1 Introduction
Lignin is one of the most abundant natural polymers on Earth and is a source of aromatic carbon [1]. It may be an inexpensive alternative to the traditional fossil fuel derivative precursors (pitch and PAN), however lignin by itself isn’t generally processable into filament form without the aid of a plasticizer. Typically, ex-lignin carbon fibres present low mechanical properties compared to traditional carbon fibers [2].
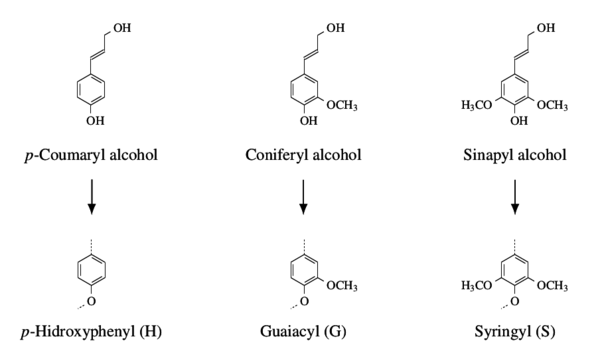
The main advantage of lignin is its availability as a by-product of the paper pulp industry, in contrast to cellulose (used as a precursor for rayon) which is the major component of the paper products. Cellulose also presents the downside of being chemically susceptible to volatilization during pyrolysis due to its high oxygen content. Figure 1 shows lignin’s phenylpropanoic precursor alcohols that polymerize in the secondary cell walls of vascular plants originating the three main lignin monomers.
Structural oxygen mostly exist as part of the intermonomeric units, primarily in the β-O-4 groups which represent over 48 % of all such units [3] [4]. This unit is the result of the formation of covalent bonds derived from the phenyl hydroxyl groups with the second carbon of the aliphatic chains [5], making it an example of an ether group in lignin, the other groups are classified as condensed carbons [5]. The predominance of ether intermonomeric units is responsible for the delignification process of lignocellulose, based on the reactivity of the C-O covalent bond (compared to the C-C bond), enabling the separation of the cellulose fibres from the remaining wood components.
Processed lignin is the designation of the product obtained by delignification reactions during paper pulp processing. The kraft process is the prevalent technique for pulp paper making, and kraft lignin is its by-product. After wood treatment kraft lignin is obtained in the form of a black liquor containing hemicellulose and the remaining reagents and other products, and thus must be isolated and purified before use.
A well purified lignin should present low inorganic content, particularly sulphur, and because of this be readily processable with a thermoplastic as a plasticizer without excessive volatilization. Examples of reported miscible polymers with lignin are polyethers (PEO [6] [7] or PEG [8] [9]) and polyesters (PET [10] [11] and PLA [12] [11] [13] in contrast to the incompatibility found in common polyolefins such as PP and PE [7].
2 Materials and methods
2.1 Processed lignin
The processed and purified lignin was obtained from West Fraser Co. Ltd. It was purified by the LignoForce process from kraft pulping. As received it contained approximately 50 % solids, ash content between 0.5 and 1.5 %, molecular weight within 6 and 10 kDa and a polydispersity from 3 to 4.6. The lignin was dried at 100 ºC for 2 h before processing and characterization, presenting a powder form as depicted in Figure 2.
2.2 Thermoplastic polymers
The PLA used was an Inzea FT HT 10 from Nurel and the PHB was the Biomer P266. They were both dried at 60 ºC for 4 h before mixing.
2.3 Lignin characterization
Lignin thermal characterization was performed by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). DSC was carried out on a Netzsch DSC 200 F3 Maia in N2 atmosphere, heating from 20 to 300 ºC at a heating rate of 10 ºC min-1 (designated as 1st, 2nd and 3rd scan respectively). TGA was carried out from 40 to 800 ºC at 10 ºC min-1 on a Q500 from TA Instruments, under N2 and under O2 atmospheres.
Chemical characterization was performed by Fourier transform infrared spectroscopy (FTIR) and elemental analysis. The infrared spectra was obtained using attenuated total reflectance (ATR) with the Pike Technologies ATR MIRacle on a Perkin Elmer Spectrum BX spectrophotometer. The elemental analysis of carbon, nitrogen, sulphate and hydrogen was obtained from a LECO TruSpec Micro CHNS and the oxygen was determined by default with the LECO TruSpec Micro O accessory.
2.4 Extrusion mixing
Various lignin:thermoplastic mixtures were produced with weight ratios of 50:50, 75:25, 85:15, 95:5. The total mass of 10 g was extruded on a micro-compounder Xplore MC15. This equipment allows the mixing of the compound for a set time before extruding, operating also as a micro-extruder.
3 Results and discussion
3.1 Lignin thermal characterization
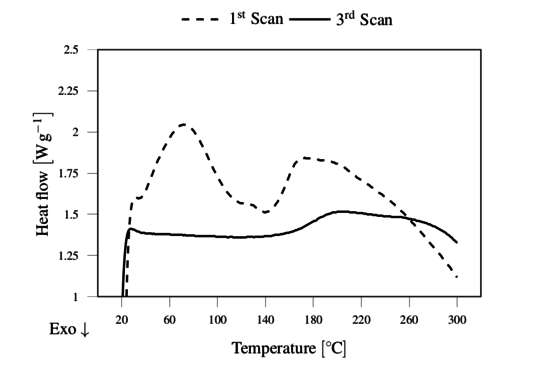
The DSC of as received lignin was characterized by a single wide peak in the 1st scan showing the evaporation of moisture around 110 ºC, setting the basis for the drying conditions. After drying, the lignin showed several endothermic reactions during the 1st scan, as illustrated in Figure 3. However, for the purpose of melt extrusion the 3rd scan was the most relevant, showing the glass transition temperature Tg in the range of 170 to 180 ºC,, as shown in Figure 3. This physical transition is the lower limit of processability of lignin, and the upper limit is set by its thermal degradation suggested here to be approximately at 230 ºC as observed in TGA (Figure 4).
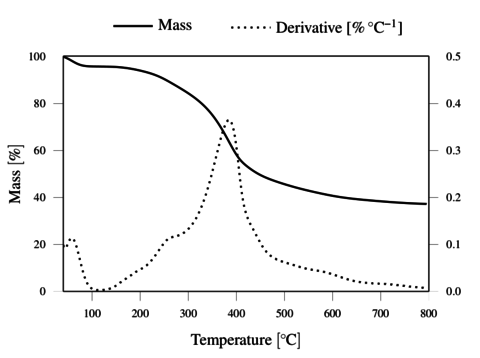
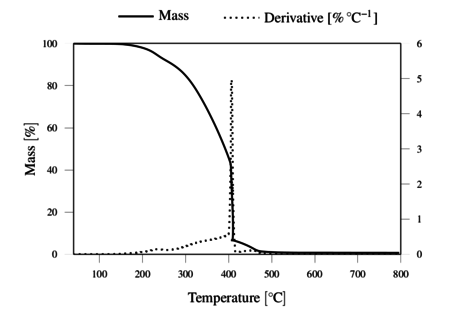
The TGA results, obtained under inert and oxidative atmospheres, are represented in Figures 4 and 5, respectively. The results demonstrate the thermal resistance of the lignin, with degradation onset just above 200 ºC and rapid degradation above 300 ºC. The lignin shows a total weight loss above 400 ºC in oxygen, and a residual mass of 36.2 wt. % at 800 ºC in nitrogen.
The thermal resistance of lignin under oxygen was observed to be at least as good as under nitrogen atmosphere up to 300 ºC. This justifies the application of the thermostabilization step under oxidative conditions at temperatures up to 250 ºC, to promote oxidative crossliking. It should be noted that allowing insipient degradation in O2 may render the filaments infusible, and thus allow further carbonization process under inert atmosphere.
3.2 Lignin chemical characterization
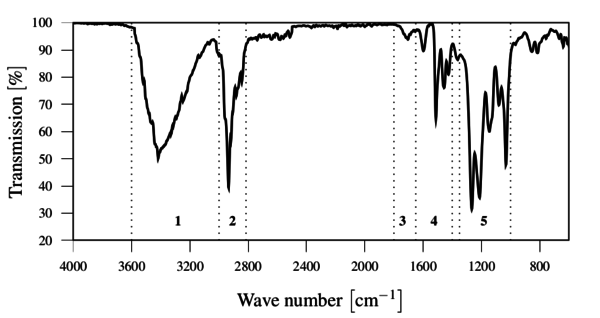
The FTIR spectrum showed the presence of the expected chemical groups without any relevant contaminations, as show in Figure 6.
The elemental analysis results were interpreted based on the assumption that lignin originated from a softwood source, which is almost exclusively made of guaiacyl, derived from coniferyl alcohol. Hence a structural formula was estimated, based on the chemical elemental composition obtained, and compared to the structural formula of this precursor alcohol, C10H12O3.The comparison is presented in Table 1.
| C [%] | H [%] | N [%] | S [%] | O [%] | |
| Theoretical | 66.7 | 6.7 | 0.0 | 0.0 | 26.6 |
| Measured | 63.7 | 6.1 | 0.2 | 1.7 | 28.3 |
The chemistry of this kraft lignin is within the expected composition of a softwood lignin, showing a relatively low content of inorganic elements, suggesting it went through an adequate purification process.
3.3 Extrusion mixing
The selection of PHB and PLA was based on the expected compatibility of polyesters with lignin, enabling the formation of hydrogen bonds with the lignin hydroxyl groups, and these two polymers may be processed within the operational window of the kraft lignin used in this article (just above lignin’s Tg).
After drying, the mixtures of lignin:thermoplastic with compositions 50:50, 75:25, 85:15 and 95:5 (w/w) where prepared, each with a total mass of 10 g. Before initiating the composite extrusion the micro-compounder was lubricated with the thermoplastic, by performing a short extrusion process of pure plastic, followed by purging the material. The thermoplastic was always fed first, followed by the lignin powder, and the composition was mixed for 1.5 min before extruding the composite filament. Both polymers were processed under the same processing conditions: 50 RPM, 190 ºC on the top zone, 180 ºC on both mid and bottom zones. Two dies were used for this study, one with a diameter of 3 mm and another with 250 μm.
Both thermoplastics produced blends with lignin and formed an uniform filament using the 3 mm die, the reduction of the die diameter lead to obstruction and prevented the formation of a continuous filament with PHB. Whenever the filament could be extruded the results were identical for PHB and PLA within similar concentrations, an example is presented in Figure 7.
The major limitation with both polymers was associated with their brittleness, forming filaments that could not be stretched even when heated. The lignin:PLA filaments produced using the 250 μm die could be slightly stretched probably due to their thin section leading to a higher melt plasticity, as shown in Figure 8. The increase of processing temperature, within the lignin operational range, did not alter this behaviour.
The obtained PLA filaments had a diameter of about 125 μm. They where all generally brittle compared to the filaments obtained with the larger die.
4 Conclusions
The lignin used in this work was derived from a softwood species, which was confirmed by elemental analysis, and corroborated with its relatively high Tg and absence of a melting peak in DSC. The thermal degradation processes associated to this lignin were observed to be exothermic. The effective operational window of this lignin was studied by DSC and TGA and was found to be between 180 ºC and 230 ºC. Drying this lignin at 100 ºC for 2 h allowed to process it with a plastcizer, which was a lower drying time than reported in literature. Selecting thermoplastics based on the ability to form hydrogen bonds with lignin is a viable strategy to produce homogeneous green fibre with high lignin content. Lignin shows a relatively high residual mass by TGA, suggesting a high carbon content, justifying it’s suitability as a carbon fibre precursor.
References
| [1] | W. Boerjan, J. Ralph e M. Baucher, “Lignin biosynthesis,” Annual Review of Plant Biology, vol. 54, nº 1, pp. 519-546, 2003. |
| [2] | D. A. Baker, N. C. Gallego e F. S. Baker, “On the characterization and spinning of an organic-purified lignin toward the manufacture of low-cost carbon fiber,” Journal of Applied Polymer Science, vol. 124, nº 1, pp. 227-234, 2011. |
| [3] | H. Chen, “Chemical composition and structure of natural Lignocellulose,” em Biotechnology of lignocellulose: theory and practice, Dordrecht, Netherlands, Springer, 2014, pp. 25-57. |
| [4] | J. Braun, K. M. Holtman e J. F. Kadla, “Lignin-based carbon fibers: oxidative thermostabilization of kraft lignin,” Carbon, vol. 43, nº 2, pp. 385-394, 2015. |
| [5] | E. Adler, “Lignin chemistry: past, present and future,” Wood Science and Technology, vol. 11, nº 3, pp. 169-218, 1977. |
| [6] | J. F. Kadla, S. Kubo, R. A. Venditti, R. D. Gilbert, A. L. Compere e W. Griffith, “Lignin-based carbon fibers for composite fiber applications,” Carbon, vol. 40, nº 15, pp. 2913-2920, 2002. |
| [7] | J. F. Kadla, S. Kubo, R. D. Gilbert e R. A. Venditti, “Lignin-based carbon fibers,” em Chemical modification, properties, and usage of lignin, New York, USA, Springer, 2002, pp. 124-130. |
| [8] | S. Kubo e J. F. Kadla, “Kraft lignin/poly(ethylene oxide) blends: effect of lignin structure on miscibility and hydrogen bonding,” Journal of Applied Polymer Science, vol. 98, nº 3, pp. 1437-1444, 2005. |
| [9] | J. F. Kadla e S. Kubo, “Kraft lignin/poly(ethylene oxide) blends: Effect of lignin structure on miscibility and hydrogen bonding,” Composites Part A: Applied Science and Manufacturing, vol. 35, nº 3, pp. 395-400, 2004. |
| [10] | N. Meek, D. Penumadu, O. Hosseinaei, D. Harper, S. Young e T. Rials, “Synthesis and characterization of lignin carbon fiber and composites,” Composites Science and Technology, vol. 137, pp. 60-68, 2016. |
| [11] | S. Kubo e J. F. Kadla, “Lignin-based carbon fibers: effect of synthetic polymer blending on fiber properties,” Journal of polymers and the environment, vol. 13, nº 2, pp. 97-105, 2005. |
| [12] | C. Mu, L. Xue, J. Zhu, M. Jiang e Z. Zhou, “Mechanical and thermal properties of toughened poly(L-ic) acid and lignin blends,” BioResources, vol. 9, nº 3, pp. 5557-5566, 2014. |
| [13] | S. Wang, Y. Li, H. Xiang, Z. Zhou, T. Chang e M. Zhu, “Low cost carbon fibers from bio-renewable lignin/poly(lactic acid) (PLA) blends,” BioResources, vol. 119, pp. 20-25, 2015. |
Document information
Published on 02/07/22
Accepted on 02/07/22
Submitted on 02/07/22
Volume 04 - Comunicaciones Matcomp19 (2020), Issue Núm. 3 - Materiales bioinsipirados, 2022
DOI: 10.23967/r.matcomp.2022.07.009
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?