| Line 3: | Line 3: | ||
Keywords: pharmacoepidemiology, type 2 diabetes mellitus, primary healthcare, meta-bolic control, oral hypoglycemic agents | Keywords: pharmacoepidemiology, type 2 diabetes mellitus, primary healthcare, meta-bolic control, oral hypoglycemic agents | ||
| − | + | 1. Introduction | |
| − | + | Diabetes mellitus (DM) comprises a group of metabolic disorders characterized by impaired carbohydrate metabolism, resulting in chronic hyperglycemia due to reduced glucose utilization and increased production via gluconeogenesis and glycogenolysis [1,2]. Diagnosis relies on measurements of venous plasma glucose and elevated glycated hemoglobin (HbA1c) levels, the latter serving as a key indicator for disease monitoring within Chile’s Family Health Centers (CESFAM) [3]. | |
| − | + | This study focuses on type 2 diabetes mellitus (T2DM), which has shown a marked rise among adults aged 25–40 years in Antofagasta, Chile, with a prevalence of 6.3% nearly double the 3.8% reported in 2010 [3]. The increasing incidence of early-onset T2DM is concerning, as the disease in young adults tends to progress more aggressively and is associated with severe long-term complications [4]. Contributing factors include the high prevalence of obesity (34.4%) and sedentary lifestyles (≈90%) among Chilean adults, both of which are major determinants of chronic disease development [3]. | |
| − | + | Early-onset T2DM has significant public health implications, being linked to an ele-vated risk of macrovascular complications (coronary artery disease, stroke, peripheral ar-terial disease) and microvascular complications (retinopathy, nephropathy, neuropathy) [5,6]. It also accelerates the development of metabolic comorbidities such as hypertension and dyslipidemia and is frequently associated with non-metabolic conditions like depres-sion, collectively reducing quality of life and life expectancy [5,6]. Despite its growing prevalence, evidence on the effectiveness of pharmacological treatments particularly oral hypoglycemic agents in this age group remains limited, constraining evidence-based therapeutic decision-making in clinical practice [7,8]. | |
| − | + | Chile’s health system is structured across three levels of care to ensure comprehen-sive access. Primary Health Care (PHC) serves as the foundation, emphasizing disease prevention, health promotion, and chronic disease management [9,10]. Within PHC, Fam-ily Health Centers (CESFAM) play a pivotal role by providing continuous, communi-ty-based care and monitoring patients with chronic diseases such as T2DM [9,10]. How-ever, the range of pharmacological options available at this level is often restricted, under-scoring the need to evaluate the real-world effectiveness of current treatments. PHC also includes rural health posts and Public Emergency Care Services (SAPU), which extend care to underserved or urgent cases. The secondary and tertiary levels of care encompass medium- and high-complexity hospitals, respectively, providing more advanced diagnos-tics and specialized treatments [9–11]. | |
| − | + | Pharmacoepidemiology provides a vital framework for assessing medication use, ef-fectiveness, adherence, and safety in real-world populations [12]. Within this context, the present study aims to evaluate the effectiveness of oral hypoglycemic therapy in achieving metabolic control among adults aged 25–40 years with T2DM treated in Antofagasta’s PHC system, using changes in HbA1c levels as the primary indicator of metabolic im-provement. | |
| − | + | 2. Materials and Methods | |
| − | + | 2.1 Study Design and Population | |
| − | + | A retrospective longitudinal cohort study was conducted from January 2021 to De-cember 2023 in eight Family Health Centers (CESFAM) located in Antofagasta, Chile. The study population comprised adults aged 25–40 years with a confirmed diagnosis of type 2 diabetes mellitus (T2DM) who attended routine follow-up visits during the study period. | |
| − | + | 2.2 Inclusion and Exclusion Criteria | |
| − | 2. | + | Eligible participants met the diagnostic criteria of the American Diabetes Association (ADA) for T2DM, defined as fasting plasma glucose ≥126 mg/dL, HbA1c ≥6.5%, or 2-hour plasma glucose ≥200 mg/dL during an oral glucose tolerance test. Only patients treated exclusively with oral hypoglycemic agents and possessing complete annual HbA1c rec-ords for each of the three study years were included. |
| − | + | Exclusion criteria comprised treatment with insulin, pregnancy, and incomplete or inconsistent medical records. | |
| − | + | 2.3 Data Collection | |
| − | + | A total of 500 anonymized electronic medical records were retrieved from CESFAM information systems with prior ethical approval. Extracted variables included demo-graphic characteristics (age, sex, nationality, education level), clinical parameters (body mass index [BMI], family history of diabetes, duration since diagnosis), pharmacological details (type and combination of oral hypoglycemic therapy), presence of comorbidities, and annual HbA1c measurements. | |
| − | + | All data were stored and analyzed within a secure secondary database provided by the Municipal Health Corporation of Antofagasta (CMDS) to ensure confidentiality and data integrity. | |
| − | + | 2.4 Statistical Analysis | |
| − | + | Data analysis was performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). Descriptive statistics were expressed as means ± standard deviations (SD) or frequencies, as appropriate. Longitudinal changes in HbA1c levels were evaluated using repeated-measures ANOVA with Tukey’s post hoc comparisons for within-group differ-ences over time. Associations between clinical and demographic variables were examined using Pearson’s correlation coefficients. Statistical significance was established at p < 0.05. | |
| − | + | 2.5 Ethical Considerations | |
| − | + | The study protocol was reviewed and approved by the Health Ethics Committee of the Universidad Internacional Iberoamericana de México (approval No. CR-234) and the Local Ethics Committee of the University of Antofagasta (file No. 469/2024). All data were anonymized prior to analysis to ensure participant confidentiality and compliance with ethical standards. | |
| − | + | 2.6 Use of Generative Artificial Intelligence | |
| − | + | Generative artificial intelligence (AI) tools were employed exclusively for language refinement and formatting of the manuscript. No AI-based systems were used in study design, data collection, statistical analysis, or interpretation. | |
| − | + | 3. Results | |
| − | + | 3.1 Epidemiological Description of the Study Population | |
| − | 2. | + | A total of 15,170 electronic medical records were initially retrieved from the national RAYEN health information platform, all corresponding to patients diagnosed with type 2 diabetes mellitus (T2DM). Of these, 2,456 records were identified from individuals aged 25–40 years who attended follow-up visits at eight Family Health Centers (CESFAM) in Antofagasta, Chile, between 2021 and 2023. |
| − | + | To ensure analytical consistency and data completeness, a final sample of 500 rec-ords was selected. These represented patients who attended all annual follow-up ap-pointments during the three-year study period, ensuring reliable evaluation of treatment effectiveness under continuous primary care management. | |
| − | + | 3.2 Sociodemographic Characteristics | |
| − | + | Among the 500 participants, 63.6% were women (n = 318) and 36.4% were men (n = 182). The highest representation was from CESFAM Corvallis (21%), followed by CESFAM Juan Pablo II (16%), CESFAM Rendic (13%), and CESFAM Valdivieso (11%). | |
| − | + | Age distribution revealed that 75.2% of patients were aged 35–40 years, 15.4% were 30–34 years, and 9.4% were 25–29 years. A family history of T2DM was reported by 78.6% of participants. | |
| − | + | The mean duration of disease was 4.06 ± 0.98 years (range: 3–8 years). Stratified by health center, the longest average duration since diagnosis was observed in CESFAM Val-divieso (5 years), while CESFAM Oriente and CESFAM Centro-Sur reported the shortest mean duration (3 years) (Table 1). | |
| − | + | Table 1. Sociodemographic and Clinical Characteristics of the Study Population (n = 500) | |
| − | + | Variable Category N Mean ± SD % | |
| − | + | Sex Male 182 — 36.4 | |
| − | + | Female 318 — 63.6 | |
| − | 1. | + | Age range (years) 25–29 47 27 ± 1.53 9.4 |
| − | + | 30–34 77 32 ± 1.38 15.4 | |
| − | 2. | + | 35–40 376 38 ± 1.87 75.2 |
| − | + | Nationality Chilean 245 — 49.0 | |
| − | 2. | + | Peruvian 18 — 3.6 |
| − | + | Bolivian 17 — 3.4 | |
| − | + | Colombian 60 — 12.0 | |
| − | + | Venezuelan 160 — 32.0 | |
| − | + | Educational level Incomplete primary 30 — 6.0 | |
| − | + | Completed primary 20 — 4.0 | |
| − | + | Incomplete second-ary 48 — 9.6 | |
| − | + | Completed secondary 174 — 34.8 | |
| − | + | Incomplete higher education 6 — 1.2 | |
| − | + | Completed higher education 13 — 2.6 | |
| − | + | Not reported 209 — 41.8 | |
| − | + | Occupational status Salaried employment 464 — 92.8 | |
| − | + | Self-employed 23 — 4.6 | |
| − | + | Homemaker 13 — 2.6 | |
| − | + | CESFAM affiliation Valdivieso 54 — 10.8 | |
| + | María Cristina 47 — 9.4 | ||
| + | Juan Pablo II 80 — 16.0 | ||
| + | Norte 50 — 10.0 | ||
| + | Rendic 67 — 13.4 | ||
| + | Corvallis 103 — 20.6 | ||
| + | Oriente 49 — 9.8 | ||
| + | Centro-Sur 50 — 10.0 | ||
| + | BMI classification Normal weight 0 — 0.0 | ||
| + | Overweight 358 — 71.6 | ||
| + | Obesity I 90 — 18.0 | ||
| + | Obesity II 52 — 10.4 | ||
| + | Comorbidities Present 287 — 57.4 | ||
| + | Absent 213 — 42.6 | ||
| + | Type of comorbidity Hypertension 80 — 27.9 | ||
| + | Dyslipidemia 125 — 43.5 | ||
| + | Hypertension + Dyslipidemia 28 — 9.8 | ||
| + | Obesity 142 — 28.4 | ||
| + | Other comorbidities Depression 54 — 18.8 | ||
| + | Disease characteristics Duration of T2DM (years) 500 4.06 ± 0.98 — | ||
| + | Family history (mother and/or father) Present 393 — 78.6 | ||
| + | Absent 107 — 21.4 | ||
| + | Oral hypoglycemic treat-ment Metformin 419 — 83.8 | ||
| + | Glibenclamide 6 — 1.2 | ||
| + | Vildagliptin 1 — 0.2 | ||
| + | Metformin + Glibenclamide 74 — 14.8 | ||
| + | HbA1c (%) 2021 500 8.91 ± 0.57 — | ||
| + | 2022 500 8.92 ± 0.55 — | ||
| + | 2023 500 7.41 ± 0.28 — | ||
| + | Table 1. Sociodemographic and clinical characteristics of the study population (n = 500). The ta-ble presents distributions of sex, age, nationality, educational level, occupational activity, and Family Health Center (CESFAM) of origin, as well as relevant clinical indicators: body mass index (BMI), presence and type of comorbidities, family history of type 2 diabetes mellitus (T2DM), time since diagnosis, oral hypoglycemic treatment, and glycated hemoglobin (HbA1c) levels dur-ing 2021–2023. Data are expressed as absolute frequencies (N), percentages (%), means, and standard deviations (SD), according to the analyses performed. | ||
| + | 3.2 Nationality and Educational Level | ||
| + | The most frequent nationality was Chilean (49%), followed by Venezuelan (32%) and Colombian (12%). Regarding educational attainment, secondary education completion was the most common level (34.8%). However, 41.8% of participants did not report their educational background, limiting stratified analyses by this variable. | ||
| + | 3.3 Employment Status | ||
| + | Most participants reported dependent employment (92.8%), while 4.6% were self-employed, and 2.6% identified as homemakers. This reflects a predominantly eco-nomically active population within the working-age range. | ||
| + | 3.4 Baseline Metabolic Profile | ||
| + | At baseline (2021), the mean glycated hemoglobin (HbA1c) was 8.12 ± 1.25%, indi-cating suboptimal metabolic control according to ADA criteria. The mean fasting plasma glucose level was 165.3 ± 27.8 mg/dL, consistent with hyperglycemia and insufficient therapeutic response at the beginning of observation. | ||
| + | 3.5 Evolution of HbA1c Levels (2021–2023) | ||
| + | A progressive and statistically significant reduction in HbA1c was observed over the three-year period. Mean values decreased from 8.12% (2021) to 7.68% (2022) and 7.24% (2023). | ||
| + | Repeated measures ANOVA indicated a significant effect of time on HbA1c levels, p < 0.001). Tukey’s post hoc analysis confirmed significant differences between all consecu-tive years (p < 0.05). | ||
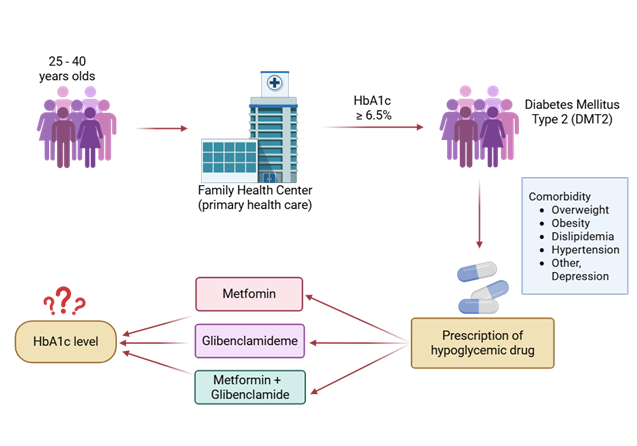
| + | Figure 1. Illustrates the decreasing trend in HbA1c across the study period, suggesting that regular follow-up and adherence to pharmacological treatment were associated with improved glycemic control. | ||
| + | [[File:Draft_Davies_505636215_8045_Picture1.png]] | ||
| + | Figure 1. Longitudinal cohort study design based on 500 anonymized electronic records of patients aged 25–40 years treated at CESFAM in Antofagasta (2021–2023), including HbA1c levels, oral hypoglycemic therapy, comorbidities, and associated treatments.3.7 Pharmacological Treatment Patterns | ||
| + | 3.5.1 Distribution of Therapy Regimens | ||
| + | Monotherapy was prescribed to 46.2% of participants, with metformin being the most common agent (42.8%). The remaining 53.8% received combination therapy, predomi-nantly metformin + glibenclamide (32%) and metformin + sitagliptin (14%). A smaller subset (7.8%) received triple therapy, typically metformin + glibenclamide + pioglitazone. | ||
| + | 3.5.2 Relationship Between Therapy Type and HbA1c Change | ||
| + | Patients on combination therapy achieved a greater mean reduction in HbA1c com-pared to those on monotherapy (ΔHbA1c = –0.94 ± 0.35 vs. –0.52 ± 0.29; p < 0.01). Never-theless, interindividual variability was notable, and treatment adherence emerged as a significant determinant of metabolic response. | ||
| + | Pearson’s correlation analysis identified a moderate positive correlation between body mass index (BMI) and HbA1c levels (r = 0.46, p < 0.001), indicating that higher BMI values were associated with poorer glycemic control. Conversely, a negative correlation was observed between duration of diabetes and HbA1c reduction (r = –0.31, p < 0.01), suggesting that patients with longer disease duration experienced smaller improvements in metabolic control. | ||
| + | No statistically significant correlations were found between HbA1c variation and sex, nationality, or employment status. | ||
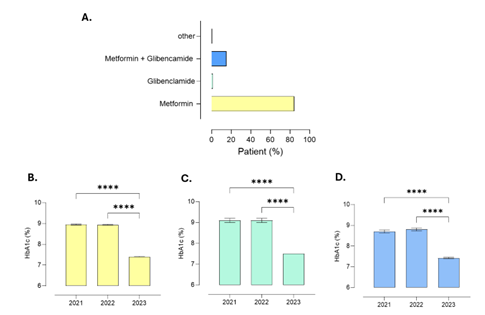
| + | 3.6 Relationship of HbA1c Levels with Hypoglycemic Drugs | ||
| + | Glycated hemoglobin (HbA1c) levels among patients remained stable between 2021 and 2022, with mean values of 8.91% ± 0.57 and 8.92% ± 0.55, respectively. In 2023, however, a reduction in HbA1c was observed, reaching a mean of 7.41% ± 0.28, indicating improved metabolic control over the follow-up period. Regarding pharmacotherapy, most patients received metformin monotherapy (84%), followed by the combination of metformin and glibenclamide (14.8%). Monotherapy with glibenclamide was minimal (1%), as was the use of vildagliptin (0.2%). Concerning comorbidities potentially affecting metabolic con-trol, 57.4% of participants had at least one comorbidity. Dyslipidemia was the most prev-alent (43.5%), followed by hypertension (27.9%). Depression, a condition not directly re-lated to metabolic control, was reported in 18.8% of participants. The most prescribed treatments for these conditions were atorvastatin (42%), sertraline and clonazepam (both 19%), as well as enalapril (9%) and hydrochlorothiazide (8%). | ||
| + | [[File:Draft_Davies_505636215_9838_Picture2.png]] | ||
| + | Figure 2. Evolution of glycated hemoglobin (HbA1c) levels in patients with type 2 diabetes mellitus aged 25–40 years treated at CESFAM in Antofagasta between 2021 and 2023. HbA1c levels remained stable between 2021 and 2022, followed by a significant reduction in 2023, particularly among patients receiving metformin monotherapy or combination therapy. (A) Proportion of patients by oral hypoglycemic prescription; (B) HbA1c levels in patients treated with glibenclamide; (C) HbA1c levels in patients treated with metformin plus glibenclamide; (D) HbA1c levels in patients treated with metformin. Data are pre-sented as mean ± SEM, with n representing the number of participants. Statistical analy-sis: one-way ANOVA with Tukey post hoc test. | ||
| + | 3.7 Relationship of HbA1c Levels with Patient BMI | ||
| + | Nutritional status in the study sample showed that 71.6% of patients were over-weight, 18% had obesity class I, and 10.4% had obesity class II. | ||
| + | When HbA1c levels were compared by sex, the mean values were similar between women (8.41% ± 0.86) and men (8.43% ± 0.85). Likewise, HbA1c values remained stable across BMI categories: obesity class I (8.42% ± 0.85), obesity class II (8.39% ± 0.83), and overweight (8.41% ± 0.87). | ||
| + | According to oral hypoglycemic therapy, mean HbA1c levels were 8.43% ± 0.87 for metformin, 8.57% ± 0.80 for glibenclamide, 8.0% ± 0.87 for vildagliptin, and 8.31% ± 0.82 for the metformin–glibenclamide combination. | ||
| + | [[File:Draft_Davies_505636215_1477_Picture3.png]] | ||
| + | Figure 3. Relationship between glycated hemoglobin (HbA1c) levels and body mass index (BMI) in patients with type 2 diabetes mellitus aged 25–40 years. HbA1c values remained stable across overweight, obesity class I, and obesity class II categories, with no significant differences between men and women or according to the type of oral hypoglycemic therapy. (A) Percentage distribution of participants by nutritional status; (B) HbA1c levels in overweight patients (n = 358); (C) HbA1c levels in patients with obesity class I (n = 90); (D) HbA1c levels in patients with obesity class II (n = 52). Values are presented as mean ± SEM, with n representing the number of participants. Statistical analysis: one-way ANOVA with Tukey post hoc test. | ||
| + | 3.8 Comorbidities Present in the Sample | ||
| + | In the analyzed sample, 57.4% of patients had at least one comorbidity potentially affecting metabolic control. Dyslipidemia was the most prevalent (43.5%), followed by obesity (28.4%) and hypertension (27.9%). Depression, a condition not directly re-lated to metabolic control, was reported in 18.8% of participants. These findings align with epidemiological evidence describing a close association between components of metabolic syndrome and the progression of type 2 diabetes mellitus. The most pre-scribed treatments for these conditions were atorvastatin (42%), sertraline and clonazepam (both 19%), enalapril (9%), and hydrochlorothiazide (8%). | ||
| + | [[File:Draft_Davies_505636215_9426_Picture4.png]] | ||
| + | Figure 4. Distribution of comorbidities in the study sample. Analysis was performed using simple descriptive statistics. | ||
| + | [[File:Draft_Davies_505636215_2673_Picture5.png]] | ||
| + | Figure 5. Most common treatments used in patients with type 2 diabetes mellitus presenting comorbidities at primary health care centers (APS) in the city of Antofagasta. Analysis performed using simple descriptive statistics. | ||
| + | 3.9 Summary of Key Findings | ||
| + | From 15,170 total T2DM records, 2,456 corresponded to adults aged 25–40 years; 500 with con-tinuous annual follow-up were included in the final analysis. Women represented 63.6% of the study population, and most participants were aged 35–40 years. Mean disease dura-tion was approximately four years, with the majority having a family history of diabetes. A significant reduction in HbA1c was observed from 2021 to 2023, demonstrating improved metabolic control under oral hypoglycemic therapy. | ||
| + | Combina-tion therapy (especially metformin-based) resulted in superior glycemic improvement compared to monotherapy. Higher BMI was associated with poorer glycemic control, un-derscoring the impact of obesity on diabetes management outcomes. | ||
| + | 4. Discussion | ||
| + | The present study provides valuable insights into the metabolic control and clinical characteristics of young adults (25–40 years) with type 2 diabetes mellitus (T2DM) at-tending Family Health Centers (CESFAM) in Antofagasta, Chile. Over the three-year fol-low-up period (2021–2023), mean glycated hemoglobin (HbA1c) levels significantly de-creased from approximately 8.9% to 7.4%, reflecting improved glycemic control in this population. This outcome is clinically relevant, as sustained HbA1c reductions are asso-ciated with decreased risks of both microvascular and macrovascular complications. Findings from the UKPDS and other landmark studies have shown that each 1% decrease in HbA1c corresponds to a 35–37% reduction in microvascular events, supporting the clinical importance of the observed changes. | ||
| + | A notable demographic feature of this cohort was the predominance of women (63.6%), which mirrors reports from other Latin American and global studies showing a higher prevalence of T2DM among women in certain age ranges. This may be explained by differences in hormonal profiles, fat distribution, and health service utilization pat-terns. Most participants were between 35 and 40 years of age (75.2%), consistent with an epidemiological shift toward earlier onset of T2DM in working-age adults, as observed in developing countries undergoing rapid nutritional and lifestyle transitions. | ||
| + | A strong family history of diabetes was observed in 78.6% of participants, under-scoring the role of genetic predisposition in disease development. Previous studies have shown that individuals with first-degree relatives affected by T2DM have approximately double the risk of developing the disease, particularly when combined with environmen-tal and lifestyle factors such as diet and physical inactivity. This emphasizes the need for early screening and prevention strategies in younger adults with familial risk. | ||
| + | Metformin was the most frequently prescribed oral hypoglycemic agent (84%), either as monotherapy or in combination with glibenclamide. This finding aligns with interna-tional and national guidelines recommending metformin as the first-line treatment for T2DM due to its efficacy, safety profile, and cardiovascular benefits. The ANOVA analysis demonstrated that both metformin monotherapy and combination therapy significantly improved HbA1c levels over the study period. In contrast, patients treated with glibenclamide alone exhibited less favorable glycemic control, consistent with previous evidence highlighting sulfonylureas’ higher risk of hypoglycemia and reduced long-term durability of effect. | ||
| + | Comorbid conditions were highly prevalent (57.4%), with dyslipidemia (43.5%) and hypertension (27.9%) being the most common. The widespread use of atorvastatin (42%) is consistent with international recommendations for cardiovascular risk reduction in di-abetic patients over 40 years or those with multiple risk factors. Furthermore, the presence of depression in nearly one-fifth of the cohort (18.8%) highlights the critical need for inte-grated mental health care in diabetes management, as psychological comorbidities are known to impair treatment adherence and worsen glycemic outcomes. | ||
| + | Regarding nutritional status, 71.6% of patients were overweight and 28.4% obese, re-flecting the well-established association between adiposity and T2DM risk. Interestingly, no significant association was observed between body mass index (BMI) and glycemic control in this cohort. This suggests that pharmacological therapy, rather than BMI alone, may be the dominant determinant of metabolic improvement. Emerging evidence indi-cates that central adiposity or visceral fat distribution may serve as more sensitive indica-tors of metabolic dysfunction than BMI in isolation. | ||
| + | Overall, these findings demonstrate significant improvements in glycemic control among young adults with T2DM yet highlight persistent challenges in achieving optimal metabolic targets (HbA1c ≤ 7.0%) as recommended by international guidelines. The re-sults suggest that pharmacological interventions, while essential, must be complemented by non-pharmacological strategies—such as nutritional counseling, structured physical activity, and patient education—to achieve sustainable metabolic control. | ||
| + | Study Limitations | ||
| + | This study has several limitations. The use of a non-probabilistic sample may limit generalizability to broader populations. Additionally, BMI was used as a categorical ra-ther than continuous variable, and only the most recent lipid profile and blood pressure data from 2023 were analyzed, which may affect precision. Moreover, potential selection bias could arise from including only patients with consistent annual follow-up. Future studies should incorporate longitudinal anthropometric and biochemical data, consider psychosocial variables, and explore the impact of integrated lifestyle and pharmacological interventions on long-term outcomes. | ||
| + | 5. Conclusions | ||
| + | This study highlights critical gaps in the management of young adults (25–40 years) with type 2 diabetes mellitus (T2DM) receiving care at Family Health Centers in Antofa-gasta, Chile. Although improvements in glycemic control were observed, the overall care model remains limited in scope and frequency. Current follow-up practices, focused pri-marily on annual assessments of basic metabolic parameters, contrast with international recommendations advocating for more comprehensive and frequent monitoring from the early stages of the disease. | ||
| + | One of the main challenges identified is the restricted availability of oral hypoglyce-mic agents in primary care settings, which constrains clinicians’ ability to individualize treatment and may contribute to suboptimal metabolic control and increased risk of long-term complications. Furthermore, the absence of detailed clinical records on micro-vascular and macrovascular complications, coupled with limited referral pathways to specialized services such as ophthalmology, vascular medicine, and nursing, underscores fragmented care inconsistent with global diabetes management standards. | ||
| + | Equally concerning is the lack of structured nutrition and physical activity programs, which reflect a predominantly reactive rather than preventive care model. The absence of sustained interventions targeting weight management, dietary habits, and exercise sub-stantially limits opportunities to achieve lasting behavioral change and prevent comor-bidities such as obesity and cardiovascular disease. | ||
| + | From a methodological standpoint, limitations related to data collection—such as the nominal recording of body mass index (BMI) and the use of only the most recent 2023 li-pid and blood pressure data—may have affected the precision and generalizability of findings. | ||
| + | Overall, the results indicate that the current primary care model does not fully meet international standards for integrated and preventive diabetes management. Strengthen-ing multidisciplinary care, increasing monitoring frequency, and implementing continu-ous patient education and lifestyle support are essential to improving metabolic control and preventing complications in this population. Future public health strategies should focus on early intervention, comprehensive risk assessment, and equitable access to pharmacological and non-pharmacological therapies to ensure sustainable diabetes care. | ||
| + | Author Contributions: For research articles with several authors, a short paragraph specifying their individual contributions must be provided. Conceptualization, MFS, MCA, YMC, JAY; methodology, MFS, MCA, YMC, NMD, JAY; software, MFS, MCA, YMC, JAY; validation, MFS, MCA, YMC, NMD, JAY; formal analysis, MFS, SDAA, AAR, MCA, YMC, NMD, JAY; investigation, MFS, MCA, SDAA, AAR, YMC, NMD, JAY; resources, MFS, MCA, YMC, NMD, JAY; data curation, MFS, MCA, YMC, NMD, JAY; writing—original draft preparation, MFS, SDAA, AAR, MCA, YMC, BFM, CRM, NMD, JAY; writing—review and editing, MFS, MCA, SDAA, AAR, YMC, BRM, CRM, NMD, JAY; visualization, MFS, MCA, YMC,CRM, SDAA, AAR, BFM, NMD, JAY; supervision, MFS, MCA, YMC, NMD, JAY; project administration, MFS, MCA, YMC, NMD, JAY. All authors have read and agreed to the published version of the manuscript | ||
| + | Funding: This research received no external funding. | ||
| + | Institutional Review Board Statement: The study protocol was approved by the Health Ethics Committee of the Universidad Internacional Iberoamericana de México (approval No. CR-234) and by the accredited local committee of the University of Antofagasta (file No. 469/2024). All data were anonymized to maintain confidentiality. | ||
| + | Acknowledgments: Special thanks to CMDS, the health sector of the city of Antofagasta, for granting access to the electronic medical records. Special recognition to the Universidad Inter-nacional Iberoamericana de México and the ethics committees that approved the study. This work is part of the Doctoral Thesis of Manuel Figueroa Sanchez to obtain his Ph.D. in Public Health Sciences from Universidad Internacional Iberoamericana, Mexico. | ||
| + | Conflicts of Interest: The authors declare no conflicts of interest. | ||
==3 Bibliography<!-- | ==3 Bibliography<!-- | ||
Revision as of 22:36, 17 October 2025
Title Real-World Effectiveness of Oral Hypoglycemic Agents in Young Adults with Type 2 Diabetes in Northern Chile Abstract Background/Objectives: The rising prevalence of type 2 diabetes mellitus (T2DM) among adults aged 25–40 years in Latin America has emerged as a significant public health challenge, driven by lifestyle and metabolic risk factors. Evaluating the real-world effec-tiveness of oral hypoglycemic agents within primary health care (PHC) systems is essen-tial to inform therapeutic strategies and improve glycemic control in this population. This study aimed to assess metabolic outcomes and treatment associations among young adults with T2DM managed in PHC settings in northern Chile. Methods: A retrospective longitudinal cohort study was conducted using 500 electronic medical records from pa-tients aged 25–40 years diagnosed with T2DM and treated between 2021 and 2023 across eight family health centers (CESFAM) in Antofagasta, Chile. The primary outcome was glycosylated hemoglobin (HbA1c). Secondary analyses examined relationships between treatment type, body mass index (BMI), and comorbidities including dyslipidemia, hy-pertension, and depression. Results: Among all patients, 71.6% were overweight and 28.4% obese. Comorbidities were documented as 57.4%, predominantly dyslipidemia (43.5%), hypertension (27.9%), and depression (18.8%). The most common therapies were metformin monotherapy (84%) and metformin plus glibenclamide (14.8%). Mean HbA1c values remained unchanged between 2021 (8.91 ± 0.57) and 2022 (8.92 ± 0.55) but im-proved significantly in 2023 (7.41 ± 0.28), although international glycemic targets were not met. Conclusions: Oral hypoglycemic therapy in PHC settings was partially effective in improving glycemic control among young adults with T2DM. These findings underscore the need for broader pharmacological options, enhanced follow-up, and reinforcing pa-tient education within Chile’s primary care system. Keywords: pharmacoepidemiology, type 2 diabetes mellitus, primary healthcare, meta-bolic control, oral hypoglycemic agents
1. Introduction Diabetes mellitus (DM) comprises a group of metabolic disorders characterized by impaired carbohydrate metabolism, resulting in chronic hyperglycemia due to reduced glucose utilization and increased production via gluconeogenesis and glycogenolysis [1,2]. Diagnosis relies on measurements of venous plasma glucose and elevated glycated hemoglobin (HbA1c) levels, the latter serving as a key indicator for disease monitoring within Chile’s Family Health Centers (CESFAM) [3]. This study focuses on type 2 diabetes mellitus (T2DM), which has shown a marked rise among adults aged 25–40 years in Antofagasta, Chile, with a prevalence of 6.3% nearly double the 3.8% reported in 2010 [3]. The increasing incidence of early-onset T2DM is concerning, as the disease in young adults tends to progress more aggressively and is associated with severe long-term complications [4]. Contributing factors include the high prevalence of obesity (34.4%) and sedentary lifestyles (≈90%) among Chilean adults, both of which are major determinants of chronic disease development [3]. Early-onset T2DM has significant public health implications, being linked to an ele-vated risk of macrovascular complications (coronary artery disease, stroke, peripheral ar-terial disease) and microvascular complications (retinopathy, nephropathy, neuropathy) [5,6]. It also accelerates the development of metabolic comorbidities such as hypertension and dyslipidemia and is frequently associated with non-metabolic conditions like depres-sion, collectively reducing quality of life and life expectancy [5,6]. Despite its growing prevalence, evidence on the effectiveness of pharmacological treatments particularly oral hypoglycemic agents in this age group remains limited, constraining evidence-based therapeutic decision-making in clinical practice [7,8]. Chile’s health system is structured across three levels of care to ensure comprehen-sive access. Primary Health Care (PHC) serves as the foundation, emphasizing disease prevention, health promotion, and chronic disease management [9,10]. Within PHC, Fam-ily Health Centers (CESFAM) play a pivotal role by providing continuous, communi-ty-based care and monitoring patients with chronic diseases such as T2DM [9,10]. How-ever, the range of pharmacological options available at this level is often restricted, under-scoring the need to evaluate the real-world effectiveness of current treatments. PHC also includes rural health posts and Public Emergency Care Services (SAPU), which extend care to underserved or urgent cases. The secondary and tertiary levels of care encompass medium- and high-complexity hospitals, respectively, providing more advanced diagnos-tics and specialized treatments [9–11]. Pharmacoepidemiology provides a vital framework for assessing medication use, ef-fectiveness, adherence, and safety in real-world populations [12]. Within this context, the present study aims to evaluate the effectiveness of oral hypoglycemic therapy in achieving metabolic control among adults aged 25–40 years with T2DM treated in Antofagasta’s PHC system, using changes in HbA1c levels as the primary indicator of metabolic im-provement. 2. Materials and Methods 2.1 Study Design and Population A retrospective longitudinal cohort study was conducted from January 2021 to De-cember 2023 in eight Family Health Centers (CESFAM) located in Antofagasta, Chile. The study population comprised adults aged 25–40 years with a confirmed diagnosis of type 2 diabetes mellitus (T2DM) who attended routine follow-up visits during the study period. 2.2 Inclusion and Exclusion Criteria Eligible participants met the diagnostic criteria of the American Diabetes Association (ADA) for T2DM, defined as fasting plasma glucose ≥126 mg/dL, HbA1c ≥6.5%, or 2-hour plasma glucose ≥200 mg/dL during an oral glucose tolerance test. Only patients treated exclusively with oral hypoglycemic agents and possessing complete annual HbA1c rec-ords for each of the three study years were included. Exclusion criteria comprised treatment with insulin, pregnancy, and incomplete or inconsistent medical records. 2.3 Data Collection A total of 500 anonymized electronic medical records were retrieved from CESFAM information systems with prior ethical approval. Extracted variables included demo-graphic characteristics (age, sex, nationality, education level), clinical parameters (body mass index [BMI], family history of diabetes, duration since diagnosis), pharmacological details (type and combination of oral hypoglycemic therapy), presence of comorbidities, and annual HbA1c measurements. All data were stored and analyzed within a secure secondary database provided by the Municipal Health Corporation of Antofagasta (CMDS) to ensure confidentiality and data integrity. 2.4 Statistical Analysis Data analysis was performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). Descriptive statistics were expressed as means ± standard deviations (SD) or frequencies, as appropriate. Longitudinal changes in HbA1c levels were evaluated using repeated-measures ANOVA with Tukey’s post hoc comparisons for within-group differ-ences over time. Associations between clinical and demographic variables were examined using Pearson’s correlation coefficients. Statistical significance was established at p < 0.05. 2.5 Ethical Considerations The study protocol was reviewed and approved by the Health Ethics Committee of the Universidad Internacional Iberoamericana de México (approval No. CR-234) and the Local Ethics Committee of the University of Antofagasta (file No. 469/2024). All data were anonymized prior to analysis to ensure participant confidentiality and compliance with ethical standards. 2.6 Use of Generative Artificial Intelligence Generative artificial intelligence (AI) tools were employed exclusively for language refinement and formatting of the manuscript. No AI-based systems were used in study design, data collection, statistical analysis, or interpretation. 3. Results 3.1 Epidemiological Description of the Study Population A total of 15,170 electronic medical records were initially retrieved from the national RAYEN health information platform, all corresponding to patients diagnosed with type 2 diabetes mellitus (T2DM). Of these, 2,456 records were identified from individuals aged 25–40 years who attended follow-up visits at eight Family Health Centers (CESFAM) in Antofagasta, Chile, between 2021 and 2023. To ensure analytical consistency and data completeness, a final sample of 500 rec-ords was selected. These represented patients who attended all annual follow-up ap-pointments during the three-year study period, ensuring reliable evaluation of treatment effectiveness under continuous primary care management. 3.2 Sociodemographic Characteristics Among the 500 participants, 63.6% were women (n = 318) and 36.4% were men (n = 182). The highest representation was from CESFAM Corvallis (21%), followed by CESFAM Juan Pablo II (16%), CESFAM Rendic (13%), and CESFAM Valdivieso (11%). Age distribution revealed that 75.2% of patients were aged 35–40 years, 15.4% were 30–34 years, and 9.4% were 25–29 years. A family history of T2DM was reported by 78.6% of participants. The mean duration of disease was 4.06 ± 0.98 years (range: 3–8 years). Stratified by health center, the longest average duration since diagnosis was observed in CESFAM Val-divieso (5 years), while CESFAM Oriente and CESFAM Centro-Sur reported the shortest mean duration (3 years) (Table 1). Table 1. Sociodemographic and Clinical Characteristics of the Study Population (n = 500) Variable Category N Mean ± SD % Sex Male 182 — 36.4 Female 318 — 63.6 Age range (years) 25–29 47 27 ± 1.53 9.4 30–34 77 32 ± 1.38 15.4 35–40 376 38 ± 1.87 75.2 Nationality Chilean 245 — 49.0 Peruvian 18 — 3.6 Bolivian 17 — 3.4 Colombian 60 — 12.0 Venezuelan 160 — 32.0 Educational level Incomplete primary 30 — 6.0 Completed primary 20 — 4.0 Incomplete second-ary 48 — 9.6 Completed secondary 174 — 34.8 Incomplete higher education 6 — 1.2 Completed higher education 13 — 2.6 Not reported 209 — 41.8 Occupational status Salaried employment 464 — 92.8 Self-employed 23 — 4.6 Homemaker 13 — 2.6 CESFAM affiliation Valdivieso 54 — 10.8 María Cristina 47 — 9.4 Juan Pablo II 80 — 16.0 Norte 50 — 10.0 Rendic 67 — 13.4 Corvallis 103 — 20.6 Oriente 49 — 9.8 Centro-Sur 50 — 10.0 BMI classification Normal weight 0 — 0.0 Overweight 358 — 71.6 Obesity I 90 — 18.0 Obesity II 52 — 10.4 Comorbidities Present 287 — 57.4 Absent 213 — 42.6 Type of comorbidity Hypertension 80 — 27.9 Dyslipidemia 125 — 43.5 Hypertension + Dyslipidemia 28 — 9.8 Obesity 142 — 28.4 Other comorbidities Depression 54 — 18.8 Disease characteristics Duration of T2DM (years) 500 4.06 ± 0.98 — Family history (mother and/or father) Present 393 — 78.6 Absent 107 — 21.4 Oral hypoglycemic treat-ment Metformin 419 — 83.8 Glibenclamide 6 — 1.2 Vildagliptin 1 — 0.2 Metformin + Glibenclamide 74 — 14.8 HbA1c (%) 2021 500 8.91 ± 0.57 — 2022 500 8.92 ± 0.55 — 2023 500 7.41 ± 0.28 —
Table 1. Sociodemographic and clinical characteristics of the study population (n = 500). The ta-ble presents distributions of sex, age, nationality, educational level, occupational activity, and Family Health Center (CESFAM) of origin, as well as relevant clinical indicators: body mass index (BMI), presence and type of comorbidities, family history of type 2 diabetes mellitus (T2DM), time since diagnosis, oral hypoglycemic treatment, and glycated hemoglobin (HbA1c) levels dur-ing 2021–2023. Data are expressed as absolute frequencies (N), percentages (%), means, and standard deviations (SD), according to the analyses performed. 3.2 Nationality and Educational Level The most frequent nationality was Chilean (49%), followed by Venezuelan (32%) and Colombian (12%). Regarding educational attainment, secondary education completion was the most common level (34.8%). However, 41.8% of participants did not report their educational background, limiting stratified analyses by this variable. 3.3 Employment Status Most participants reported dependent employment (92.8%), while 4.6% were self-employed, and 2.6% identified as homemakers. This reflects a predominantly eco-nomically active population within the working-age range. 3.4 Baseline Metabolic Profile At baseline (2021), the mean glycated hemoglobin (HbA1c) was 8.12 ± 1.25%, indi-cating suboptimal metabolic control according to ADA criteria. The mean fasting plasma glucose level was 165.3 ± 27.8 mg/dL, consistent with hyperglycemia and insufficient therapeutic response at the beginning of observation. 3.5 Evolution of HbA1c Levels (2021–2023) A progressive and statistically significant reduction in HbA1c was observed over the three-year period. Mean values decreased from 8.12% (2021) to 7.68% (2022) and 7.24% (2023). Repeated measures ANOVA indicated a significant effect of time on HbA1c levels, p < 0.001). Tukey’s post hoc analysis confirmed significant differences between all consecu-tive years (p < 0.05).
Figure 1. Illustrates the decreasing trend in HbA1c across the study period, suggesting that regular follow-up and adherence to pharmacological treatment were associated with improved glycemic control.
Figure 1. Longitudinal cohort study design based on 500 anonymized electronic records of patients aged 25–40 years treated at CESFAM in Antofagasta (2021–2023), including HbA1c levels, oral hypoglycemic therapy, comorbidities, and associated treatments.3.7 Pharmacological Treatment Patterns 3.5.1 Distribution of Therapy Regimens Monotherapy was prescribed to 46.2% of participants, with metformin being the most common agent (42.8%). The remaining 53.8% received combination therapy, predomi-nantly metformin + glibenclamide (32%) and metformin + sitagliptin (14%). A smaller subset (7.8%) received triple therapy, typically metformin + glibenclamide + pioglitazone. 3.5.2 Relationship Between Therapy Type and HbA1c Change Patients on combination therapy achieved a greater mean reduction in HbA1c com-pared to those on monotherapy (ΔHbA1c = –0.94 ± 0.35 vs. –0.52 ± 0.29; p < 0.01). Never-theless, interindividual variability was notable, and treatment adherence emerged as a significant determinant of metabolic response. Pearson’s correlation analysis identified a moderate positive correlation between body mass index (BMI) and HbA1c levels (r = 0.46, p < 0.001), indicating that higher BMI values were associated with poorer glycemic control. Conversely, a negative correlation was observed between duration of diabetes and HbA1c reduction (r = –0.31, p < 0.01), suggesting that patients with longer disease duration experienced smaller improvements in metabolic control. No statistically significant correlations were found between HbA1c variation and sex, nationality, or employment status. 3.6 Relationship of HbA1c Levels with Hypoglycemic Drugs Glycated hemoglobin (HbA1c) levels among patients remained stable between 2021 and 2022, with mean values of 8.91% ± 0.57 and 8.92% ± 0.55, respectively. In 2023, however, a reduction in HbA1c was observed, reaching a mean of 7.41% ± 0.28, indicating improved metabolic control over the follow-up period. Regarding pharmacotherapy, most patients received metformin monotherapy (84%), followed by the combination of metformin and glibenclamide (14.8%). Monotherapy with glibenclamide was minimal (1%), as was the use of vildagliptin (0.2%). Concerning comorbidities potentially affecting metabolic con-trol, 57.4% of participants had at least one comorbidity. Dyslipidemia was the most prev-alent (43.5%), followed by hypertension (27.9%). Depression, a condition not directly re-lated to metabolic control, was reported in 18.8% of participants. The most prescribed treatments for these conditions were atorvastatin (42%), sertraline and clonazepam (both 19%), as well as enalapril (9%) and hydrochlorothiazide (8%).
Figure 2. Evolution of glycated hemoglobin (HbA1c) levels in patients with type 2 diabetes mellitus aged 25–40 years treated at CESFAM in Antofagasta between 2021 and 2023. HbA1c levels remained stable between 2021 and 2022, followed by a significant reduction in 2023, particularly among patients receiving metformin monotherapy or combination therapy. (A) Proportion of patients by oral hypoglycemic prescription; (B) HbA1c levels in patients treated with glibenclamide; (C) HbA1c levels in patients treated with metformin plus glibenclamide; (D) HbA1c levels in patients treated with metformin. Data are pre-sented as mean ± SEM, with n representing the number of participants. Statistical analy-sis: one-way ANOVA with Tukey post hoc test.
3.7 Relationship of HbA1c Levels with Patient BMI
Nutritional status in the study sample showed that 71.6% of patients were over-weight, 18% had obesity class I, and 10.4% had obesity class II.
When HbA1c levels were compared by sex, the mean values were similar between women (8.41% ± 0.86) and men (8.43% ± 0.85). Likewise, HbA1c values remained stable across BMI categories: obesity class I (8.42% ± 0.85), obesity class II (8.39% ± 0.83), and overweight (8.41% ± 0.87).
According to oral hypoglycemic therapy, mean HbA1c levels were 8.43% ± 0.87 for metformin, 8.57% ± 0.80 for glibenclamide, 8.0% ± 0.87 for vildagliptin, and 8.31% ± 0.82 for the metformin–glibenclamide combination.
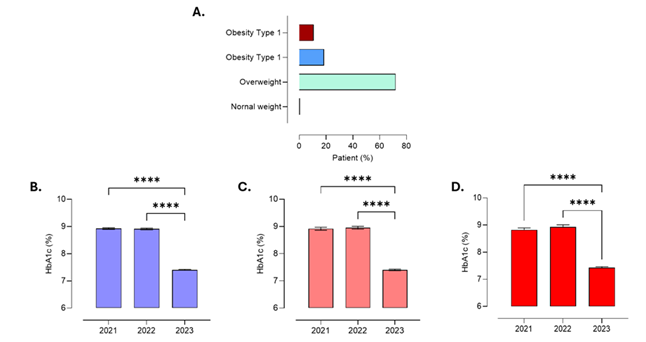 Figure 3. Relationship between glycated hemoglobin (HbA1c) levels and body mass index (BMI) in patients with type 2 diabetes mellitus aged 25–40 years. HbA1c values remained stable across overweight, obesity class I, and obesity class II categories, with no significant differences between men and women or according to the type of oral hypoglycemic therapy. (A) Percentage distribution of participants by nutritional status; (B) HbA1c levels in overweight patients (n = 358); (C) HbA1c levels in patients with obesity class I (n = 90); (D) HbA1c levels in patients with obesity class II (n = 52). Values are presented as mean ± SEM, with n representing the number of participants. Statistical analysis: one-way ANOVA with Tukey post hoc test.
3.8 Comorbidities Present in the Sample
In the analyzed sample, 57.4% of patients had at least one comorbidity potentially affecting metabolic control. Dyslipidemia was the most prevalent (43.5%), followed by obesity (28.4%) and hypertension (27.9%). Depression, a condition not directly re-lated to metabolic control, was reported in 18.8% of participants. These findings align with epidemiological evidence describing a close association between components of metabolic syndrome and the progression of type 2 diabetes mellitus. The most pre-scribed treatments for these conditions were atorvastatin (42%), sertraline and clonazepam (both 19%), enalapril (9%), and hydrochlorothiazide (8%).
Figure 3. Relationship between glycated hemoglobin (HbA1c) levels and body mass index (BMI) in patients with type 2 diabetes mellitus aged 25–40 years. HbA1c values remained stable across overweight, obesity class I, and obesity class II categories, with no significant differences between men and women or according to the type of oral hypoglycemic therapy. (A) Percentage distribution of participants by nutritional status; (B) HbA1c levels in overweight patients (n = 358); (C) HbA1c levels in patients with obesity class I (n = 90); (D) HbA1c levels in patients with obesity class II (n = 52). Values are presented as mean ± SEM, with n representing the number of participants. Statistical analysis: one-way ANOVA with Tukey post hoc test.
3.8 Comorbidities Present in the Sample
In the analyzed sample, 57.4% of patients had at least one comorbidity potentially affecting metabolic control. Dyslipidemia was the most prevalent (43.5%), followed by obesity (28.4%) and hypertension (27.9%). Depression, a condition not directly re-lated to metabolic control, was reported in 18.8% of participants. These findings align with epidemiological evidence describing a close association between components of metabolic syndrome and the progression of type 2 diabetes mellitus. The most pre-scribed treatments for these conditions were atorvastatin (42%), sertraline and clonazepam (both 19%), enalapril (9%), and hydrochlorothiazide (8%).
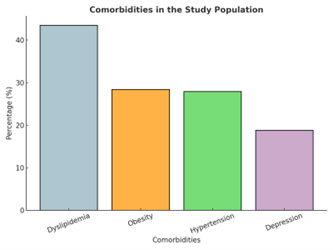 Figure 4. Distribution of comorbidities in the study sample. Analysis was performed using simple descriptive statistics.
Figure 4. Distribution of comorbidities in the study sample. Analysis was performed using simple descriptive statistics.
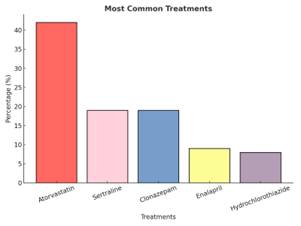 Figure 5. Most common treatments used in patients with type 2 diabetes mellitus presenting comorbidities at primary health care centers (APS) in the city of Antofagasta. Analysis performed using simple descriptive statistics.
3.9 Summary of Key Findings
From 15,170 total T2DM records, 2,456 corresponded to adults aged 25–40 years; 500 with con-tinuous annual follow-up were included in the final analysis. Women represented 63.6% of the study population, and most participants were aged 35–40 years. Mean disease dura-tion was approximately four years, with the majority having a family history of diabetes. A significant reduction in HbA1c was observed from 2021 to 2023, demonstrating improved metabolic control under oral hypoglycemic therapy.
Combina-tion therapy (especially metformin-based) resulted in superior glycemic improvement compared to monotherapy. Higher BMI was associated with poorer glycemic control, un-derscoring the impact of obesity on diabetes management outcomes.
4. Discussion
The present study provides valuable insights into the metabolic control and clinical characteristics of young adults (25–40 years) with type 2 diabetes mellitus (T2DM) at-tending Family Health Centers (CESFAM) in Antofagasta, Chile. Over the three-year fol-low-up period (2021–2023), mean glycated hemoglobin (HbA1c) levels significantly de-creased from approximately 8.9% to 7.4%, reflecting improved glycemic control in this population. This outcome is clinically relevant, as sustained HbA1c reductions are asso-ciated with decreased risks of both microvascular and macrovascular complications. Findings from the UKPDS and other landmark studies have shown that each 1% decrease in HbA1c corresponds to a 35–37% reduction in microvascular events, supporting the clinical importance of the observed changes.
A notable demographic feature of this cohort was the predominance of women (63.6%), which mirrors reports from other Latin American and global studies showing a higher prevalence of T2DM among women in certain age ranges. This may be explained by differences in hormonal profiles, fat distribution, and health service utilization pat-terns. Most participants were between 35 and 40 years of age (75.2%), consistent with an epidemiological shift toward earlier onset of T2DM in working-age adults, as observed in developing countries undergoing rapid nutritional and lifestyle transitions.
A strong family history of diabetes was observed in 78.6% of participants, under-scoring the role of genetic predisposition in disease development. Previous studies have shown that individuals with first-degree relatives affected by T2DM have approximately double the risk of developing the disease, particularly when combined with environmen-tal and lifestyle factors such as diet and physical inactivity. This emphasizes the need for early screening and prevention strategies in younger adults with familial risk.
Metformin was the most frequently prescribed oral hypoglycemic agent (84%), either as monotherapy or in combination with glibenclamide. This finding aligns with interna-tional and national guidelines recommending metformin as the first-line treatment for T2DM due to its efficacy, safety profile, and cardiovascular benefits. The ANOVA analysis demonstrated that both metformin monotherapy and combination therapy significantly improved HbA1c levels over the study period. In contrast, patients treated with glibenclamide alone exhibited less favorable glycemic control, consistent with previous evidence highlighting sulfonylureas’ higher risk of hypoglycemia and reduced long-term durability of effect.
Comorbid conditions were highly prevalent (57.4%), with dyslipidemia (43.5%) and hypertension (27.9%) being the most common. The widespread use of atorvastatin (42%) is consistent with international recommendations for cardiovascular risk reduction in di-abetic patients over 40 years or those with multiple risk factors. Furthermore, the presence of depression in nearly one-fifth of the cohort (18.8%) highlights the critical need for inte-grated mental health care in diabetes management, as psychological comorbidities are known to impair treatment adherence and worsen glycemic outcomes.
Regarding nutritional status, 71.6% of patients were overweight and 28.4% obese, re-flecting the well-established association between adiposity and T2DM risk. Interestingly, no significant association was observed between body mass index (BMI) and glycemic control in this cohort. This suggests that pharmacological therapy, rather than BMI alone, may be the dominant determinant of metabolic improvement. Emerging evidence indi-cates that central adiposity or visceral fat distribution may serve as more sensitive indica-tors of metabolic dysfunction than BMI in isolation.
Overall, these findings demonstrate significant improvements in glycemic control among young adults with T2DM yet highlight persistent challenges in achieving optimal metabolic targets (HbA1c ≤ 7.0%) as recommended by international guidelines. The re-sults suggest that pharmacological interventions, while essential, must be complemented by non-pharmacological strategies—such as nutritional counseling, structured physical activity, and patient education—to achieve sustainable metabolic control.
Study Limitations
This study has several limitations. The use of a non-probabilistic sample may limit generalizability to broader populations. Additionally, BMI was used as a categorical ra-ther than continuous variable, and only the most recent lipid profile and blood pressure data from 2023 were analyzed, which may affect precision. Moreover, potential selection bias could arise from including only patients with consistent annual follow-up. Future studies should incorporate longitudinal anthropometric and biochemical data, consider psychosocial variables, and explore the impact of integrated lifestyle and pharmacological interventions on long-term outcomes.
5. Conclusions
This study highlights critical gaps in the management of young adults (25–40 years) with type 2 diabetes mellitus (T2DM) receiving care at Family Health Centers in Antofa-gasta, Chile. Although improvements in glycemic control were observed, the overall care model remains limited in scope and frequency. Current follow-up practices, focused pri-marily on annual assessments of basic metabolic parameters, contrast with international recommendations advocating for more comprehensive and frequent monitoring from the early stages of the disease.
One of the main challenges identified is the restricted availability of oral hypoglyce-mic agents in primary care settings, which constrains clinicians’ ability to individualize treatment and may contribute to suboptimal metabolic control and increased risk of long-term complications. Furthermore, the absence of detailed clinical records on micro-vascular and macrovascular complications, coupled with limited referral pathways to specialized services such as ophthalmology, vascular medicine, and nursing, underscores fragmented care inconsistent with global diabetes management standards.
Equally concerning is the lack of structured nutrition and physical activity programs, which reflect a predominantly reactive rather than preventive care model. The absence of sustained interventions targeting weight management, dietary habits, and exercise sub-stantially limits opportunities to achieve lasting behavioral change and prevent comor-bidities such as obesity and cardiovascular disease.
From a methodological standpoint, limitations related to data collection—such as the nominal recording of body mass index (BMI) and the use of only the most recent 2023 li-pid and blood pressure data—may have affected the precision and generalizability of findings.
Overall, the results indicate that the current primary care model does not fully meet international standards for integrated and preventive diabetes management. Strengthen-ing multidisciplinary care, increasing monitoring frequency, and implementing continu-ous patient education and lifestyle support are essential to improving metabolic control and preventing complications in this population. Future public health strategies should focus on early intervention, comprehensive risk assessment, and equitable access to pharmacological and non-pharmacological therapies to ensure sustainable diabetes care.
Author Contributions: For research articles with several authors, a short paragraph specifying their individual contributions must be provided. Conceptualization, MFS, MCA, YMC, JAY; methodology, MFS, MCA, YMC, NMD, JAY; software, MFS, MCA, YMC, JAY; validation, MFS, MCA, YMC, NMD, JAY; formal analysis, MFS, SDAA, AAR, MCA, YMC, NMD, JAY; investigation, MFS, MCA, SDAA, AAR, YMC, NMD, JAY; resources, MFS, MCA, YMC, NMD, JAY; data curation, MFS, MCA, YMC, NMD, JAY; writing—original draft preparation, MFS, SDAA, AAR, MCA, YMC, BFM, CRM, NMD, JAY; writing—review and editing, MFS, MCA, SDAA, AAR, YMC, BRM, CRM, NMD, JAY; visualization, MFS, MCA, YMC,CRM, SDAA, AAR, BFM, NMD, JAY; supervision, MFS, MCA, YMC, NMD, JAY; project administration, MFS, MCA, YMC, NMD, JAY. All authors have read and agreed to the published version of the manuscript
Funding: This research received no external funding.
Institutional Review Board Statement: The study protocol was approved by the Health Ethics Committee of the Universidad Internacional Iberoamericana de México (approval No. CR-234) and by the accredited local committee of the University of Antofagasta (file No. 469/2024). All data were anonymized to maintain confidentiality.
Acknowledgments: Special thanks to CMDS, the health sector of the city of Antofagasta, for granting access to the electronic medical records. Special recognition to the Universidad Inter-nacional Iberoamericana de México and the ethics committees that approved the study. This work is part of the Doctoral Thesis of Manuel Figueroa Sanchez to obtain his Ph.D. in Public Health Sciences from Universidad Internacional Iberoamericana, Mexico.
Conflicts of Interest: The authors declare no conflicts of interest.
Figure 5. Most common treatments used in patients with type 2 diabetes mellitus presenting comorbidities at primary health care centers (APS) in the city of Antofagasta. Analysis performed using simple descriptive statistics.
3.9 Summary of Key Findings
From 15,170 total T2DM records, 2,456 corresponded to adults aged 25–40 years; 500 with con-tinuous annual follow-up were included in the final analysis. Women represented 63.6% of the study population, and most participants were aged 35–40 years. Mean disease dura-tion was approximately four years, with the majority having a family history of diabetes. A significant reduction in HbA1c was observed from 2021 to 2023, demonstrating improved metabolic control under oral hypoglycemic therapy.
Combina-tion therapy (especially metformin-based) resulted in superior glycemic improvement compared to monotherapy. Higher BMI was associated with poorer glycemic control, un-derscoring the impact of obesity on diabetes management outcomes.
4. Discussion
The present study provides valuable insights into the metabolic control and clinical characteristics of young adults (25–40 years) with type 2 diabetes mellitus (T2DM) at-tending Family Health Centers (CESFAM) in Antofagasta, Chile. Over the three-year fol-low-up period (2021–2023), mean glycated hemoglobin (HbA1c) levels significantly de-creased from approximately 8.9% to 7.4%, reflecting improved glycemic control in this population. This outcome is clinically relevant, as sustained HbA1c reductions are asso-ciated with decreased risks of both microvascular and macrovascular complications. Findings from the UKPDS and other landmark studies have shown that each 1% decrease in HbA1c corresponds to a 35–37% reduction in microvascular events, supporting the clinical importance of the observed changes.
A notable demographic feature of this cohort was the predominance of women (63.6%), which mirrors reports from other Latin American and global studies showing a higher prevalence of T2DM among women in certain age ranges. This may be explained by differences in hormonal profiles, fat distribution, and health service utilization pat-terns. Most participants were between 35 and 40 years of age (75.2%), consistent with an epidemiological shift toward earlier onset of T2DM in working-age adults, as observed in developing countries undergoing rapid nutritional and lifestyle transitions.
A strong family history of diabetes was observed in 78.6% of participants, under-scoring the role of genetic predisposition in disease development. Previous studies have shown that individuals with first-degree relatives affected by T2DM have approximately double the risk of developing the disease, particularly when combined with environmen-tal and lifestyle factors such as diet and physical inactivity. This emphasizes the need for early screening and prevention strategies in younger adults with familial risk.
Metformin was the most frequently prescribed oral hypoglycemic agent (84%), either as monotherapy or in combination with glibenclamide. This finding aligns with interna-tional and national guidelines recommending metformin as the first-line treatment for T2DM due to its efficacy, safety profile, and cardiovascular benefits. The ANOVA analysis demonstrated that both metformin monotherapy and combination therapy significantly improved HbA1c levels over the study period. In contrast, patients treated with glibenclamide alone exhibited less favorable glycemic control, consistent with previous evidence highlighting sulfonylureas’ higher risk of hypoglycemia and reduced long-term durability of effect.
Comorbid conditions were highly prevalent (57.4%), with dyslipidemia (43.5%) and hypertension (27.9%) being the most common. The widespread use of atorvastatin (42%) is consistent with international recommendations for cardiovascular risk reduction in di-abetic patients over 40 years or those with multiple risk factors. Furthermore, the presence of depression in nearly one-fifth of the cohort (18.8%) highlights the critical need for inte-grated mental health care in diabetes management, as psychological comorbidities are known to impair treatment adherence and worsen glycemic outcomes.
Regarding nutritional status, 71.6% of patients were overweight and 28.4% obese, re-flecting the well-established association between adiposity and T2DM risk. Interestingly, no significant association was observed between body mass index (BMI) and glycemic control in this cohort. This suggests that pharmacological therapy, rather than BMI alone, may be the dominant determinant of metabolic improvement. Emerging evidence indi-cates that central adiposity or visceral fat distribution may serve as more sensitive indica-tors of metabolic dysfunction than BMI in isolation.
Overall, these findings demonstrate significant improvements in glycemic control among young adults with T2DM yet highlight persistent challenges in achieving optimal metabolic targets (HbA1c ≤ 7.0%) as recommended by international guidelines. The re-sults suggest that pharmacological interventions, while essential, must be complemented by non-pharmacological strategies—such as nutritional counseling, structured physical activity, and patient education—to achieve sustainable metabolic control.
Study Limitations
This study has several limitations. The use of a non-probabilistic sample may limit generalizability to broader populations. Additionally, BMI was used as a categorical ra-ther than continuous variable, and only the most recent lipid profile and blood pressure data from 2023 were analyzed, which may affect precision. Moreover, potential selection bias could arise from including only patients with consistent annual follow-up. Future studies should incorporate longitudinal anthropometric and biochemical data, consider psychosocial variables, and explore the impact of integrated lifestyle and pharmacological interventions on long-term outcomes.
5. Conclusions
This study highlights critical gaps in the management of young adults (25–40 years) with type 2 diabetes mellitus (T2DM) receiving care at Family Health Centers in Antofa-gasta, Chile. Although improvements in glycemic control were observed, the overall care model remains limited in scope and frequency. Current follow-up practices, focused pri-marily on annual assessments of basic metabolic parameters, contrast with international recommendations advocating for more comprehensive and frequent monitoring from the early stages of the disease.
One of the main challenges identified is the restricted availability of oral hypoglyce-mic agents in primary care settings, which constrains clinicians’ ability to individualize treatment and may contribute to suboptimal metabolic control and increased risk of long-term complications. Furthermore, the absence of detailed clinical records on micro-vascular and macrovascular complications, coupled with limited referral pathways to specialized services such as ophthalmology, vascular medicine, and nursing, underscores fragmented care inconsistent with global diabetes management standards.
Equally concerning is the lack of structured nutrition and physical activity programs, which reflect a predominantly reactive rather than preventive care model. The absence of sustained interventions targeting weight management, dietary habits, and exercise sub-stantially limits opportunities to achieve lasting behavioral change and prevent comor-bidities such as obesity and cardiovascular disease.
From a methodological standpoint, limitations related to data collection—such as the nominal recording of body mass index (BMI) and the use of only the most recent 2023 li-pid and blood pressure data—may have affected the precision and generalizability of findings.
Overall, the results indicate that the current primary care model does not fully meet international standards for integrated and preventive diabetes management. Strengthen-ing multidisciplinary care, increasing monitoring frequency, and implementing continu-ous patient education and lifestyle support are essential to improving metabolic control and preventing complications in this population. Future public health strategies should focus on early intervention, comprehensive risk assessment, and equitable access to pharmacological and non-pharmacological therapies to ensure sustainable diabetes care.
Author Contributions: For research articles with several authors, a short paragraph specifying their individual contributions must be provided. Conceptualization, MFS, MCA, YMC, JAY; methodology, MFS, MCA, YMC, NMD, JAY; software, MFS, MCA, YMC, JAY; validation, MFS, MCA, YMC, NMD, JAY; formal analysis, MFS, SDAA, AAR, MCA, YMC, NMD, JAY; investigation, MFS, MCA, SDAA, AAR, YMC, NMD, JAY; resources, MFS, MCA, YMC, NMD, JAY; data curation, MFS, MCA, YMC, NMD, JAY; writing—original draft preparation, MFS, SDAA, AAR, MCA, YMC, BFM, CRM, NMD, JAY; writing—review and editing, MFS, MCA, SDAA, AAR, YMC, BRM, CRM, NMD, JAY; visualization, MFS, MCA, YMC,CRM, SDAA, AAR, BFM, NMD, JAY; supervision, MFS, MCA, YMC, NMD, JAY; project administration, MFS, MCA, YMC, NMD, JAY. All authors have read and agreed to the published version of the manuscript
Funding: This research received no external funding.
Institutional Review Board Statement: The study protocol was approved by the Health Ethics Committee of the Universidad Internacional Iberoamericana de México (approval No. CR-234) and by the accredited local committee of the University of Antofagasta (file No. 469/2024). All data were anonymized to maintain confidentiality.
Acknowledgments: Special thanks to CMDS, the health sector of the city of Antofagasta, for granting access to the electronic medical records. Special recognition to the Universidad Inter-nacional Iberoamericana de México and the ethics committees that approved the study. This work is part of the Doctoral Thesis of Manuel Figueroa Sanchez to obtain his Ph.D. in Public Health Sciences from Universidad Internacional Iberoamericana, Mexico.
Conflicts of Interest: The authors declare no conflicts of interest.
3 Bibliography
4 Acknowledgments
5 References
Document information
Published on 01/01/2025
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?