Abstract
The characteristics of CO2 reacting with CaO in a molten eutectic mixture of CaF2 and NaF has been investigated. Calculations of the Gibbs free energy, temperature analysis of the decomposition of the formed carbonates, and XRD analyses of quenched samples taken during CO2 absorption or desorption were employed to identify the phases present in the melt. Efficient CO2 absorption from a simulated flue gas was observed, due to a combined reaction where CaO initially reacts with CO2 and forms CaCO3. Subsequently, Na2CO3 is formed by an ion exchange reaction between CaCO3 and NaF. It was found that the CaO activity is highest in the temperature range 826–834°C. Increasing the CaO concentration from 5 to 20 wt% in the molten salt resulted in reduced CO2 reactivity efficiency, probably because of precipitation and agglomeration of the sorbent. The total carbonation conversion was independent of the CO2 concentration in the inlet gas, and the sorbent carrying capacity was in the range 0.722–0.743 g CO2/g CaO corresponding to 0.037–0.144 g CO2/g total liquid. Decarbonation was conducted by raising the temperature. 40% conversion back to CaO was recorded at 1160°C. The recorded curves for the CO2 concentration in the outlet gas exhibited a rapid desorption step followed by a slow step.
Introduction
Sorption of CO2 by CaO-based solid-state substances is being developed for the use in cyclic absorption–desorption processes for capture of CO2 from waste gases emitted by a wide range of industrial processes [1, 2]. Examples are power generation [3], cement production [4, 5], and metallurgical industry [6]. CaO from naturally occurring limestone sources may be used directly with high absorption capacity. However, these materials exhibit degradation upon cycling with limited sustained capacity; after 10 cycles, around 10% of the initial capacity is maintained without H2O present in the gas. With water present, around 25% sustained capacity is obtained after 10 cycles [7]. Solid CaO-based materials with enhanced properties both with regards to cycling and carrying capacity are being developed [8-10]. In this work, we explore a somewhat different approach. The dissolution and/or dispersion of sorbent particles in a molten salt matrix may enhance the reactivity of the sorbent with CO2 because efficient gas-liquid-solid reactions can be employed [11-13]. Various alkali and alkaline-earth metal halide salts (CaCl2, CaCl2–CaF2, CaF2–NaF and CaF2–LiF) have previously been studied for the use as solvents for dissolution and/or dispersion of CaO to enhance sorbent activities in the CO2 capture process [11-13]. CaF2–NaF and CaF2–LiF eutectic mixtures have been used. The results indicate that the highest carrying capacity (0.722–0.743 g CO2/g CaO) may be achieved with the CaO–CaF2/NaF system due to thermodynamically stable Na2CO3 being formed through an ion exchange reaction [11, 14]. Studies of cyclic CO2 absorption/desorption by 5.32 wt% CaO in molten CaCl2 showed a stable carrying capacity of 0.504 g CO2/g CaO [12]. In this case, the decomposition of the formed carbonates proceeded rapidly and completely, but when using alkali and alkaline-earth metal halide salts, full decomposition of the carbonates formed in the melt could not be achieved [11].
Notably, besides the technical design, the selection of appropriate molten salt composition for dissolution or partial dissolution of the active sorbent is an essential aspect in development of a technologically and economically efficient CO2 capture process. Therefore, the aim of this work was to investigate systematically the efficiency of a process based on CaO dissolved/dispersed in a eutectic mixture of CaF2 and NaF. Previously, the phase transitions of CaO, CaCO3 and Na2CO3 in CaF2/NaF were investigated experimentally and mapped using FactSage software [14]. The eutectic composition of CaF2/NaF (41.85 wt% CaF2 in NaF, 815°C) was chosen because of its suitable melting point for the carbonation reaction and low vapor pressure, which allows low-pressure operation [15, 16]. The CO2 sorption/desorption behavior was studied using a Fourier transform infrared (FTIR) gas detector and gravimetric analysis. Particularly, the impacts of the CaO mass proportion in the melt, the temperatures of the system for the carbonation/decarbonation reactions, the CO2 content and the simulated flue gas flow rate were examined to determine the efficiency of the CO2 sorption process by the CaO–CaF2–NaF chemical system.
Materials and Methods
Materials
CaO (Sigma-Aldrich reagent, 96–100% purity), CaF2 (Sigma-Aldrich reagent, 99.0–100.0% purity), and NaF (Sigma-Aldrich reagent, 98.5–100.0% purity) were used for preparing the molten salt mixtures. The powders were dried at 200°C for 24 h. All sorbents were prepared by direct mixing of NaF–CaF2 (41.85 wt% CaF2 and 48.15 wt% NaF) and CaO (5, 10, 15, 20 wt%) powders to form a 10 cm high column in a nickel crucible (52 mm dia. × 350 mm height) in the liquid state. The weight varied from 630 to 650 g depending on the composition, unless otherwise mentioned. The mixtures were melted in argon atmosphere and kept at 970°C for 10 h before carbonation, unless otherwise mentioned. Pure gases provided by AGA were used for all experiments and the mixtures of carbon dioxide (purity of 99.99%) in nitrogen (purity of 99.999%, H2O ≤ 3 ppm, O2 ≤ 3 ppm, CnHm ≤ 1 ppm) were controlled by mass flow controllers (MASS-STREAM, M+W Instruments GmbH, Allershausen, Germany).
Experimental set-up and methods
Absorption and desorption of CO2 were performed in an automated one-chamber atmospheric pressure reactor. The nickel crucible mentioned above was placed inside a stainless steel outer chamber [12]. This assembly was fixed in a vertical ceramic tube furnace. The carbonation/decarbonation experiments were monitored using a FTIR gas analyzer (Nicolet 6700, Omnic 8 software; Thermo Scientific, Waltham, MA, USA) and an industrial weighing scale (MS8001S, accuracy of 0.1 g; Mettler Toledo, Columbus, OH, USA). Before each experiment the melts were stirred by bubbling N2 (0.5 L/min) through the liquid at the chosen temperature (820–950°C) for 2 h before carbonation. The N2 and CO2-N2 gas mixtures (total flow 0.6 L/min, 1.3–14.0 vol % CO2) were bubbled into the CaO–CaF2–NaF melt via a nickel pipe passed through the top of the sealed reactor assembly and immersed to a depth of 1 cm from the bottom of the crucible. A constant flow of argon (0.20 L/min) was applied as a purge flow over the outer stainless steel assembly to minimize corrosion of the chamber. The temperature measurements were performed using a type S (Pt−Pt10Rh, ±1°C) thermocouple immersed in the melt. The tightness of the system was controlled by a mass flow meter (Sierra 820 Series; Sierra Instruments, Inc., Monterey, CA, USA) supplied at the outlet of the FTIR gas cell. In each test, the carbonation process was considered to be complete when the inlet and outlet CO2-N2 stream compositions were nearly matching. Decomposition of the formed carbonates back to oxide was conducted at temperatures ranging from of 994 to 1163°C. The total weight of the assembly as well as the gas composition was continuously monitored. The experimental parameters (gas flow, temperature) were controlled and data collected by a computer integrated system (National Instruments, Austin, TX, USA).
Weight increase due to absorption of CO2 (carbonation), and weight decrease due CO2 liberation during thermal decomposition of the formed carbonates (decarbonation) were recorded during the experiments. Correction for the weight change due to oxidation of the stainless steel compartment was taken into the calculations. For this purpose, a baseline study of the oxidation of the reactor was performed at different temperatures. Weight loss due to evaporation of the liquids was deemed negligible as no condensate was found anywhere outside the reaction chamber.
Characterization
Powder X-ray diffraction (XRD) analysis was performed for phase identification. Samples were collected both during the carbonation and the decarbonation stages of 20 wt% CaO in NaF–CaF2. The samples were collected by quenching the melts on a stainless steel cylinder. XRD data were collected from 5° ≤ 2θ ≤ 110° (scan rate 0.01 degree per second) using Ni-filtered Cu Kα on a Bruker D8 Advance diffractometer working in Bragg-Brentano (θ/2θ) geometry. All XRD measurements were performed at room temperature and ambient pressure in air.
Results and discussion
Evaluation of the CaO–CaF2–NaF system for the CO2 sorption processes
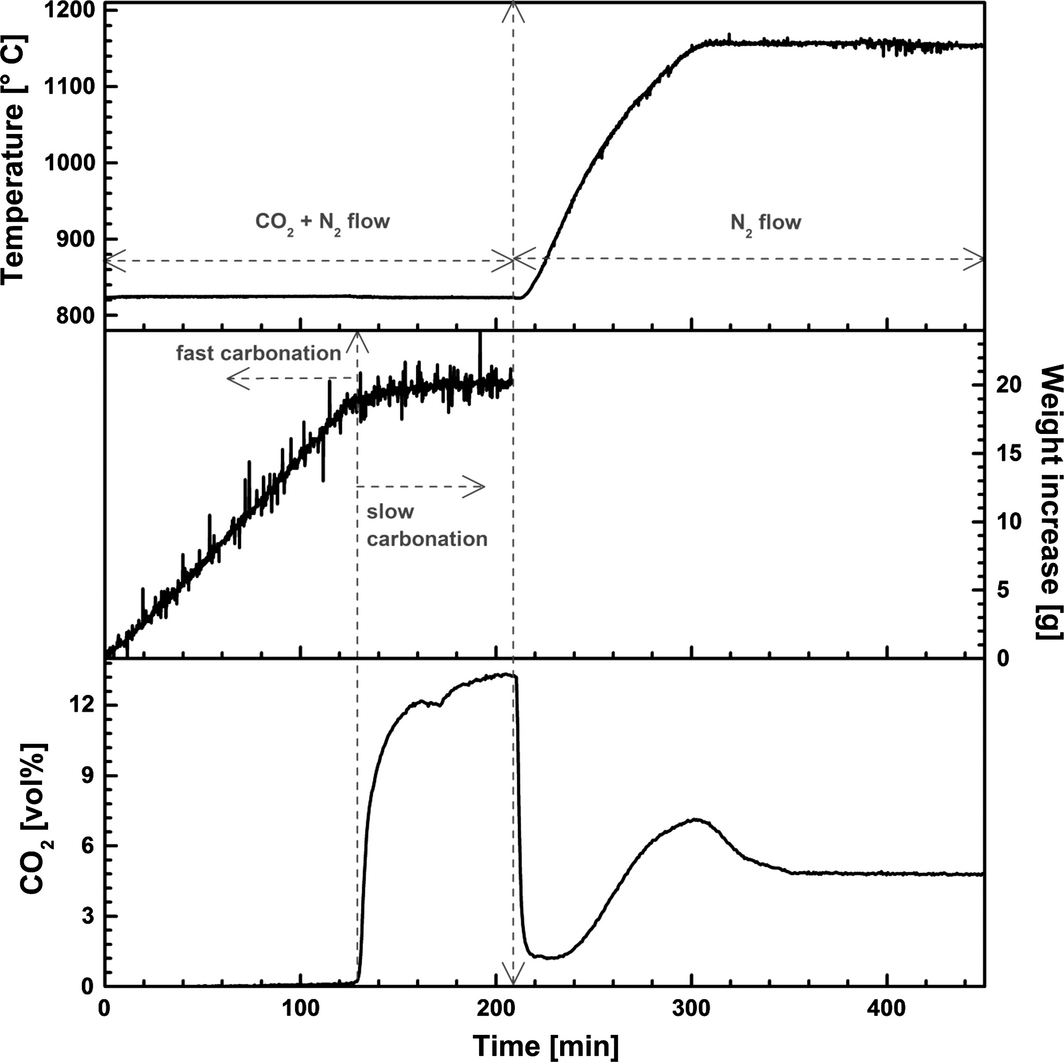
Figure 1 depicts measurements of the melt temperature, CO2 concentration in N2 leaving the sealed reactor, and the weight changes of the reactor assembly detected during the experiments. First, the melt consisting of 5 wt% CaO, 50.8 wt% NaF, and 44.2 wt% CaF2 was kept under N2 atmosphere at 824°C for 2 h before initiating the carbonation. The CO2 flow was started at time t = 0. This caused temperature increase, which can be attributed to the exothermic reaction of CaO with CO2,
|
|
|
Figure 1. Separation of CO2 from a simulated flue gas (N2 + 13 vol% CO2) by CaO/CaF2/NaF (5/44.2/50.8 wt%) at 824°C. Decomposition of the formed carbonates was conducted at 1156°C under N2 atmosphere. |
|
|
(1) |
The weight and CO2 concentration curves reveal a period with fast carbonation followed by slow carbonation. The initial fast carbonation reaction represents a highly efficient CO2 removal stage where close to 99.9% of the applied CO2 is captured; this stage can be related to CO2 reaction with dispersed CaO. The second carbonation stage may be assigned to the reaction with dissolved CaO, and possibly with deposited CaO (which may be covered by a layer of carbonate). This slow stage is considered to be completed when the CO2 concentration recorded by the FTIR analyzer becomes similar to the inlet concentration. Decomposition of the formed carbonates was conducted by raising the temperature to 1156°C and bubbling pure N2 through the melt, which also resulted in a rapid step and a slow step (Fig. 1). The total conversion efficiency of CaO to carbonates at 824°C was around 94 wt%, but complete decomposition of the formed carbonates was not reached (65 wt% of CO2 released). The high carbonation efficiency may be enabled by the ion exchange reaction described by equations (2) and (3):[12]
|
|
(2) |
|
|
(3) |
At temperatures lower than 335°C, formation of shortite (Na2Ca2(CO3)3) may take place (eq. 4), but above this temperature shortite decomposes to nyerereite (Na2Ca(CO3)2) which melts at 817°C (eq. 5) [17].
|
|
(4) |
|
|
(5) |
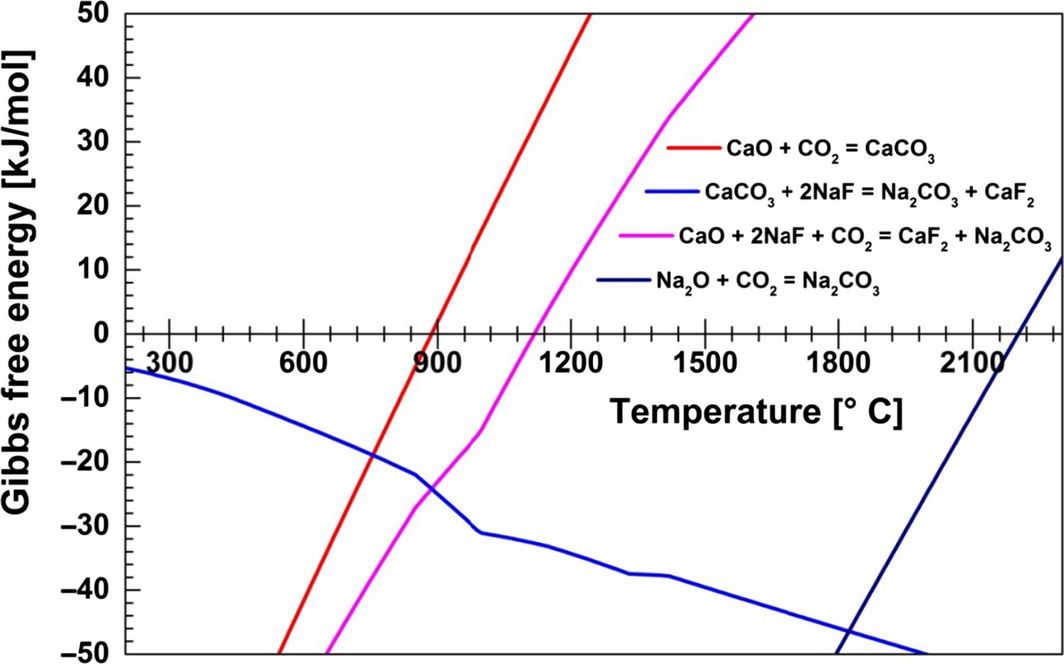
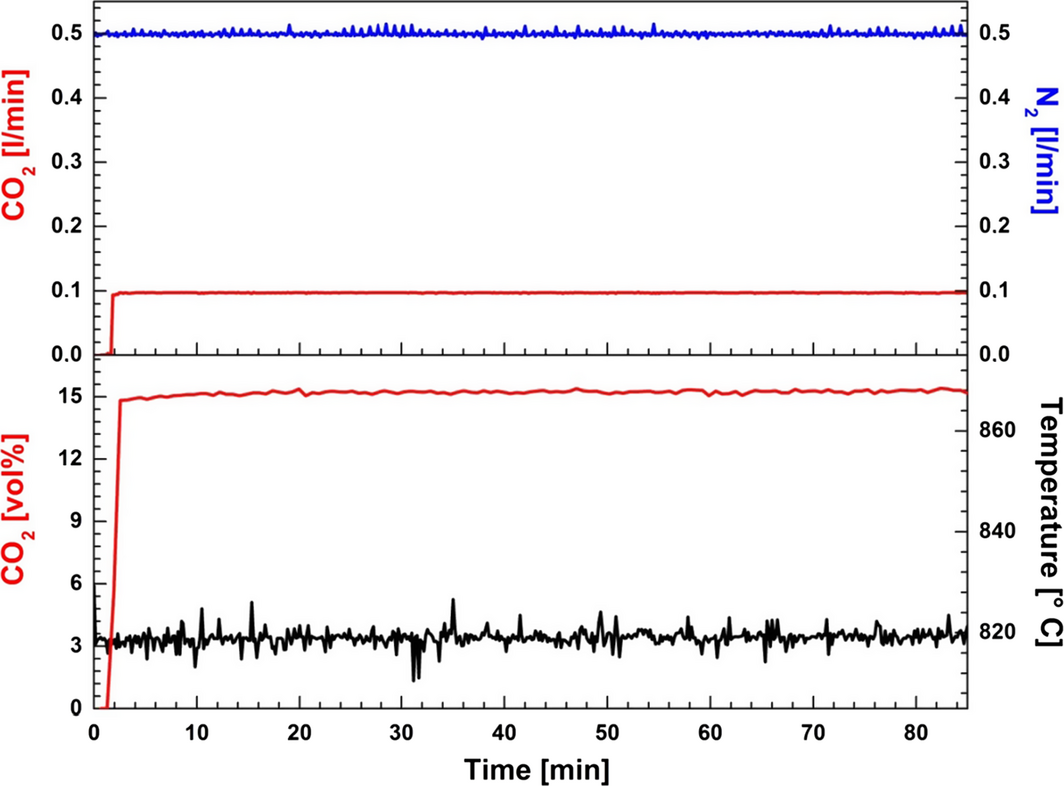
The Gibbs free energy for possible carbonation/decarbonation reactions, as described by equations (1), (2), and (3), was calculated using the HSC Chemistry program (version 6.1, Outotec, Espoo, Finland), and the results are provided in Figure 2. In addition, the Gibbs free energy for the reaction between solid sodium oxide and CO2 was calculated. The results show that Na2CO3 is thermodynamically more stable than CaCO3, because the Gibbs free energy for equation (2) is negative in the temperature range 400–2300°C. Decomposition of CaCO3 occurs at temperatures above 900°C. The value of the Gibbs free energy for the combined, total reaction (eq. 3) is negative at low temperatures and becomes positive above 1120°C, indicating that the equilibrium will be shifted toward the left as the temperature increases. The occurrence of this reaction was supported experimentally by conducting a baseline study using the CaF2–NaF system without addition of CaO (Fig. 3). In this study, 14 vol% CO2 in N2 was bubbled through the CaF2–NaF melt at 820°C after approximately 2 min of pure N2 flow. CO2 removal from the gas mixture could not be observed.
|
|
|
Figure 2. Gibbs free energy versus temperature for possible carbonation/decarbonation reactions in the system CaO–CaF2–NaF and in solid Na2O. |
|
|
|
Figure 3. Baseline study of CO2 capture at 820°C by bubbling 15 vol% CO2 in N2 through the melt consisting of 48.15 wt% NaF in CaF2. |
Structural analysis
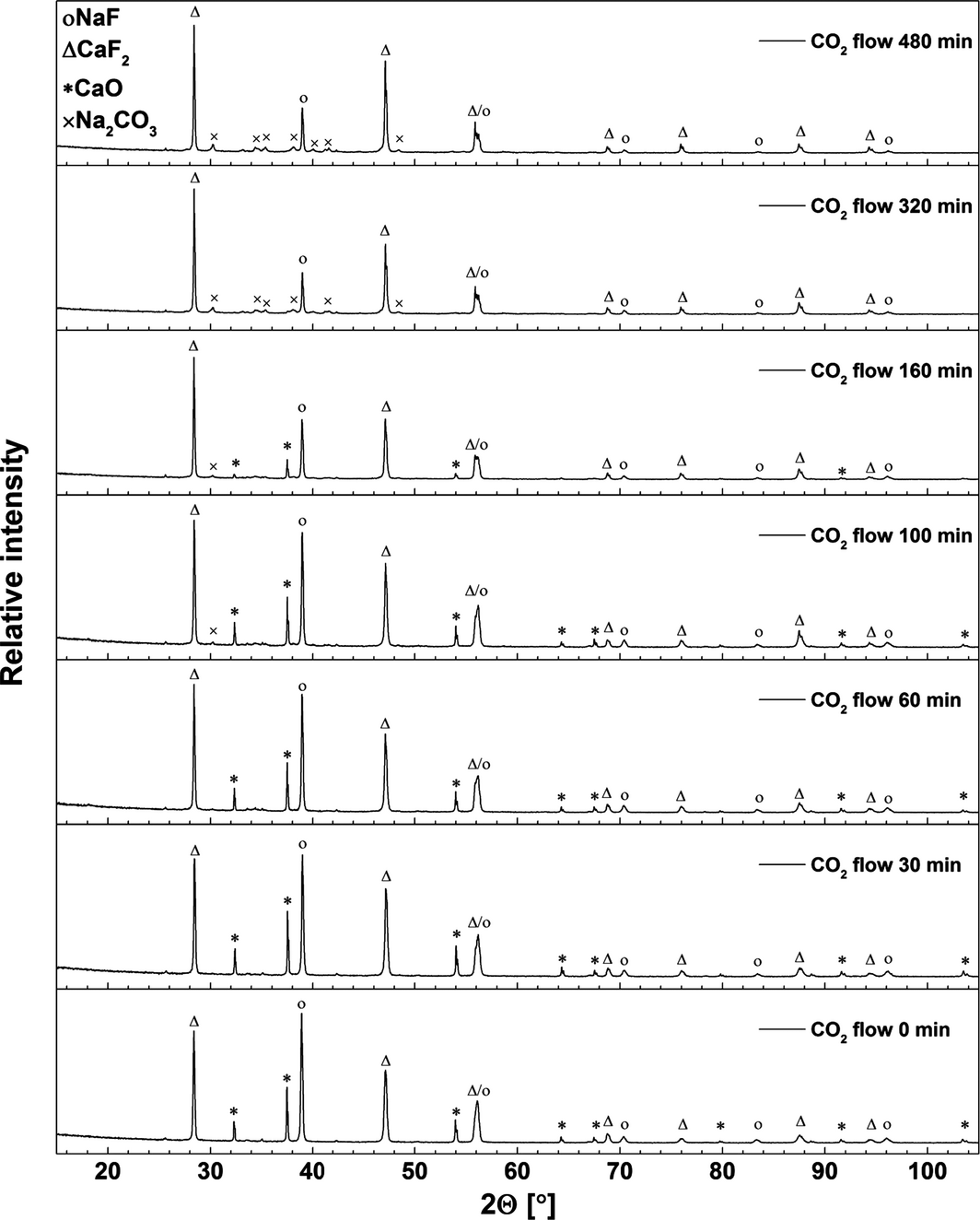
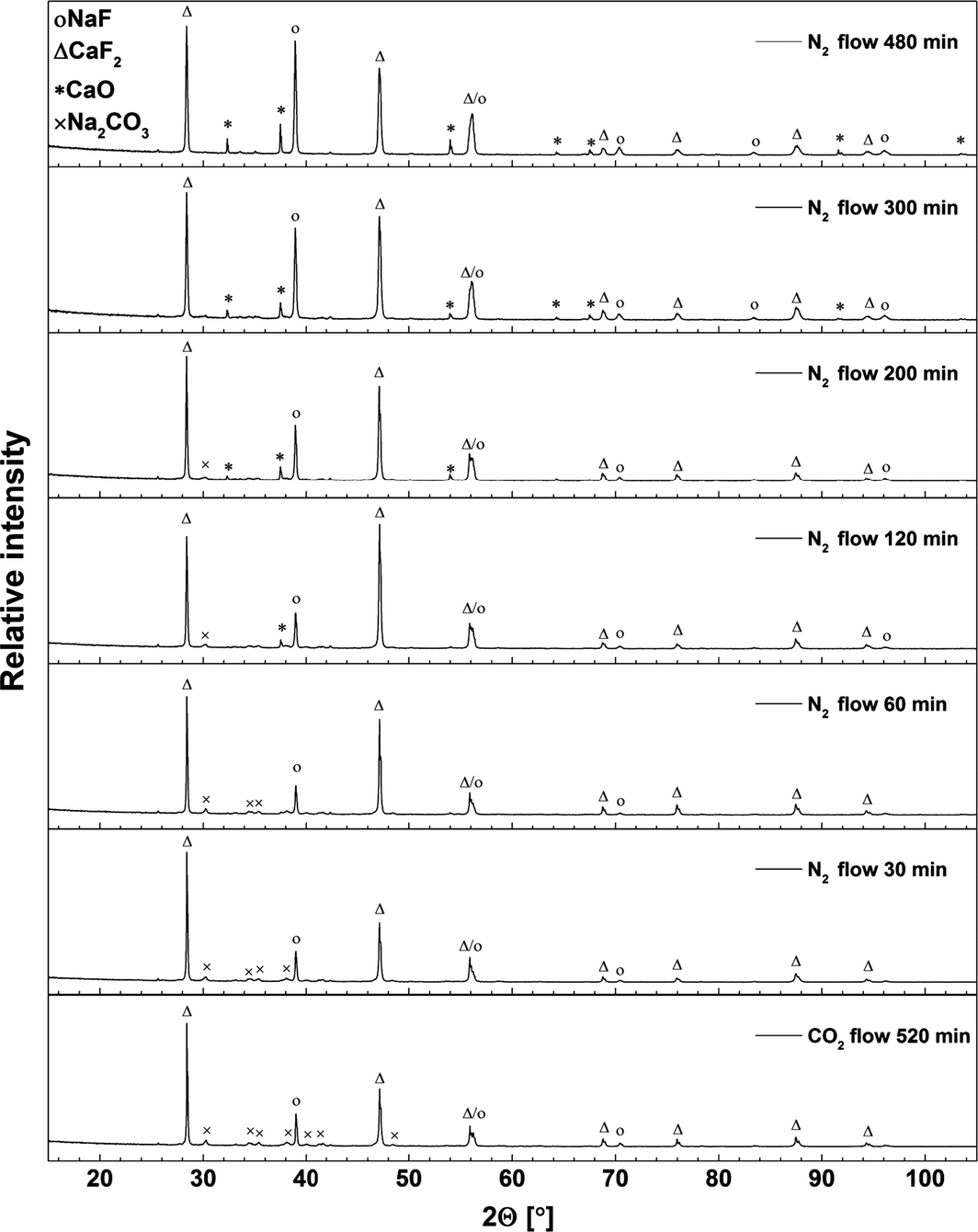
X-ray diffraction analysis was performed on quenched samples collected during the carbonation step using a melt consisting of CaO/CaF2/NaF (20/37.2/42.8 wt%) and 14 vol% CO2 in N2 at 830°C. The results are shown in Figure 4. For the sample taken before CO2 was passed through the melt, the XRD pattern peaks can be assigned to CaO, CaF2, and NaF. The intensity of the peaks attributed to NaF and CaO decreased systematically as carbonation commenced. The X-ray diffraction pattern of the sample taken after 100 min of CO2 absorption indicates formation of Na2CO3 (space group P6(3)/m) [18]. We suggest that the ion exchange reaction forming Na2CO3 takes place immediately during the carbonation step (eq. 2) [14]. The total reaction occurring can be expressed as equation (3). After 480 min of CO2 absorption most of the CaO was converted to CaF2, and the NaF has been partly converted to Na2CO3 as shown in Figure 4. No peaks attributable to CaCO3, Na2Ca(CO3)2, or Na2Ca2(CO3)3 were found during carbonation. The formation of Na2Ca(CO3)2 and Na2Ca2(CO3)3 may be expected at temperatures lower than 400 and 817°C, respectively [17]. We suggest that complete sodium cation substitution in CaCO3 occurs, and that Na2CO3 and CaF2 are formed as described by equation (3).
|
|
|
Figure 4. X-ray diffraction (XRD) patterns of the quenched samples collected during the carbonation step. Initial composition of the melt: 20 wt% CaO and 37.2 wt% CaF2 in NaF. Carbonation of CaO–CaF2/NaF was conducted by bubbling 14 vol% CO2 in N2 through the melt at 830°C. |
The formed carbonates were decomposed by increasing the temperature to 1170°C under pure N2 atmosphere. Samples were removed from the melt as described above. The X-ray diffraction patterns of the samples are shown in Figure 5. The intensity of the Na2CO3 peaks decreased with increasing decarbonation time. In addition, the presence of CaO can clearly be seen after 120 min of CO2 desorption. As expected, the intensity of peaks characteristic for CaO increased with time, indicating that the reversible reaction occurs (eq. 3). Finally, there were no intensive Na2CO3 peaks detected in the XRD pattern after 480 min of decarbonation, which indicates that full decomposition of the formed carbonates was accomplished.
|
|
|
Figure 5. X-ray diffraction patterns of the quenched samples collected during the decarbonation step, after carbonation of the sample (CaO/CaF2/NaF =20/37.2/42.8 wt%) for 520 min under 14 vol% CO2 in N2. Decarbonation of the samples was carried out at 1170°C under pure N2 for the time indicated. |
Because of the complexity in the undergoing reactions between CaCO3 and NaF, it is difficult to calculate the amount of CaO that reacts in the carbonation/decarbonation process based on the diffraction data. The formation of small amounts of complex carbonates in the CaO–CaF2–NaF system not detectable in the XRD pattern may influence the results. Therefore, the conversions of the sorbent to carbonates for all tests were calculated on the basis of the initially added amounts of CaO in the CaF2–NaF eutectic mixture.
Effect of carbonation temperature
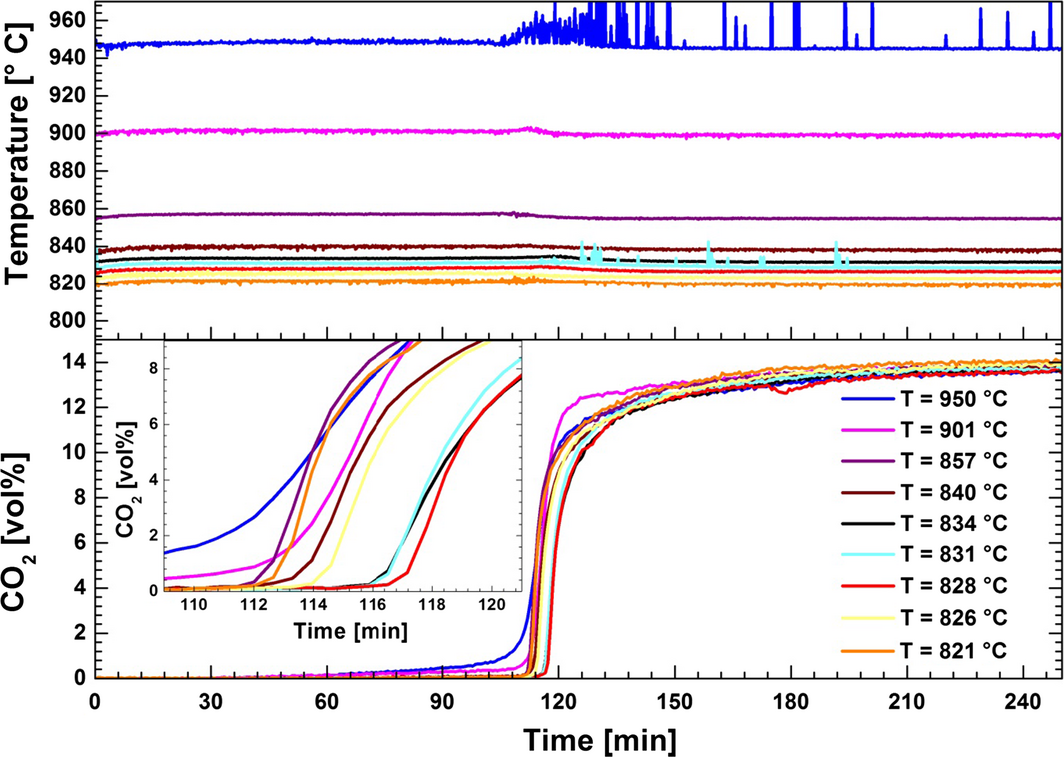
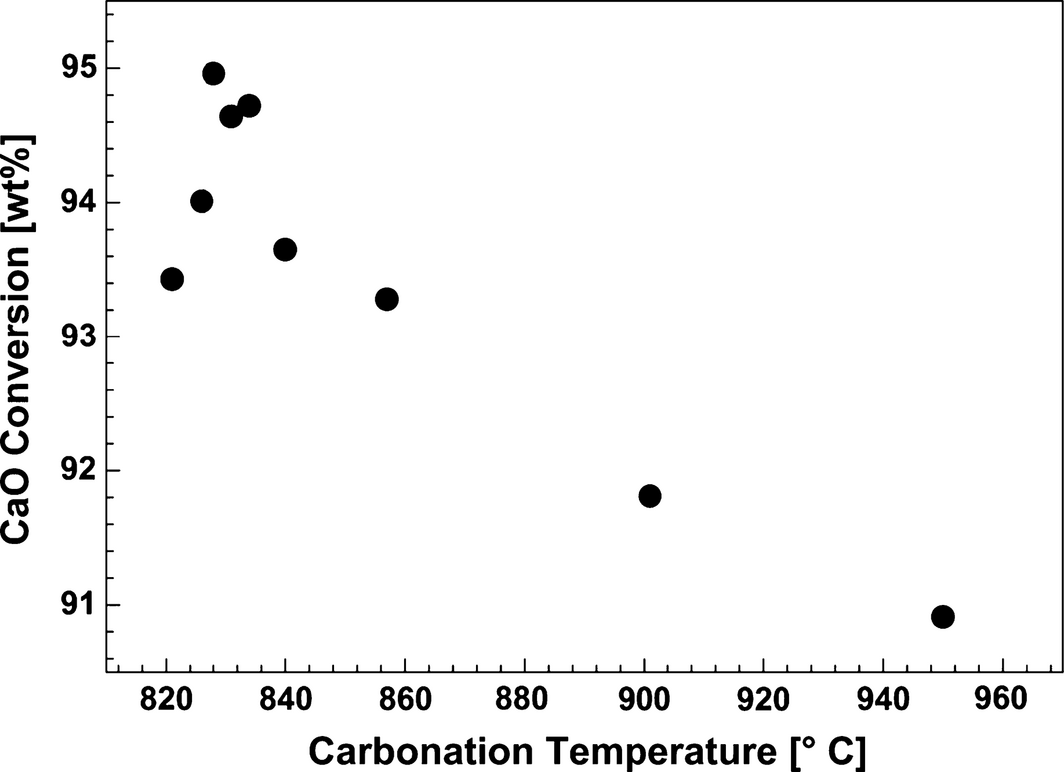
The effect of temperature on the carbonation reaction in CaO/CaF2/NaF (5/44.2/50.8 wt%) was investigated to determine the most efficient conditions for the sorbent conversion (Fig. 6). A temperature increase of 4–6°C was detected upon start of carbonation. The slow carbonation reaction stage starts when a rapid increase in the recorded CO2 concentration in the gas stream from the reactor is observed. It can be suggested that the fast reaction stage can be related to the heterogeneous reaction between CO2 and dispersed CaO, whereas the slow stage is related to the homogeneous reaction between CO2 and dissolved CaO, or between CO2 and agglomerated CaO where there will be strong mass transfer limitations. The results calculated from the amount of CO2 having been absorbed from the gas fed to the reactor indicate that CaO conversion in the initial fast carbonation reaction is close to 90 wt% of total reacted sorbent (Fig. 7). The highest carbonation efficiency was observed at 826–834°C, where the fast reaction was completed after 117 min and the final CaO conversion was 94–95 wt%. At temperatures higher than 834°C the total conversion efficiency decreased from 95 to 91 wt%. The carbonation conversion observed in the temperature range 821–950°C indicates formation of the thermodynamically stable carbonate (Na2CO3, see eqs (2) and (3)).
|
|
|
Figure 6. Effect of the temperature on the carbonation reaction in CaO/CaF2/NaF (5/44.2/50.8 wt%). The carbonation step was conducted by transporting 14 vol% CO2 in N2 through the melt. Inset: CO2 concentration versus time (109–121 min). |
|
|
|
Figure 7. CaO conversion dependence on carbonation temperature. Effect of the temperature on the carbonation reaction in CaO/CaF2/NaF (5/44.2/50.8 wt%) was investigated by bubbling 14 vol% CO2 in N2 through the melt for 300 min at temperatures in the range 821–950°C. |
Effect of decarbonation temperature
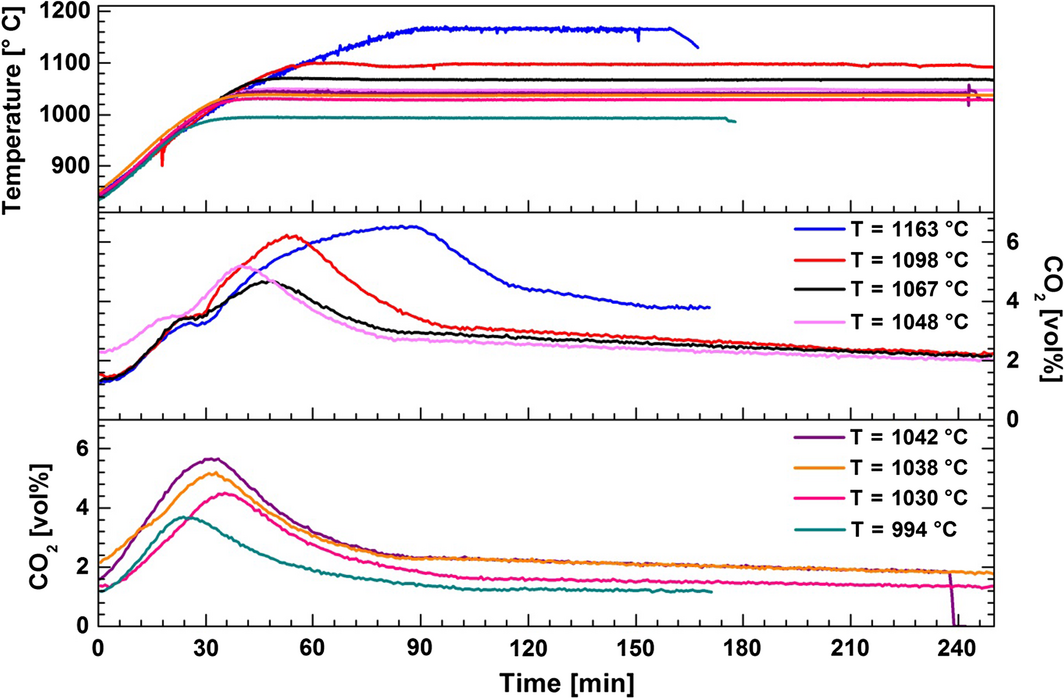
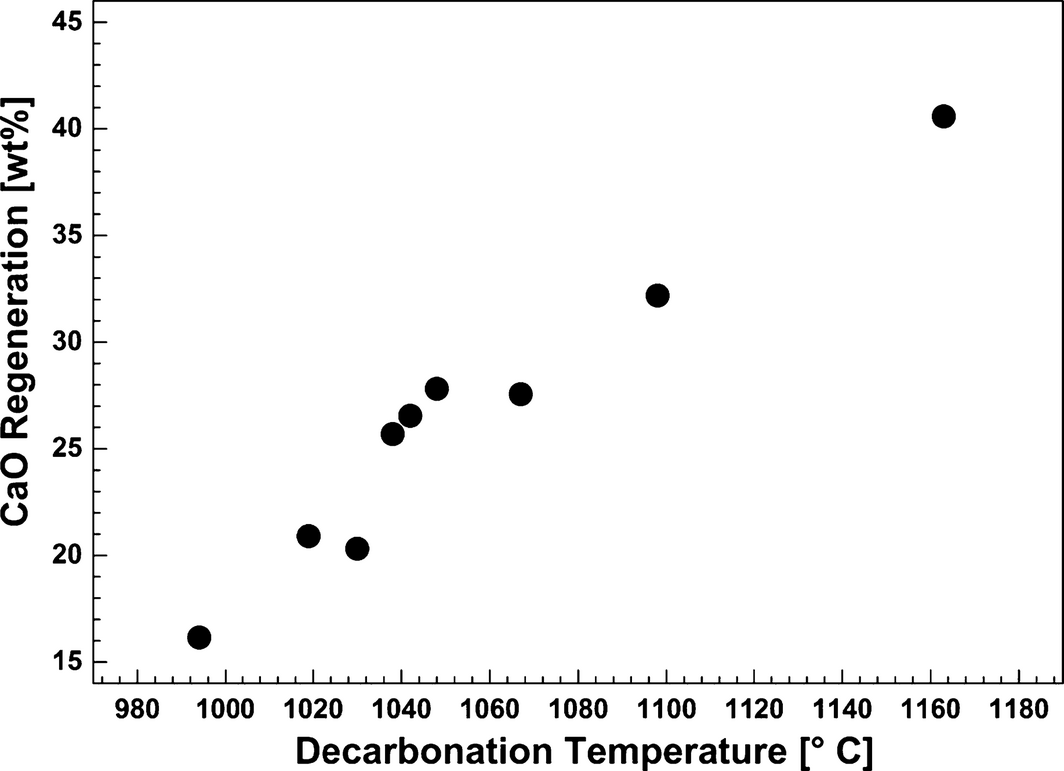
To investigate the decarbonation reaction mechanism, decomposition of the carbonates was carried out while flowing pure N2 through the reactor at temperatures ranging from 994 to 1163°C (Fig. 8). The initial carbonation was conducted at 830°C by bubbling 14 vol% CO2 in N2 through the melt consisting of dissolved/dispersed 5 wt% CaO in CaF2–NaF for 300 min. The decarbonation was performed by stopping the flow of CO2 and increasing the temperature. It was observed that the CO2 concentration curves recorded at 1048–1163°C exhibit two peaks, and the curves recorded at the lower temperature show one single peak (Fig. 8). As described above, the calculation of the standard Gibbs free energy for the reaction where CaO reacts with CO2 and NaF to form CaF2 and Na2CO3 (eq. 3) indicates that the equilibrium may be achieved at around 1120°C (Fig. 2). Therefore, the faster decomposition of the formed sodium carbonate is supposed to appear at temperatures higher than 1120°C (this temperature is not corrected for activities lower than unity for all substances other than CaO). We suggest that the first peak in the CO2 concentration curve may be assigned to the decomposition of a small amount of CaCO3, while the second peak is because of decomposition of Na2CO3. The results in Figure 9 demonstrate the effect of decarbonation temperature on CaO regeneration. The degree of conversion of the formed carbonates was calculated from the amount of CO2 released from the reactor during 170 min. It can be observed that the release of CO2 increases rapidly at higher temperatures. However, complete decomposition of the formed carbonates was not observed because of the reactor design; it was observed that the nickel tube which was used to transport the gases into the melt was blocked due to precipitation of CaO.
|
|
|
Figure 8. Effect of the temperature on decarbonation in the melt consisting of CaO/CaF2/NaF (5/44.2/50.8 wt%). Carbonation was conducted by bubbling 14 vol% CO2 in N2 through the melt at 830°C for 300 min. The decarbonation step was performed under pure N2. |
|
|
|
Figure 9. CaO regeneration as a function of the decarbonation temperature. The decarbonation efficiency was determined after 170 min. Carbonation was conducted by bubbling 14 vol% CO2 in N2 through the melt consisting of CaO/CaF2/NaF (5/44.2/50.8 wt%) at 830°C for 300 min. The decarbonation step was subsequently performed under pure N2. |
Effect of variation in CaO content in the fluoride melts
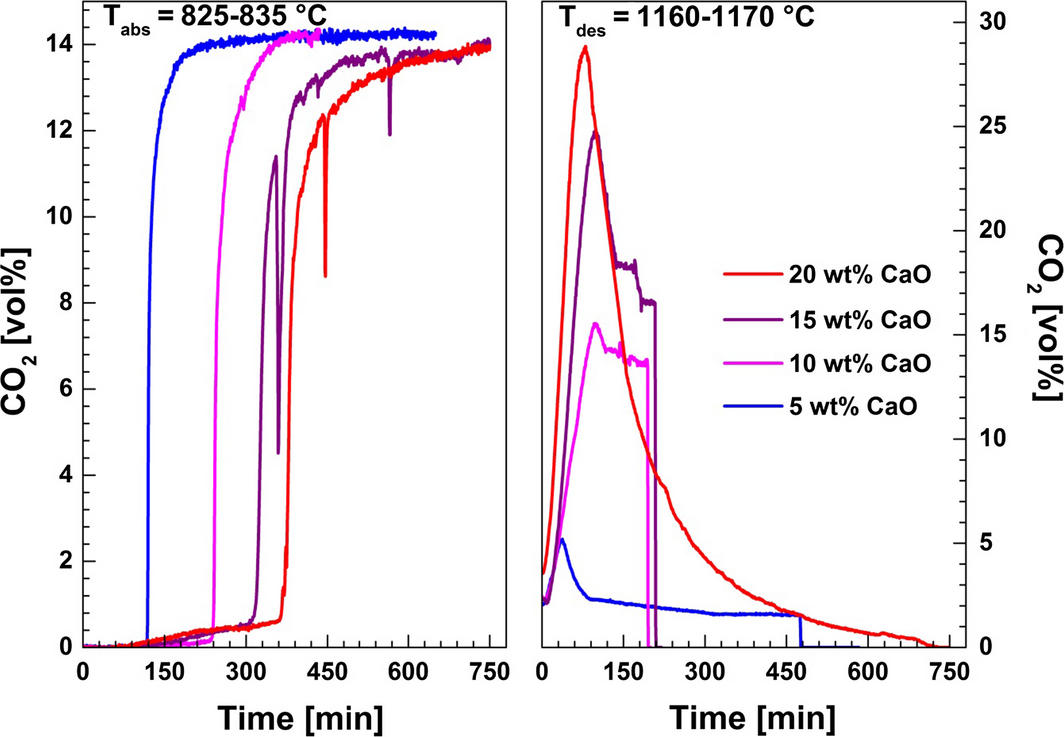
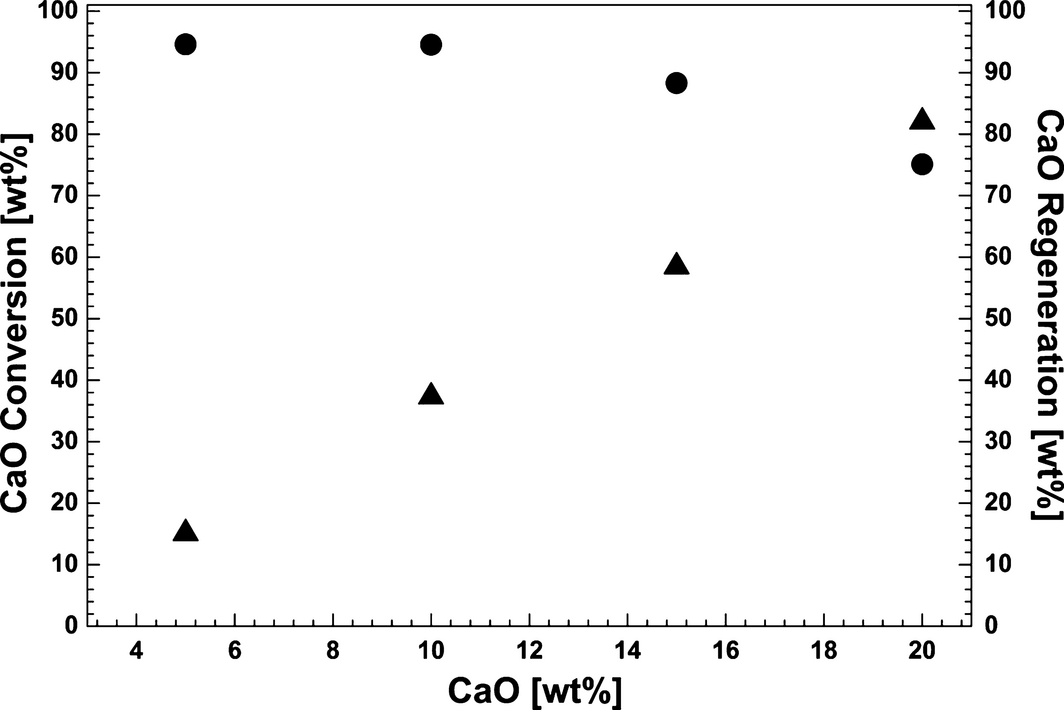
The influence of the CaO content in the CaF2/NaF (46.5/53.5 wt%) eutectic mixture on the CO2 uptake is shown in Figure 10. As noted above, the conversion of CaO in the molten fluoride based salt was found to be highest between 826 and 834°C (Figs. 6 and 7). Accordingly, the experiments with variable CaO concentration were carried out within this temperature range. The gas composition in the carbonation step was 14 vol% CO2 in N2. It was found that 99.9% of the total applied CO2 was removed from the inlet gas in the initial carbonation stage with 5 wt% CaO in CaF2/NaF. The initial, fast CO2 absorption efficiency decreased to 98.1 % by increasing the CaO concentration from 5 to 20 wt%. The total CaO conversion efficiency decreased rapidly from 94.6 to 75.2 wt% when increasing the CaO concentration from 10 to 20 wt% (Fig. 11). The loss in CaO activity above 10 wt% may be attributed to inactivation of CaO due to deposition and agglomeration of solid deposits of CaO (s) in stagnant parts of the crucible with low circulation with these highly supersaturated liquids.
|
|
|
Figure 10. The influence of the CaO concentration on CO2 capture in a CaF2–NaF eutectic mixture The carbonation step was conducted by bubbling 14 vol% CO2 in N2 through the melt at 825–835°C. The decarbonation step was performed at 1160–1170°C under pure N2. |
|
|
|
Figure 11. CaO conversion (●) and regeneration (of total reacted CaO, ▲) as a function of the CaO concentration in the CaF2–NaF eutectic mixture. |
Regeneration of the CaO was performed by raising the temperature to 1160–1170°C under pure N2 (Fig. 10). Incomplete CO2 desorption was recorded by gas analysis for CaO contents of 5, 10, and 15 wt%. This was due to clogging of the purge gas tube due to deposition of CaO in the outlet as previously mentioned. This is evidenced by the abrupt change in recorded value after the initial, rapid release of CO2. In this case, the regeneration efficiency of CaO was calculated as an estimate from the measured amount of CO2 released, for the total reacted CaO with CO2 (Fig. 11). The highest CO2 release rate from the carbonated sample (84.5% of total captured CO2) was achieved with 20 wt% CaO in CaF2–NaF. The formation of carbonates in the CaO–NaF–CaF2 system may influence the liquidus temperature of the mixture, and also the solubility of CaO. The carbonates have higher solubility than CaO, so higher carbonate concentration would lead to lower melting points and maybe to higher solubility of CaO or a more complex substance being formed. However, these observations are hard to explain and will be the subject for further studies.
Effect of CO2 concentration in the simulated flue gas
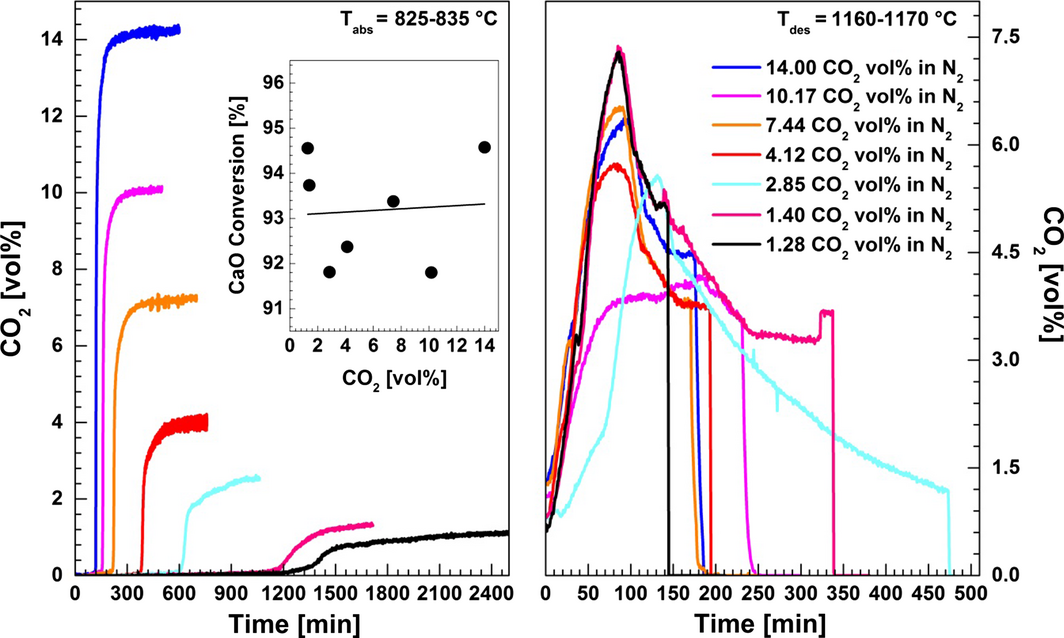
The impact of the inlet gas composition on sorbent conversion efficiency was examined by varying the CO2 concentration in N2 between 1.3 and 14 vol% at 825–835°C. For this purpose, a melt containing 5 wt% CaO was used. Figure 12 shows the concentration of CO2 versus time in the outlet gas as recorded by the FTIR gas analyzer. It was found that the total carbonation conversion is independent on the inlet CO2 concentration in N2, and it was around 93.3 wt% (0.733 g CO2/g of CaO). However, a closer examination of these results reveals that the regions of fast and slow carbonation reaction are affected by the inlet gas composition. The conversion of CaO in the fast carbonation stage is reduced from 89.5 to 70.1% by decreasing the CO2 concentration from 14 vol% to 1.3 vol%. In addition, the duration of the slow carbonation reaction was prolonged when the CO2 concentration decreased. This may be related to phase transitions in the CaO–NaF–CaF2 system because of the formation of carbonates which influence the solubility of unreacted CaO as well as the liquidus temperature of the melt. As mentioned above, the fast carbonation reaction may be attributed to reaction between CO2 and dispersed, solid CaO. Therefore, the additional dissolution/dispersion of solid CaO could influence the fast as well as the slow step in the carbonation reaction.
|
|
|
Figure 12. The influence of the CO2 concentration in N2 on carbonation/decarbonation reactions in CaO/CaF2/NaF (5/44.2/50.8 wt%). The carbonation step was conducted by bubbling 1.3–14 vol% CO2 in N2 through the melt at 825–835°C. The decarbonation step was performed at 1160–1170°C under pure N2. Inset; CaO conversion as a function of the CO2 concentration. |
Effect of CO and HF formation
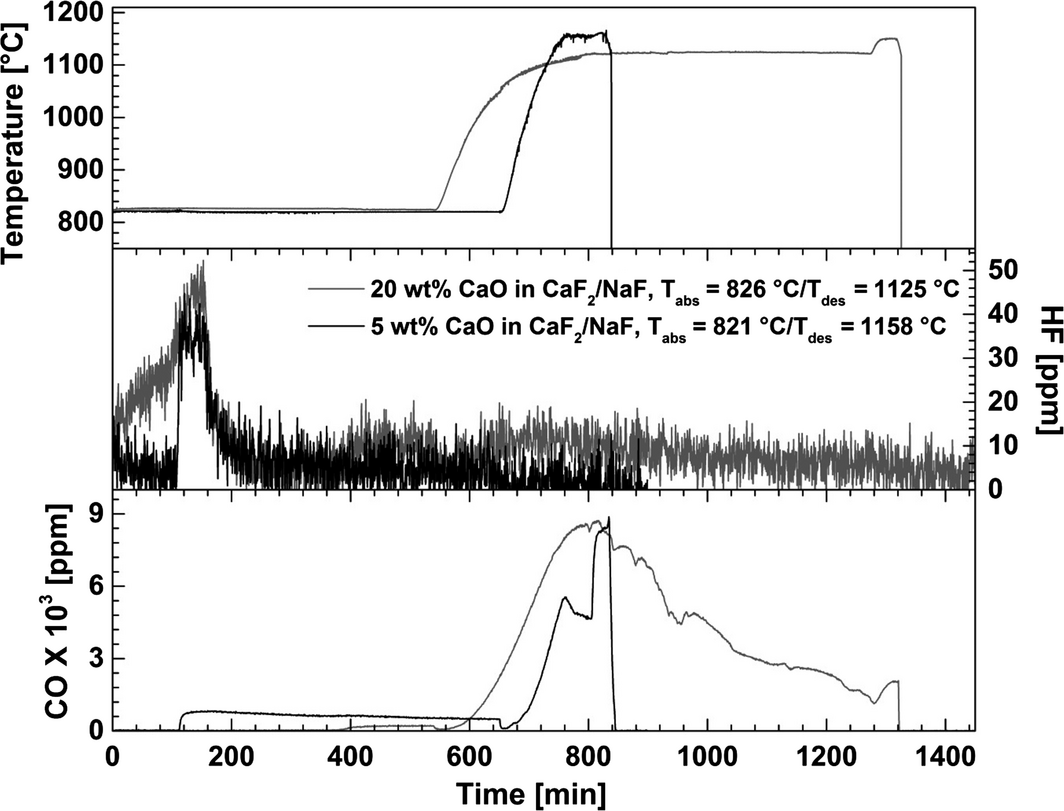
The concentrations of carbon monoxide (CO, from the reduction in CO2) and hydrofluoric acid (HF, from the hydrolysis of fluoride salts) are shown in Figure 13. The data were recorded during carbonation experiments using 14 vol% CO2 in N2 and 5 or 20 wt% CaO in CaF2–NaF. Desorption of CO2 was conducted under pure N2. The results indicate that the CaO concentration has little or no effect on the HF formation. The HF peak at 50 ppm was detected after 100 min of carbonation at 821 and 826°C, decreasing to 10 ppm. The same value was found during the decarbonation process at elevated temperature (1125 and 1158°C). This indicates that metal fluoride hydrolysis is independent on the temperature in the range 821–1158°C, and it may be related to minor contents of moisture in the inlet gas. The weight change because of the hydrolysis of the fluoride salts was considered negligible.
|
|
|
Figure 13. Hydrofluoric acid (HF) and carbon monoxide (CO) concentration in the outlet gas during CO2 absorption/desorption with 5 and 20 wt% of CaO in a CaF2–NaF eutectic mixture. The carbonation was carried out at 821 and 826°C, and the decarbonation temperatures were 1125 and 1158°C, respectively. 14 vol% CO2 in N2 was applied in the carbonation step and pure N2 in the decarbonation step. |
The carbon monoxide concentration curves indicate that the formation of CO was affected by the CaO concentration (Fig. 13). The CO emission from the reactor in the initial fast carbonation step is close to 0 ppm for both compositions (5 and 20 wt% of CaO in CaF2–NaF), but during the slow carbonation step, a CO peak of 470 ppm is detected at low CaO concentration. However, during the decarbonation step CO contents up to 9000 ppm are observed, depending on the CO2 release from the reactor. The carbon monoxide behavior may be explained by CO2 reduction by iron at elevated temperatures, or by the Boudouard reaction.
Concluding Remarks
The purpose of the present research is to contribute in the ongoing development of CO2 capture by the calcium-or carbonate looping process. The aim is to investigate if using CaO in a liquid as being dissolved or partly dissolved in molten CaF2–NaF may be an alternative to solid CaO-based sorbents. By dissolving the active substances in a liquid, the total carrying capacity by total mass is reduced accordingly. However, this may be an alternative approach to modifying solid sorbents by adding secondary oxides or other substances in order to enhance the physical stability of the particles upon cycling. We regard the process as being promising; the laboratory data reported here indicate that carbonation was fast and efficient, and the total efficiency did not deteriorate with increasing number of cycles. Presently, the bottleneck seems to be the decomposition of the carbonates formed, as CO2 desorption was incomplete because of sedimentation and deposition of CaO. However, this might be possible to solve with a better reactor design, since structure analysis of melt samples indicated that full decomposition of the formed carbonates should be possible. The formation of Na2CO3 seems to enhance the absorption, however, the desorption temperature is increased due to the higher stability of sodium carbonate. This will lead to higher energy consumption in a potentially larger scale process.
It should be kept in mind that the present research is still at its fundamental stage. In future investigations it will be necessary to analyze the effect of side reactions on the stability of the molten salt and the efficiency of the overall process. Particularly, reactions involving water vapor, which is always present in real flue gases, may be important. Also, there is still room for improvement of the salt mixture and optimization of the process conditions. Moreover, further research using different reactor designs is needed, and it can be foreseen that the choice of materials will be an important issue in the future as metallic construction materials may not show long-physical stability at these elevated temperatures.
Acknowledgments
The authors thank the Research Council of Norway for financial support through the CLIMIT research program.
Conflict of Interest
None declared.
References
- MacDowell, N., N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson et al. 2010. An overview of CO2 capture technologies. Energy Environ. Sci.3:1645–1669.
- Wang, M., A. Lawal, P. Stephenson, J. Sidders, and C. Ramshaw. 2011. Post-combustion CO2 capture with chemical absorption: a state-of-the-art review. Chem. Eng. Res. Des.89:1609–1624.
- Zaman, M., and J. H. Lee. 2013. Carbon capture from stationary power generation sources: a review of the current status of the technologies. Korean J. Chem. Eng.30:1497–1526.
- Barker, D. J., S. A. Turner, P. A. Napier-Moore, M. Clark, and J. E. Davison. 2009. CO(2) capture in the cement industry. Energy Procedia1:87–94.
- Naranjo, M., D. T. Brownlow, and A. Garza. 2011. CO2 capture and sequestration in the cement industry. 10th Int. Conf. Greenhouse Gas Control Technol.4:2716–2723.
- Kuramochi, T., A. Ramirez, W. Turkenburg, and A. Faaij. 2012. Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog. Energy Combust.38:87–112.
- Donat, F., N. H. Florin, E. J. Anthony, and P. S. Fennell. 2012. Influence of high-temperature steam on the reactivity of CaO sorbent for CO2 capture. Environ. Sci. Technol.46:1262–1269.
- Liu, W. Q., J. J. Yin, C. L. Qin, B. Feng, and M. H. Xu. 2012. Synthesis of CaO-based sorbents for CO2 capture by a spray-drying technique. Environ. Sci. Technol.46:11267–11272.
- Valverde, J. M.2013. Ca-based synthetic materials with enhanced CO2 capture efficiency. J. Mater. Chem. A1:447–468.
- Gupta, H., and L. S. Fan. 2002. Carbonation-calcination cycle using high reactivity calcium oxide for carbon dioxide separation from flue gas. Ind. Eng. Chem. Res.41:4035–4042.
- Olsen, E., and V. Tomkute. 2013. Carbon capture in molten salts. Energy Sci. Eng.1:144–150.
- Tomkute, V., A. Solheim, and E. Olsen. 2013. Investigation of high-temperature CO2 capture by CaO in CaCl2 molten salt. Energy Fuel27:5373–5379.
- Tomkute, V., A. Solheim, and E. Olsen. 2014. CO2 capture by CaO in molten CaF2–CaCl2: optimization of the process and cyclability of CO2 capture. Energy Fuel28:5345–5353.
- Tomkute, V., A. Solheim, S. Sakirzanovas, B. Oye, and E. Olsent. 2014. Phase equilibria evaluation for CO2 capture: CaO–CaF2–NaF, CaCO3–NaF–CaF2, and Na2CO3–CaF2–NaF. J. Chem. Eng. Data59:1257–1263.
- Forsberg, C. W., P. F. Peterson, and H. H. Zhao. 2007. High-temperature liquid-fluoride-salt closed-Brayton-cycle solar power towers. J. Solar Energy Eng.129:141–146.
- Beilmann, M., O. Benes, R. J. M. Konings, and T. Fanghanel. 2011. Thermodynamic investigation of the (LiF + NaF + CaF2 + LaF3) system. J. Chem. Thermodyn.43:1515–1524.
- Cooper, A. F., J. Gittins, and O. F. Tuttle. 1975. System Na2CO3–K2CO3–CaCO3 at 1 Kilobar and Its Significance in Carbonatite Petrogenesis. Am. J. Sci.275:534–560.
- Swainson, I. P., M. T. Dove, and M. J. Harris. 1995. Neutron powder diffraction study of the ferroelastic phase-transition and lattice melting in sodium-carbonate, Na2CO3. J. Phys. Condens. Matter7:4395–4417.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?