Summary
Background
Massive hemorrhage and the need for blood transfusion carry a high rate of morbidity and mortality after hepatectomy. The aim of this study was to evaluate the safety and potential benefit of infrahepatic inferior vena cava (IVC) clamping in hepatectomy for tumors involving hepatocaval confluence.
Methods
We conducted a retrospective analysis of 113 consecutive patients who underwent hepatectomy with infrahepatic IVC clamping (n = 60, Group A) and without infrahepatic IVC clamping (n = 53, Group B) as the initial treatment for tumors involving hepatocaval confluence.
Results
In Group A, central venous pressure reduced from 7.6 ± 3.2 to 4.4 ± 2.7 cm H2O (p < 0.001). Patients in Group A experienced less blood loss (477.3 ± 340.3 vs. 794.5 ± 602.7 mL, p = 0.001), fewer blood transfusion requirements (8.3% vs. 22.6%, p = 0.034), lower postoperative complications (40% vs. 60.4%, p = 0.031), and shorter hospital stay (10.7 ± 2.2 vs. 12.9 ± 4.8 days, p = 0.008) than those in Group B.
Conclusion
Infrahepatic IVC clamping is generally effective and safe in controlling bleeding during hepatectomy for tumors involving hepatocaval confluence.
Keywords
central venous pressure;hemorrhage;hepatectomy;inferior vena cava
1. Introduction
Massive hemorrhage and the need for blood transfusion carry a high rate of morbidity and mortality after hepatectomy. Controlling the blood inflow using the Pringle maneuver does not prevent hemorrhage from either the inferior vena cava (IVC) or the hepatic veins. The pressure within the hepatic veins is directly related to the central venous pressure (CVP).1 Several studies have shown that infrahepatic (IVC) clamping can potentially result in a greater decrease in blood loss during transection of the liver parenchyma by decreasing the CVP.2; 3; 4 ; 5
Surgery for tumors involving hepatocaval confluence presents a great risk of excessive intraoperative bleeding via the hepatic veins.6 In the present study, we analyzed our experience on hepatic resection with and without infrahepatic IVC clamping to elucidate the safety and potential benefit of infrahepatic IVC clamping for treatment of tumors involving hepatocaval confluence.
2. Materials and methods
2.1. Patients
The outcome of 60 consecutive patients who underwent hepatectomy with inflow blood exclusion (Pringle maneuver) and infrahepatic IVC clamping (Group A) between June 2010 and July 2012 was compared with that of 53 consecutive patients who received hepatectomy with Pringle maneuver only between January 2007 and April 2010 (Group B). All patients were operated on for tumors involving hepatocaval confluence, which was assessed by abdominal ultrasonography, abdominal computed tomography, and/or magnetic resonance imaging (Fig. 1). Liver function was assessed using Child–Pugh grading, serum liver biochemistry tests, and indocyanine green clearance test. Only Child–Pugh class A patients were considered for major hepatectomy (resection of ≥3 liver segments). Exclusion criteria were as follows: extrahepatic metastases in patients with malignancy, peripherally located lesion that did not involve hepatocaval confluence, tumors originating in the caudate lobe, or patients with severe comorbid conditions.
|
|
|
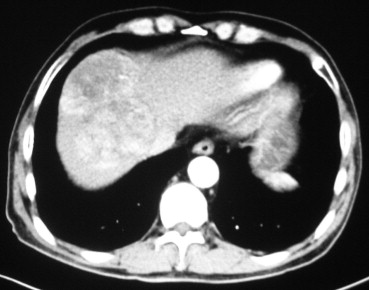
Figure 1. Abdominal computed tomography image of a 9-cm tumor located in segment 8. |
2.2. Surgical technique
The abdomen was entered via a bilateral subcostal incision or a right subcostal incision. After an exploratory laparotomy, intraoperative ultrasonography was used to assess the extent of the disease and its relationship to vascular structures and to mark the demarcation line of parenchymal transection. The hepatic pedicle was isolated and encircled with a rubber tube. In Group A, the infrahepatic IVC was dissected and taped with a vessel loop. Resection was initiated with Pringle maneuver in cycles of clamp/unclamp time of 15/5 minutes. In Group A, the infrahepatic IVC was clamped throughout the liver resection including the interval of declamping of hepatic pedicle. In both groups, transection of the liver parenchyma was performed along the demarcation line by a clamp crushing method using Kelly forceps. Attempts were made to obtain a tumor-free margin during parenchymal transaction. According to the Couinaud classification of liver segments, resection of three or more segments is considered a major resection, whereas a resection of two or fewer segments is considered a minor resection.
Arterial pressure was measured by puncture of radial artery. CVP was measured via a catheter inserted into the patients right subclavian vein.
Operative mortality was defined as death occurring within 30 days of the operation.
2.3. Statistical analyses
Categorical variables were compared by using the chi-square test or Fishers exact test as appropriate. Continuous data were expressed as the mean ± standard deviation and compared using one-way analysis of variance. The Mann–Whitney U test was used to evaluate the differences between groups. All statistical analyses were performed using SPSS for Windows (version 11.0; SPSS Institute, Chicago, IL, USA). A p value <0.05 was considered statistically significant.
3. Results
The clinical clinicopathologic features were not different between Group A and Group B (Table 1).
| Variables | Group A (n = 60) | Group B (n = 53) | p |
|---|---|---|---|
| Age (y) | 48.7 ± 10.8 | 49.5 ± 12.1 | 0.355 |
| Sex | |||
| Male/female | 43/17 | 38/15 | 0.997 |
| Child–Pugh classification | |||
| A/B | 58/2 | 49/4 | 0.319 |
| HbsAg-positive | 42 | 39 | 0.673 |
| Cirrhosis | 30 | 28 | 0.764 |
| Indications | |||
| Hepatocellular carcinoma | 31 | 26 | 0.782 |
| Cholangiocarcinoma | 7 | 9 | 0.419 |
| Combined hepatocellular cholangiocarcinoma | 4 | 5 | 0.588 |
| Metastatic cancer | 7 | 7 | 0.804 |
| Hepatic epithelioid hemangioendothelioma | 3 | 1 | 0.371 |
| Hepatic carcinosarcoma | 1 | 0 | 0.345 |
| Hemangioma | 4 | 3 | 0.825 |
| Focal nodular hyperplasia | 2 | 1 | 0.633 |
| Hepatic adenoma | 1 | 1 | 0.929 |
| Tumor size (cm) | 7.5 ± 3.4 | 6.9 ± 2.3 | 0.315 |
| Tumor number | |||
| Single/multiple | 47/13 | 42/11 | 0.906 |
In Group A, all patients tolerated clamping of the IVC well. CVP reduced from 7.6 ± 3.2 to 4.4 ± 2.7 cm H2O (p < 0.001). The mean IVC clamping time was 28.2 ± 2.4 (range 17–38) minutes. Surgery and perioperative outcomes between the two groups are shown in Table 2. The two groups had a similar extent of hepatectomy, operative time, and inflow occlusion time. However, patients in Group A experienced less blood loss (477.3 ± 340.3 vs. 794.5 ± 602.7 mL, p = 0.001), fewer blood transfusion requirements (8.3% vs. 22.6%, p = 0.034), lower postoperative complications (40% vs. 60.4%, p = 0.031), and shorter hospital stay (10.7 ± 2.2 vs. 12.9 ± 4.8 days, p = 0.008) than those in the Group B.
| Variables | Group A (n = 60) | Group B (n = 53) | p |
|---|---|---|---|
| Extent of hepatectomy (major/minor resection) | |||
| Right trisectionectomy S 4, 5, 6, 7, 8 ± 1 | 4 | 4 | 0.948 |
| Left trisectionectomy S 2, 3, 4, 5, 8 ± 1 | 3 | 2 | |
| Right hemihepatectomy S 5, 6, 7, 8 ± 1 | 14 | 16 | |
| Left hemihepatecomy S 2, 3, 4 ± 1 | 8 | 7 | |
| Mesohepatectomy S 4, 5, 8 | 8 | 4 | |
| S4a + S8 resection | 12 | 10 | |
| S4 resection | 2 | 0 | |
| S7 resection | 3 | 3 | |
| S8 resection | 2 | 2 | |
| Others resection | 4 | 5 | |
| Operative time (min) | 178.1 ± 44.8 | 166.6 ± 3 5.7 | 0.180 |
| Inflow occlusion time (min) | 22.6 ± 7.1 | 18.9 ± 5.8 | 0.191 |
| Intraoperative blood loss (mL) | 477.3 ± 340.3 | 794.5 ± 602.7 | 0.001 |
| Perioperative blood transfusion | 5 | 12 | 0.034 |
| Complications | 24 | 32 | 0.031 |
| Reoperations for bleeding | 0 | 1 | |
| Liver dysfunction | 3 | 2 | |
| Ascites | 7 | 13 | |
| Bile leak | 2 | 1 | |
| Pleural effusion | 8 | 10 | |
| Pneumonia | 3 | 4 | |
| Wound events | 4 | 6 | |
| Others | 6 | 7 | |
| Operative mortality | 0 | 0 | 0.999 |
| Stay in hospital (d) | 10.7 ± 2.2 | 12.9 ± 4.8 | 0.008 |
Operative death was nil. No patients developed renal insufficiency. The levels of serum urea nitrogen and creatinine on postoperative days 1, 3, and 7 were comparable between the two groups.
4. Discussion
Hemorrhage control during liver resection of the tumors involving hepatocaval confluence often presents a technical challenge. Total hepatic vascular exclusion (THVE), combining the Pringle maneuver and concomitant clamping of the IVC below and above the liver, allows resection of this type of tumors.6 However, this procedure may result in unpredictable hemodynamic intolerance, and is associated with increased morbidity and hospital stay.7 Selective hepatic vascular exclusion (SHVE), which occludes blood inflow and outflow with simultaneous preservation of IVC flow, avoids the drawbacks of THVE.8 ; 9 Unfortunately, outside the liver, the hepatic veins have a short course and are situated in a strategically difficult location. For bulky fragile tumors involving hepatocaval confluence, extraparenchymal dissection and isolation of major hepatic veins may cause tumor rupture or vein injury.
Bleeding from the hepatic veins is closely related to the CVP. In 2004, Otsubo et al,2 in a study of 103 consecutive patients with CVP >5 cmH2O, documented that infrahepatic IVC clamping is associated with lower CVP and less blood loss during hepatectomy. Chen et al3 and Uchiyama et al4 also showed the beneficial effects of this procedure on reducing blood loss. In current study, we confirmed the advantages of infrahepatic IVC clamping. Patients in Group A experienced less blood loss and fewer blood transfusion requirements than those in Group B. Once hepatic inflow of blood is controlled by Pringle maneuver, the risk of intraoperative bleeding is primarily from the hepatic veins.10 By using infrahepatic IVC clamping, the operation is carried out in a bloodless surgical field. We were able to clarify the relationship between the tumors and the major vascular architecture around the hepatocaval confluence and selectively clip or ligate intrahepatic structures, therefore limiting blood loss during liver transection. Less blood loss has a direct impact on lower incidence of postoperative complications, and this allows shorter hospitalization.
In a prospective randomized controlled study (RCT) of 85 patients, Kato et al11 found that the amount of bleeding during hepatectomy tended to be smaller in the IVC clamping group than that in the IVC nonclamping group (233 [40–875] vs. 285 [8–1397] mL), but the difference did not reach significant levels (p = 0.47). These results could be biased by the small number of patients in their study.
IVC clamping was tolerated well by all patients in our series. Although infrahepatic IVC is occluded, blood draining from the subphrenic vein, adrenal veins, and lumbar veins may contribute to the maintenance of hemodynamics.11 Unlike THVE or SHVE, infrahepatic IVC clamping is a simple technique that does not require the complete mobilization of the liver. IVC can be easily dissected below the liver and taped. The main concern about the use of infrahepatic IVC clamping is the risk of air embolism due to the necessity of maintaining a low CVP during liver resection. There is evidence that Trendelenburgs posture was helpful in preventing low CVP-induced air embolism.12 In our practice, any holes in the hepatic veins were immediately repaired with fine sutures during parenchymal dissection. Of the 60 patients in this series, none have experienced air embolism. Evidence from previous studies also showed that infrahepatic IVC clamping does not increase the incidence of this hazardous complication during parenchymal transaction.2; 3; 4 ; 11 Another concern is the renal function after IVC clamping accompanied with an increase of renal venous pressure. In this study, no patients developed renal insufficiency. The postoperative levels of serum urea nitrogen and creatinine were comparable between the two groups.
Previous studies have suggested that the safe limit of intermittent Pringle maneuver at the time of liver transaction was 120 minutes.13 Because the longest time span for infrahepatic IVC clamping has not been defined, further investigation needs to be conducted in order to address this issue.
Traditionally, CVP reduction was achieved with anesthesiological interventions (i.e., fluid restriction or combined with diuretics, nitroglycerin, and opioids) during liver surgery.12 ; 14 However, this method requires intimate collaboration between the surgeon and the anesthetist. One most recent RCT from Germany found that inferior IVC clamping was superior to anesthesiological interventions in controlling blood loss during hepatic resection.5 Moreover, because there is no fluid restriction, intraoperative hemodynamic instability occurred less frequently in patients with infrahepatic IVC clamping.
The major drawback of the present study is its retrospective nature. Bias is likely to have been introduced by variation in surgical skill and perioperative care. However, our results were strengthened by a recent large RCT of 192 patients conducted by Zhu et al.15 These scholars indicated that portal triad clamping combined with infrahepatic IVC clamping is more efficacious in controlling bleeding during complex hepatectomies than portal triad clamping with low CVP.
In conclusion, infrahepatic IVC clamping is a safe procedure, which efficiently prevented bleeding during hepatectomy and should be more widely adopted in liver surgery, especially for tumors involving hepatocaval confluence.
References
- 1 M. Johnson, R. Mannar, A.V. Wu; Correlation between blood loss and inferior vena caval pressure during liver resection; Br J Surg, 85 (1998), pp. 188–190
- 2 T. Otsubo, K. Takasaki, M. Yamamoto, et al.; Bleeding during hepatectomy can be reduced by clamping the inferior vena cava below the liver; Surgery, 135 (2004), pp. 67–73
- 3 X.P. Chen, Z.W. Zhang, B.X. Zhang, et al.; Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis; Langenbecks Arch Surg, 391 (2006), pp. 209–215
- 4 K. Uchiyama, M. Ueno, S. Ozawa, et al.; Half clamping of the infrahepatic inferior vena cava reduces bleeding during a hepatectomy by decreasing the central venous pressure; Langenbecks Arch Surg, 394 (2009), pp. 243–247
- 5 N.N. Rahbari, M. Koch, J.B. Zimmermann, et al.; Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial; Ann Surg, 253 (2011), pp. 1102–1110
- 6 T. Berney, G. Mentha, P. Morel; Total vascular exclusion of the liver for the resection of lesions in contact with the vena cava or the hepatic veins; Br J Surg, 85 (1998), pp. 485–488
- 7 J. Belghiti, R. Noun, E. Zante, T. Ballet, A. Sauvanet; Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study; Ann Surg, 224 (1996), pp. 155–161
- 8 V.E. Smyrniotis, G.G. Kostopanagiotou, J.C. Contis, et al.; Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study; World J Surg, 27 (2003), pp. 765–769
- 9 A.J. Li, Z.Y. Pan, W.P. Zhou, et al.; Comparison of two methods of selective hepatic vascular exclusion for liver resection involving the roots of the hepatic veins; J Gastrointest Surg, 12 (2008), pp. 1383–1390
- 10 H. Chen, N.B. Merchant, M.S. Didolkar; Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality; J Gastrointest Surg, 4 (2000), pp. 162–167
- 11 M. Kato, K. Kubota, J. Kita, M. Shimoda, K. Rokkaku, T. Sawada; Effect of infra-hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study; World J Surg, 32 (2008), pp. 1082–1087
- 12 W.D. Wang, L.J. Liang, X.Q. Huang, X.Y. Yin; Low central venous pressure reduces blood loss in hepatectomy; World J Gastroenterol, 12 (2006), pp. 935–939
- 13 K. Man, S.T. Fan, I.O. Ng, et al.; Tolerance of the liver to intermittent Pringle maneuver in hepatectomy for liver tumors; Arch Surg, 134 (1999), pp. 533–539
- 14 J.A. Melendez, V. Arslan, M.E. Fischer, et al.; Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction; J Am Coll Surg, 187 (1998), pp. 620–625
- 15 P. Zhu, W.Y. Lau, Y.F. Chen, et al.; Randomized clinical trial comparing infrahepatic inferior vena cava clamping with low central venous pressure in complex liver resections involving the Pringle manoeuvre; Br J Surg, 99 (2012), pp. 781–788
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?