Highlights
- 15O-labeled water positron emission tomography (PET) is a tool to compare directly reperfused segments with normal segments simultaneously in each patients hearts.
- 15O-labeled water PET permits noninvasive quantitative measurement of myocardial blood flow during the subacute phase of myocardial infarction.
- 15O-labeled water PET detects restoration of myocardial blood flow after intracoronary administration of nicorandil in STEMI patients.
Abstract
Background
The impact of nicorandil as adjunctive therapy for percutaneous coronary intervention (PCI) in patients with ST-elevation myocardial infarction (STEMI) is controversial. We performed 15O-labeled water positron emission tomography (PET) to quantify regional myocardial perfusion in patients with STEMI who received nicorandil or no adjunctive therapy during PCI.
Methods
PCI was performed within 8 h after STEMI onset in 33 patients. 14 patients received intracoronary nicorandil 2 mg immediately after recanalization of the culprit lesion (Nico group). After 3–4 weeks, PET was performed in which myocardial blood flow (MBF) was measured at baseline and during adenosine triphosphate (ATP)-induced hyperemia. Myocardial vascular resistance (MVR) was calculated for all segments. Data were obtained from the reperfused (Rep) and normal segments (Cont) in each patient.
Results
In patients not given nicorandil (No-Nico group), the MBF was significantly lower in Rep than that in Cont at baseline and during hyperemia (Cont vs. Rep: 0.82 ± 0.14 vs. 0.68 ± 0.11, P = 0.001, ATP-Cont vs. ATP-Rep: 2.00 ± 0.72 vs. 1.52 ± 0.61, P = 0.017), which was restored in the Nico group (Cont vs. Rep: 0.79 ± 0.17 vs. 0.78 ± 0.20; ATP-Cont vs. ATP-Rep: 2.02 ± 0.84 vs. 1.84 ± 0.62). MVR was elevated in Rep at baseline and during hyperemia in the No-Nico group. MVR elevation in Rep was prevented in the Nico group.
Conclusions
15O-labeled water PET was feasible for segmental analysis of MBF during the subacute phase of STEMI. It revealed that intracoronary administration of nicorandil to STEMI patients who underwent PCI prevented MVR elevation and thus restored MBF in the reperfused segments to a level similar to that in the normal segments.
Keywords
Nicorandil;Myocardial infarction;Myocardial blood flow;Positron emission tomography
1. Introduction
Nicorandil is a potassium channel opener with nitrate-like actions that dilate coronary arteries. This agent is also known to prevent reperfusion injury and promote ischemic preconditioning [1]. It has been expected to reduce infarction size and improve clinical outcomes in patients with acute myocardial infarction [2] ; [3]. The value of nicorandil as an adjunctive therapy to primary PCI, however, is still controversial. In fact, a randomized clinical trial showed that intravenous or intracoronary injection of nicorandil after coronary reperfusion did not reduce infarct size as assessed with a total creatine kinase leak [4] ; [5]. In contrast, several reports have shown salutary effects on coronary perfusion and clinical outcomes by intravenous administration before reperfusion or intracoronary administration during primary PCI [6] ; [7]. Sakata et al. have reported that intracoronary administration of nicorandil improved incomplete restoration of myocardial perfusion after coronary revascularization and functional recovery of the jeopardized myocardium, as shown by myocardial contrast echocardiography in convalescents [8]. Ishii et al. reported that single intravenous administration of nicorandil before reperfusion improved coronary flow as evaluated by the corrected thrombolysis in myocardial infarction frame count, leading to improved clinical outcomes and prevention of cardiovascular events and death in patients with ST-elevation myocardial infarction (STEMI) [9]. This may explain why the administration of nicorandil has shown inconsistent outcomes in patients with STEMI. The studies have provided different timing for nicorandil administration and/or different methods for evaluating myocardial perfusion.
Positron emission tomography (PET) with 15O-labeled water is a noninvasive method for accurately quantifying regional myocardial blood flow (MBF) presented as the regional perfusion (mL·min− 1) per gram of myocardium [10] ; [11]. This method has been validated in previous reports [12] ; [13]. Previous studies showed a reduction of basal MBF in patients who smoked or had diabetes mellitus. Furthermore, there are still concerns regarding confounding factors affecting MBF such as hypertension, dyslipidemia, sex, and age [14] ; [15]. Therefore, to clarify the effect of nicorandil on MBF, it is important to measure the MBF in the normal segments to compare them with the reperfused segments of the left ventricle in each patient. This study was designed to estimate MBF in all segments at basal and during adenosine triphosphate disodium hydrate (ATP)-induced hyperemia. ATP is a short-acting drug that mainly dilates the coronary arterial smooth muscle. Therefore, ATP-induced hyperemic blood flow is used to evaluate the coronary vasculature [16].
Based on these previous studies, the main aim of this study is to evaluate the regional myocardial blood flow in the reperfused segments compared with those in the normal segments by 15O-labeled water PET after intracoronary administration of nicorandil during percutaneous coronary intervention (PCI) procedures in patients with STEMI.
2. Materials and methods
The eligibility criteria were the presence of STEMI in a patient admitted to the hospital within 8 h of the onset of symptoms. The exclusion criteria were a history of myocardial infarction; left main trunk stenosis; severe liver or kidney dysfunction, or both; suspected aortic dissection; previous coronary artery bypass grafting; and/or a history of drug allergy. All patients gave written informed consent immediately after admission to the hospital.
The Institutional Review Board and Ethics Committee of Kagawa University Hospital approved the study protocol in accordance with the Declaration of Helsinki.
We introduced an adjunctive treatment with nicorandil to the standard PCI. Nicorandil 2 mg was given by intracoronary injection immediately after recanalization of the culprit lesion. We applied this treatment to 14 consecutive patients from June 2011, who comprised the nicorandil (Nico) group. An earlier group of 19 consecutive patients who underwent standard primary PCI only, served as the no-nicorandil (No-Nico) group. The difference between the two groups was the presence or absence of the adjunctive treatment with intracoronary administration of nicorandil. Standard PCI, including nitrate administration, was performed in all patients within 8 h after the onset of STEMI.
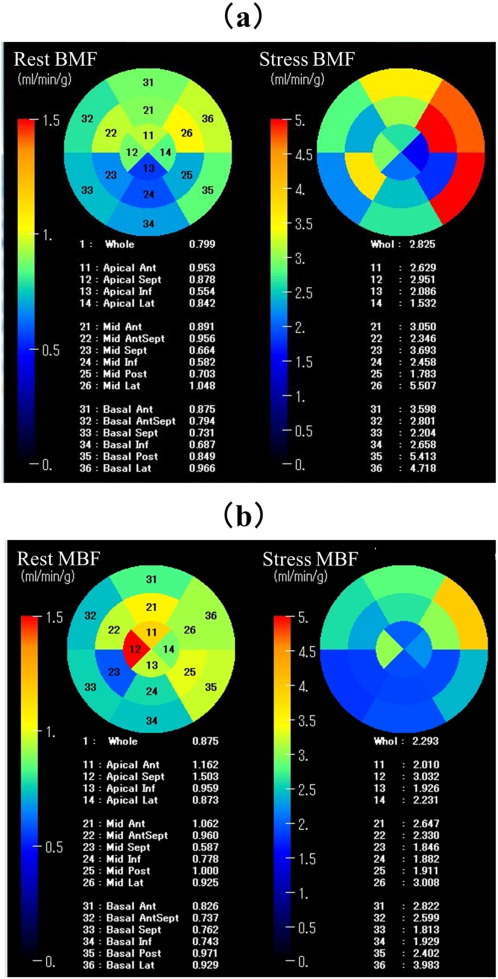
We evaluated the regional MBF and myocardial vascular resistance (MVR) by 15O-labeled water PET between 3 and 4 weeks after PCI. MVR is calculated as the mean arterial blood pressure divided by the MBF. For the analysis of MBF, we performed segment-based measurements. As shown in Fig. 1, the left ventricle was divided into 16 segments according to the model recommended by the American Society of Echocardiography and American Heart Association [17]. These segments included the anterior, anteroseptal, septal, inferior, posterior, and lateral walls, where each was subdivided into basal and mid portions, as well as the anterior apex, septal apex, inferior apex, and lateral apex. Data were obtained from each segment, and MBF was calculated by mean value in each coronary artery territories [18]. Furthermore, to avoid the effect of ischemia on MBF, we categorized the myocardial segments into three groups according to the most recent coronary angiography before the 15O-labeled water PET: those perfused insufficiently by a stenosed coronary artery (Ischemia group); those with a nonstenosed coronary artery (controls, Cont); and those reperfused by PCI (Rep). The definition of stenosis is more than 75% of stenosis according to the definition of the American Heart Association [19]. The Ischemia group was excluded from the analysis.
|
|
|
Fig. 1. Myocardial blood flow images by 15O-labeled water PET using a 16-segment model at rest (left) and during hyperemia (right) in patients with acute inferior myocardial infarction treated with (a) or without (b) nicorandil to the standard PCI. MBF = myocardial blood flow; PET = positron emission tomography and PCI = percutaneous coronary intervention. |
2.1. PET image processing.
PET image processing and analysis were accomplished as described previously [20]. PET was performed using a whole-body scanner (Siemens/CTI, Knoxville, TN, USA) equipped with germanium-68 retractable line sources for transmission scans. All emissions and transmissions were reconstructed using filtered back-projection. The full-width at half maximum at the center of the field of view was 4.7 mm. The optimal imaging positron was determined by a 5-min rectilinear scan after exposure of an external 68Ga ring source. A 6-min transmission scan was then acquired for the purpose of attenuation correction of all subsequent emission scans.
Blood volume images were produced in the following manner. During the 5-min scanning period, venous blood samples were obtained every 2 min, and radioactivity in the whole blood was measured with an automatic gamma counter (Fukuda Electric Company, Saitama, Japan).
15O radioactivity returned to background level 15 min after the blood volume scan. Then, 15O-labeled water was slowly (over 2 min) infused into an antecubital vein. 15O-labeled water was administered twice during the study. The administered dose of 15O-labeled water was 500 MBq/min. A 20-frame dynamic PET scan was performed for 6 min consisting of six frames for 5 s, six frames for 15 s, and eight frames for 30 s.
The MBF (in milliliters per gram per minute) of the whole left ventricle was measured using 15O-labeled water as the flow tracer and previously validated 15O radioactivity. We repeated the MBF measurement during ATP-induced hyperemia. ATP was infused for 9 min at 0.16 mg/kg/min, according to a standard protocol. PET acquisition was started 3 min after beginning the ATP infusion. Blood pressure was recorded at 1-min intervals. The patients electrocardiogram was monitored continuously throughout the procedure at baseline and every minute during ATP administration.
2.2. Production of 15O-labeled water PET
A low-energy deuteron accelerator was used to produce the 15O compounds (CYPRIS-HM18 cyclotron; Sumitomo Heavy Industries, Tokyo, Japan). 15O-labeled water was produced with a dialysis technique in a continuously working water module. Sterility and pyrogen tests were performed daily to verify the purity of the product.
2.3. Statistical analysis
Data were analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). All data are expressed as the mean ± standard deviation. The baseline clinical and angiographic characteristic parameters between the Nico group and the No-Nico group in all groups were compared with an unpaired t-test. The MBF and MVR at baseline and during ATP-induced hyperemia were compared in each group with the unpaired t-test. The PET-derived parameters (MBF and MVR) were compared between the two groups by repeated-measures analysis of variance. P < 0.05 was considered statistically significant.
3. Results
The baseline characteristics of the patients and the baseline and PET procedure drug information are shown in Table 1 and 2. There were no differences between the two groups in PCI procedure. The ratio of patients with obesity and hyperlipidemia in the No-Nico were significantly higher than that in the Non-Nico group.
| Nicorandil | No-Nicorandil | P value | |
|---|---|---|---|
| n = 14 | n = 19 | ||
| Age, y | 69.9 ± 12.7 | 63.5 ± 12.0 | 0.149 |
| Sex, F/M | 2/12 | 5/14 | – |
| BMI, kg/m2 | 23.3 ± 3.30 | 22.7 ± 2.35 | 0.562 |
| Obesity, n (%) | 6 (42.9) | 2 (10.5) | 0.047 |
| Hypertension, n (%) | 10 (71.4) | 7 (36.8) | 0.080 |
| Diabetes mellitus, n (%) | 6 (42.9) | 6 (31.6) | 0.716 |
| Hyperlipidemia, n (%) | 9 (64.3) | 5 (26.3) | 0.040 |
| Smoking, n (%) | 8 (57.1) | 10 (52.6) | 1.000 |
| Onset-to-balloon time (min) | 302 ± 148 | 306 ± 158 | 0.939 |
| Onset-to-balloon time < 4 h, n | 8 (57.1) | 10 (52.6) | 1.000 |
| TIMI grade before PCI (0/1/2/3), n | 9/4/1/0 | 10/6/3/0 | – |
| Previous PCI, n | 1 (7.14) | 1 (5.3) | 1.000 |
| PTCR, n | 0 | 0 | – |
| Peak CPK, IU | 2798 ± 1364 | 3523 ± 2174 | 0.281 |
| Final achievement of TIMI flow III grade | 14 (100) | 18 (94.7) | 1.000 |
| Culprit vessel, n (%) | |||
| LAD | 8 | 9 | |
| LCX | 2 | 2 | – |
| RCA | 4 | 8 | |
| PCI procedure | |||
| Main vessel | |||
| POBA/BMS/DES, n | 0/2/10 | 1/18/0 | – |
| Thromboaspiration, n | 8 (57.1) | 8 (42.1) | 0.491 |
| Stent length, mm | 25.7 ± 7.2 | 19.7 ± 10.4 | 0.073 |
| Stent diameter, mm | 3.14 ± 0.49 | 3.28 ± 0.36 | 0.376 |
| Procedure time, min | 96.4 ± 28.0 | 93.2 ± 24.3 | 0.723 |
| Restenosis at 6 months, n | 1 (7.14) | 4 (21.1) | 0.366 |
Values are mean ± SD or n (%). Nicorandil vs. No-Nicorandil. BMI, body mass index; PTCR, Percutaneous Transluminal Coronary Recanalization; TIMI, thrombolysis in myocardial infarction trial; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; CPK, creatine phosphokinase; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; POBA and percutaneous old balloon angioplasty. Values are mean ± SD or n (%). BMS, bare metal stent and DES, drug eluting stent.
| Nicorandil | No-Nicorandil | P value | |
|---|---|---|---|
| n = 14 | n = 19 | ||
| Baseline | |||
| ARB, n | 4 (28.6) | 2 (10.5) | 0.363 |
| ACE-I, n | 0 | 4 (21.1) | 0.119 |
| CCB, n | 4 (28.6) | 3 (15.8) | 0.422 |
| Statins, n | 2 (14.3) | 0 | 0.172 |
| Aspirin, n | 2 (14.3) | 3 (15.8) | 1.000 |
| Clopidogrel, n | 0 | 0 | – |
| Ticlopidine, n | 0 | 0 | – |
| Antiplatelet agents, n | 2 (14.3) | 3 (15.8) | 1.000 |
| β-Blocker, n | 2 (14.3) | 1 (5.3) | 0.561 |
| Nitrates, n | 2 (14.3) | 2 (10.5) | 1.000 |
| Nicorandil, n | 0 | 2 (10.5) | 0.496 |
| Diuretic, n | 0 | 0 | – |
| At PET procedure | |||
| ARB, n | 11 (78.6) | 11 (57.9) | 0.278 |
| ACE-I, n | 4 (28.6) | 7 (36.8) | 0.719 |
| CCB, n | 2 (14.3) | 2 (10.5) | 1.000 |
| Statins, n | 13 (92.9) | 9 (47.4) | 0.009 |
| Aspirin, n | 14 (100) | 18 (94.7) | 1.000 |
| Clopidogrel, n | 13 (92.9) | 0 | 0.0001 |
| Ticlopidine, n | 0 | 15 (78.9) | 0.0001 |
| Antiplatelet agents, n | 14 (100) | 19 (100) | – |
| β-Blocker, n | 13 (92.9) | 4 (21.1) | 0.0001 |
| Nitrates, n | 1 (7.14) | 4 (21.1) | 0.366 |
| Nicorandil, n | 2 (14.3) | 12 (63.2) | 0.011 |
| Diuretic, n | 4 (28.6) | 4 (21.1) | 0.695 |
n(%). Nicorandil vs. No-Nicorandil. ARB, angiotensin II receptor blocker; ACE-I, angiotensin converting enzyme inhibitor; CCB, calcium channel blocker and PET, positron emission tomography.
The mean MBF at baseline in the normal segments was 0.79 ± 0.15 mL/min/g (95% confidence interval, 0.74–0.83 mL/min/g) in this study. Because variations of about 10% of the MBF value were seen among the patients at baseline, it was important to compare the MBF in the normal segments with that in the reperfused segments in each patient.
We collected a total of 137 normal segments in Cont, 82 reperfused segments in Rep, and 5 exclusion segments from 14 patients in the Nico group. We also collected a total of 150 normal segments in Cont, 105 reperfused segments in Rep, and 49 exclusion segments from 19 patients in the No-Nico group.
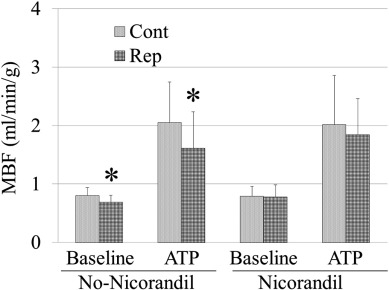
As shown in Fig. 2, in the No-Nico group, the MBF was significantly lower in the reperfused segments than that in the normal segments at baseline. The difference was augmented during ATP-induced hyperemia (Cont vs. Rep: 0.82 ± 0.14 vs. 0.68 ± 0.11, P = 0.001; ATP-Cont vs. ATP-Rep: 2.00 ± 0.72 vs. 1.52 ± 0.61, P = 0.017). In contrast, in the Nico group, the MBF in the reperfused segments was restored to a level similar to those in the normal segments at baseline and during ATP-induced hyperemia (Cont vs. Rep: 0.79 ± 0.17 vs. 0.78 ± 0.20; ATP-Cont vs. ATP-Rep: 2.02 ± 0.84 vs. 1.84 ± 0.62, P = NS for both measurements). The Rep/Cont, which indicates the ratio of salvage of MBF, tended to be restored in the Nico group at baseline and during ATP-induced hyperemia (baseline: No-Nico Rep/Cont vs. Nico Rep/Cont: 0.89 ± 0.24 vs. 0.99 ± 0.23, P = 0.237; ATP: No-Nico Rep/Cont vs. Nico Rep/Cont: 0.77 ± 0.26 vs. 0.92 ± 0.21, P = 0.091).
|
|
|
Fig. 2. Comparison of myocardial blood flow between normal segments and reperfused segments. *P < 0.05 vs. control segments. MBF = myocardial blood flow; Cont = normal segments and Rep = reperfused segments. |
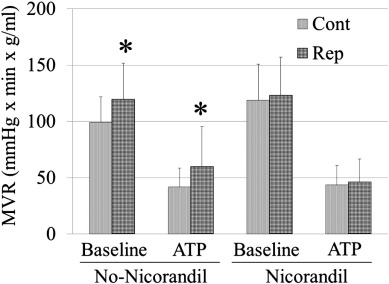
In addition, to avoid the effect of perfusion pressure on MBF, we calculated the MVR in all segments. The MVR was elevated in the reperfused segments at baseline and during ATP-induced hyperemia in the No-Nico group (Cont vs. Rep: 98.8 ± 22.9 vs. 119.5 ± 32.3, P = 0.01; ATP-Cont vs. ATP-Rep: 41.6 ± 17.0 vs. 59.7 ± 35.7, P = 0.02). However, such an elevation of MVR in the reperfused segments was successfully prevented in the Nico group(Cont vs. Rep: 115.7 ± 33.4 vs. 121.2 ± 34.8; ATP-Cont vs. ATP-Rep: 43.5 ± 17.5 vs. 45.9 ± 20.6) (Fig. 3).
|
|
|
Fig. 3. Comparison of myocardial vascular resistance between normal control segments and reperfused segments. *P < 0.05 vs. control segments. MVR = myocardial vascular resistance; Cont = normal segments and Rep = reperfused segments. |
Nevertheless, there was no reduction of the maximum creatine phosphokinase leak and improvement of the left ventricular ejection fraction valued by echocardiography during the subacute phase of myocardial infarction (Nico: 49.0 ± 8.38% vs. No-Nico: 55.0 ± 16.6%).
4. Discussion
We have clearly shown that intracoronary administration of nicorandil preserved the regional microcirculation in the reperfused segments in patients with STEMI. One other finding was that 15O-labeled water PET was feasible for segmental analyses of MBF at the subacute phase of STEMI. Although a left bundle branch block (LBBB) exhibits reduced MBF both at stress and rest [21], there were no patients with LBBB in this study. Our protocol was quite simple, involving a single intracoronary injection. The most likely mechanism for the salutary effects of nicorandil on restoration of MBF might be that the pharmacologic vasodilation for the coronary vasculature resulted in a maintained vascular lumen. Hence, nicorandil prevented increased MVR after reperfusion. Nicorandil increases coronary blood flow, particularly to small vessels (< 100 μm) [22]. It is similar to a potassium channel opener with nitrate-like actions that dilate coronary arteries. In the ischemic myocardium, K-ATP channels are activated by intracellular ATP depletion, with increased outflow of potassium, shortening the duration of the action potential and reducing calcium inflow to myocytes [23]. Inhibition and reduction of calcium inflow to myocytes may have cardioprotective effects on the ischemic heart, and K-ATP channel openers help this action [24]. In one study, mitochondrial K-ATP channels were further shown to be related to preconditioning as the end factor of many signal transduction systems. Thus, nicorandil might exert salutary effects through its activation [25].
The timing of intracoronary administration of nicorandil might be an important factor for decreasing the infarction size and improve clinical outcome. Nicorandil was injected immediately after recanalization of culprit lesion. Timing the injection so it is “before the onset of reperfusion injury” and targeting the point of injection (i.e., “selective intracoronary injection”) may be key to the beneficial effect that prevents reperfusion injury and preserves the regional microcirculation. Additionally, as intracoronary administration of nicorandil did not reduce the maximum creatine phosphokinase leak, it is possible that it improved the microcirculation.
Previous reports have indicated that MBF is reduced in remote areas in patients with ischemic heart disease, probably as a result of decreased coronary vasodilator reserve [26] ; [27]. Early coronary arteriosclerosis and altered endothelial function also are found before the development of gross coronary artery disease (CAD) in patients with hyperlipidemia, diabetes mellitus, and hypertension [28]; [29] ; [30]. The present data are consistent with previous reports. Early microvascular change may reduce MBF in these segments despite the lack of significant stenosis in major coronary arteries. Ischemic changes and coronary risk factors might alter endothelial function. Such subtle changes in MBF with PET can be identified.
Noninvasive measurement of MBF by 15O-labeled water PET accurately reflects the results of invasive techniques such as measurements using an intracoronary Doppler guide wire [31]. PET can compare directly reperfused segments with normal segments simultaneously, a capability that is limited when using the intracoronary Doppler guide wire. PET permits quantitative measurements of MBF, which generate accurate data about multi-vessel and diffuse CAD. Therefore, 15O-labeled water PET may detect small regional differences in MBF and document early microvascular changes sensitively that cannot be detected by stress perfusion, single-photon emission computed tomography.
5. Study limitations
The number of subjects in this study was small, so it will be necessary to increase the number of subjects for further comparison of the two methods. This study is not randomized and double blind, so the difference in baseline patient characteristics and device might affect these results. In this study, patients continued to take their usual medications. We cannot rule out that these medications selectively affected the coronary vasodilator reserve. Because most of the patients had severe CAD and were taking several medications, we decided to continue their usual medications to maintain stable conditions during the studies. Finally, the definition of “onset” may vary, which means that the difference in treatment times may also vary.
6. Conclusions
Noninvasive measurement of myocardial blood flow by PET might be feasible and valuable for assessing the impact of adjunctive therapy at the subacute phase in patients with ST-elevation myocardial infarction. 15O-labeled water PET revealed that the targeted administration of nicorandil could provide salutary adjunctive therapy to primary PCI for STEMI by preserving the MBF in the reperfused segments.
References
- [1] J. Imagawa, G.F. Baxter, D.M. Yellon; Myocardial protection afforded by nicorandil and ischaemic preconditioning in a rabbit infarct model in vivo; J. Cardiovasc. Pharmacol., 31 (1998), pp. 74–79
- [2] H. Ito, Y. Taniyama, K. Iwakura, N. Nishikawa, T. Masuyama, T. Kuzuya, et al.; Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction; J. Am. Coll. Cardiol., 33 (1999), pp. 654–660
- [3] H. Ono, T. Osanai, H. Ishizaka, H. Hanada, T. Kamada, H. Onodera, et al.; Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation; Am. Heart J., 148 (2004) E15
- [4] M. Kitakaze, M. Asakura, J. Kim, Y. Shintani, H. Asanuma, T. Hamasaki, et al.; Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials; Lancet, 370 (2007), pp. 1483–1493
- [5] S.-J. Kim, W. Kim, J.-S. Woo, S.-J. Ha, W.-Y. Kang, S.-H. Hwang, et al.; Effect of myocardial protection of intracoronary adenosine and nicorandil injection in patients undergoing non-urgent percutaneous coronary intervention: a randomized controlled trial; Int. J. Cardiol., 158 (2012), pp. 88–92
- [6] A. Okamura, H. Rakugi, M. Ohishi, Y. Yanagitani, M. Shimizu, T. Nishii, et al.; Additive effects of nicorandil on coronary blood flow during continuous administration of nitroglycerin; J. Am. Coll. Cardiol., 37 (2001), pp. 719–725
- [7] B. Luo, P. Wu, T. Bu, Z. Zeng, D. Lu; All-cause mortality and cardiovascular events with nicorandil in patients with IHD: systematic review and meta-analysis of the literature; Int. J. Cardiol., 176 (2014), pp. 661–669
- [8] Y. Sakata, K. Kodama, K. Komamura, Y.J. Lim, F. Ishikura, A. Hirayama, et al.; Salutary effect of adjunctive intracoronary nicorandil administration on restoration of myocardial blood flow and functional improvement in patients with acute myocardial infarction; Am. Heart J., 133 (1997), pp. 616–621
- [9] H. Ishii, S. Ichimiya, M. Kanashiro, T. Amano, K. Imai, T. Murohara, et al.; Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction; Circulation, 112 (2005), pp. 1284–1288
- [10] L.I. Araujo, A.A. Lammertsma, C.G. Rhodes, E.O. McFalls, H. Iida, E. Rechavia, et al.; Noninvasive quantification of regional myocardial blood flow in coronary artery disease with oxygen-15-labeled carbon dioxide inhalation and positron emission tomography; Circulation, 83 (1991), pp. 875–885
- [11] F. Hermansen, S.D. Rosen, F. Fath-Ordoubadi, J.S. Kooner, J.C. Clark, P.G. Camici, et al.; Measurement of myocardial blood flow with oxygen-15 labelled water: comparison of different administration protocols; Eur. J. Nucl. Med., 25 (1998), pp. 751–759
- [12] H. Iida, I. Kanno, A. Takahashi, S. Miura, M. Murakami, K. Takahashi, et al.; Measurement of absolute myocardial blood flow with H215O and dynamic positron-emission tomography. Strategy for quantification in relation to the partial-volume effect; Circulation, 78 (1988), pp. 104–115
- [13] C. Katoh, U. Ruotsalainen, H. Laine, S. Alenius, H. Iida, P. Nuutila, et al.; Iterative reconstruction based on median root prior in quantification of myocardial blood flow and oxygen metabolism; J. Nucl. Med., 40 (1999), pp. 862–867
- [14] Y. Iwado, K. Yoshinaga, H. Furuyama, Y. Ito, K. Noriyasu, C. Katoh, et al.; Decreased endothelium-dependent coronary vasomotion in healthy young smokers; Eur. J. Nucl. Med. Mol. Imaging, 29 (2002), pp. 984–990
- [15] R. Huang, S.S. Abdelmoneim, L.F. Nhola, S.L. Mulvagh; Relationship between HgbA1c and myocardial blood flow reserve in patients with type 2 diabetes mellitus: noninvasive assessment using real-time myocardial perfusion echocardiography; J. Diabetes Res., 2014 (2014), pp. 1–8
- [16] M. Miyagawa, S. Kumano, M. Sekiya, K. Watanabe, H. Akutzu, T. Imachi, et al.; Thallium-201 myocardial tomography with intravenous infusion of adenosine triphosphate in diagnosis of coronary artery disease; J. Am. Coll. Cardiol., 26 (1995), pp. 1196–1201
- [17] N.B. Schiller, P.M. Shah, M. Crawford, A. DeMaria, R. Devereux, H. Feigenbaum, et al.; Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-dimensional Echocardiograms; J. Am. Soc. Echocardiogr., 2 (1989), pp. 358–367
- [18] D.S. Segar, S.E. Brown, S.G. Sawada, T. Ryan, H. Feigenbaum; Dobutamine stress echocardiography: correlation with coronary lesion severity as determined by quantitative angiography; J. Am. Coll. Cardiol., 19 (1992), pp. 1197–1202
- [19] W.G. Austen, J.E. Edwards, R.L. Frye, G.G. Gensini, V.L. Gott, L.S. Griffith, et al.; A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association; Circulation, 51 (1975), pp. 5–40
- [20] M. Ohara, K. Yukiiri, H. Masugata, Y. Iwado, H. Takinami, Y. Nishiyama, et al.; Relationship between myocardial flow reserve by oxygen-15 water positron emission tomography in the subacute phase of myocardial infarction and left ventricular remodeling in the chronic phase; Hypertens. Res., 31 (2008), pp. 1307–1313
- [21] O. Lindner, J. Vogt, D. Baller, A. Kammeier, P. Wielepp, J. Holzinger, et al.; Global and regional myocardial oxygen consumption and blood flow in severe cardiomyopathy with left bundle branch block; Eur. J. Heart Fail., 7 (2005), pp. 225–230
- [22] K. Akai, Y. Wang, K. Sato, N. Sekiguchi, A. Sugimura, T. Kumagai, et al.; Vasodilatory effect of nicorandil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potassium channels; J. Cardiovasc. Pharmacol., 26 (1995), pp. 541–547
- [23] C.G. Nichols, C. Ripoll, W.J. Lederer; ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction; Circ. Res., 68 (1991), pp. 280–287
- [24] J.A. Auchampach, M. Maruyama, I. Cavero, G.J. Gross; Pharmacological evidence for a role of ATP-dependent potassium channels in myocardial stunning; Circulation, 86 (1992), pp. 311–319
- [25] T. Sato, N. Sasaki, B. O'Rourke, E. Marbán; Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels; J. Am. Coll. Cardiol., 35 (2000), pp. 514–518
- [26] N.G. Uren, T. Crake, D.C. Lefroy, R. de Silva, G.J. Davies, A. Maseri; Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction; N. Engl. J. Med., 331 (1994), pp. 222–227
- [27] N.G. Uren, P. Marraccini, R. Gistri, R. de Silva, P.G. Camici; Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease; J. Am. Coll. Cardiol., 22 (1993), pp. 650–658
- [28] I. Yokoyama, S. Momomura, T. Ohtake, K. Yonekura, J. Nishikawa, Y. Sasaki, et al.; Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus; J. Am. Coll. Cardiol., 30 (1997), pp. 1472–1477
- [29] H. Laine, O.T. Raitakari, H. Niinikoski, O.P. Pitkänen, H. Iida, J. Viikari, et al.; Early impairment of coronary flow reserve in young men with borderline hypertension; J. Am. Coll. Cardiol., 32 (1998), pp. 147–153
- [30] I. Yokoyama, T. Ohtake, S. Momomura, J. Nishikawa, Y. Sasaki, M. Omata; Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis; Circulation, 94 (1996), pp. 3232–3238
- [31] P. Merlet, B. Mazoyer, L. Hittinger, H. Valette, J.P. Saal, B. Bendriem, et al.; Assessment of coronary reserve in man: comparison between positron emission tomography with oxygen-15-labeled water and intracoronary Doppler technique; J. Nucl. Med., 34 (1993), pp. 1899–1904
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?