut, 54 (2005), pp. 1151–1156
- [3] J.H. Wang, C.C. Wang, C.H. Hung, C.L. Chen, S.N. Lu; Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma; J Hepatol, 56 (2012), pp. 412–418
- [4] S. Terraz, C. Constantin, P. Majno, L. Spahr, G. Mentha, C.D. Becker; Image-guided multipolar radiofrequency ablation of liver tumours: initial clinical results; European Radiology, 17 (2007), pp. 2253–2261
- [5] Y. Osaki, K. Ikeda, N. Izumi, S. Yamashita, H. Kumada, S. Hatta, et al.; Clinical effectiveness of bipolar radiofrequency ablation for small liver cancers; J Gastroenterol, 48 (2013), pp. 874–883
- [6] J.D. Perkins; Seeding risk following percutaneous approach to hepatocellular carcinoma; Liver Transplantation, 13 (2007), pp. 1603–1607
- [7] T. Livraghi, S. Lazzaroni, F. Meloni, L. Solbiati; Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma; Br J Surg, 92 (2005), pp. 856–858
- [8] S. Mulier, P. Mulier, Y. Ni, Y. Miao, B. Dupas, G. Marchal, et al.; Complications of radiofrequency coagulation of liver tumours; Br J Surg, 89 (2002), pp. 1206–1222
- [9] O. Seror, G. N'Kontchou, B. Fathallal, J. Tran van Nhieu, A. Laurent, J. Trinchet, et al.; Results of radiofrequency ablation of hepatocellular carcinoma with a multipolar multielectrode technique: pathological assessment in explanted livers; ASCO (2009) Poster 279
- [10] J. Bruix, M. Sherman, American Association for the Study of Liver Diseases; Management of hepatocellular carcinoma: an update; Hepatology, 53 (2011), pp. 1020–1022
- [11] G. N'Kontchou, A. Mahamoudi, M. Aout, N. Ganne-Carrié, V. Grando, E. Coderc, et al.; Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 western patients with cirrhosis; Hepatology, 50 (2009), pp. 1475–1483
- [12] Y.S. Kim, W.J. Lee, H. Rhim, H.K. Lim, D. Choi, J.Y. Lee; The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion; AJR Am J Roentgenol, 195 (2010), pp. 758–765
- [13] H. Rhim, H.K. Lim; Radiofrequency ablation of hepatocellular carcinoma: pros and cons; Gut Liver, 4 (Suppl. 1) (2010), pp. S113–S118
- [14] T. de Baere, F. Deschamps, P. Briggs, C. Dromain, V. Boige, L. Hechelhammer, et al.; Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion; Radiology, 248 (2008), pp. 1056–1066
- [15] D.S.K. Lu, S.S. Raman, P. Limanond, D. Aziz, J. Economou, R. Busuttil, et al.; Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors; J Vasc Interv Radiol, 14 (2003), pp. 1267–1274
- [16] J.M. Llovet, R. Vilana, C. Brú, L. Bianchi, J.M. Salmeron, L. Boix, et al.; Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma; Hepatology, 33 (2001), pp. 1124–1129
- [17] T.M. Chen, P.T. Huang, L.F. Lin, J.N. Tung; Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience; J Gastroenterol Hepatol, 23 (2008), pp. e445–e450
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?