(Created page with " ==Summary== ====Background/Objective==== Postoperative nausea and vomiting (PONV) is one of the most common and distressing adverse events after laparoscopic cholecystecto...") |
m (Scipediacontent moved page Draft Content 891189147 to Si et al 2014a) |
(No difference)
| |
Latest revision as of 12:31, 26 May 2017
Summary
Background/Objective
Postoperative nausea and vomiting (PONV) is one of the most common and distressing adverse events after laparoscopic cholecystectomy (LC). A meta-analysis of randomized clinical trials (RCTs) was performed to determine the efficacy and safety of dexamethasone combined with other antiemetic in the prevention of PONV in patients undergoing LC.
Methods
A systematic literature search was conducted to identify all relevant RCTs. The primary outcome was PONV in the early period (0–3 hours, 0–4 hours, or 0–6 hours), late period (>6 hours), and the overall period (0–24 hours).
Results
Nine RCTs with a total of 1089 patients were included in the analysis. Pooled analysis showed that dexamethasone combined with other antiemetics provided significantly better prophylaxis than single antiemetics in the early period [odds ratio (OR): 0.34; 95% confidence interval (CI): 0.21–0.55; p < 0.001], late period (OR: 0.35; 95% CI: 0.22–0.57; p < 0.001), and the overall period (OR: 0.36; 95% CI: 0.27–0.49; p < 0.001). Correspondingly, rescue antiemetic usage was significantly less in the combination therapy group (OR: 0.22; 95% CI: 0.12–0.41; p < 0.001). The most frequently reported adverse events were headache, dizziness, and itching. The incidence of adverse events did not differ between the two groups.
Conclusion
Dexamethasone combined with other antiemetics was significantly better than single antiemetics for prophylaxis of PONV in patients undergoing LC, without apparent side effects.
Keywords
dexamethasone;laparoscopic cholecystectomy;postoperative nausea and vomiting
1. Introduction
Postoperative nausea and vomiting (PONV) is one of the most common and distressing adverse events after laparoscopic cholecystectomy (LC).1 Dexamethasone, a corticosteroid, can effectively prevent PONV.2 To improve antiemetic efficacy, clinicians often add another agent to the monotherapy.3 Although there have been several randomized controlled trials (RCTs) evaluating efficacy of the combination of dexamethasone with other antiemetics for the prevention of PONV in patients undergoing LC, the number of patients in the individual trial is often small.4; 5; 6; 7 ; 8 In such settings, the use of a meta-analysis has been advocated to obtain a more precise estimate of effect size.9
Therefore, we performed a meta-analysis to evaluate the available evidence regarding the antiemetic efficacy of dexamethasone combined with other antiemetics for PONV compared with single antiemetics in patients undergoing LC. This study was undertaken following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
2. Materials and methods
2.1. Literature search
A computerized search of Medline and Embase databases as well as the Cochrane Library was performed. The following MeSH search headings were used: “laparoscopic cholecystectomy”, “dexamethasone”, “nausea”, “vomiting”, “postoperative”, and “postoperative nausea and vomiting”. Reference lists in the selected articles were manually searched for additional studies. The electronic search was performed from 1 January 1966 to 30 October 2012.
2.2. Inclusion and exclusion criteria
All studies published as full reports of RCTs in the English language that evaluated the efficacy of prophylactic dexamethasone combined with other antiemetics compared with single antiemetics on PONV in patients undergoing LC were included. Abstracts, reviews, letters to the editor, retrospective studies, and animal data were excluded. No attempts were made to obtain unpublished studies.
2.3. Data extraction
Two reviewers (BL and LW) independently extracted the following parameters from each study: first author, year of publication, study population characteristics, study design, number of patients in each arm, sex, age, inclusion and exclusion criteria, and outcomes of interest. The incidence of PONV was extracted at three time points: early period (0–3 hours, 0–4 hours, or 0–6 hours), late period (>6 h), and overall period (0–24 hours). If the incidence of events in the overall period was not reported in a study, we extracted data from the time points with the highest event rate. Discrepancies between the reviewers were resolved through discussion until consensus was achieved.
2.4. Qualitative analysis
The RCTs were scored using the Jadad scale,10 which evaluates studies based on randomization (0–2 points), double-blinding (0–2 points), and withdrawals and dropouts (0–1 point). Studies achieving ≥3 points were considered to be of higher quality.
2.5. Outcomes of interest
The primary outcome was PONV, which included both nausea and vomiting.
Secondary outcomes were adverse effects and rescue antiemetic usage.
2.6. Statistical analysis
Dichotomous variables were summarized using odds ratio (OR) with a 95% confidence interval (CI). For each comparison, heterogeneity was evaluated by χ2 and I2. If the statistical test for heterogeneity was present (p < 0.1), a random effects model was used. If the data were not significantly heterogeneous (p > 0.1), a fixed effects model was used. Publication bias was assessed visually using a funnel plot. All data were analyzed using Review Manager version 5.0 (Cochrane Collaboration, Software Update, Oxford, UK) and p < 0.05 was considered statistically significant.
3. Results
3.1. Eligible studies
From the electronic databases, we initially identified 10 RCTs that met the eligibility criteria. One study was excluded because it presented PONV as continuous data,8 so a final total of nine studies published between 2000 and 2012 was included in the present analysis.4; 5; 6; 7; 11; 12; 13; 14 ; 15 The study characteristics and patient demographic data are shown in Table 1 ; Table 2. Sample size ranged from 80 to 150, with a total of 1089 patients, of whom 526 received prophylactic dexamethasone plus other antiemetics (combination therapy group) and 563 received a single antiemetic (monotherapy group). All of the studies had higher quality. There were no significant demographic differences between patients randomized to the combination therapy group versus the monotherapy group in all the trials.
| Study | Year | Country | Combination therapy | Monotherapy | Quality score |
|---|---|---|---|---|---|
| Fujii4 | 2000 | Japan | D 8 mg + G 40 μg/kg IV 15 min prior to induction | G 40 μg/kg IV prior to induction | 4 |
| Coloma5 | 2002 | USA | D 4 mg IV at induction + Do 12.5 mg IV when the gallbladder was removed | Do 12.5 mg IV when the gallbladder was removed | 4 |
| Elhakim6 | 2002 | Egypt | D 2 mg + O 4 mg IV prior to induction; D 4 mg + O 4 mg IV prior to induction; D 8 mg + O 4 mg IV prior to induction; D 16 mg + O 4 mg IV prior to induction; | O 4 mg IV prior to induction | 5 |
| Biswas7 | 2003 | India | D 8 mg + G 40 μg/kg IV 15 min prior to induction | G 40 μg/kg IV prior to induction | 5 |
| Nesek-Adam11 | 2007 | Croatia | D 8 mg IV after induction of anesthesia + M 10 mg IV at the end of surgery | M 10 mg IV at the end of surgery; D 8 mg after induction | 4 |
| Bano12 | 2008 | Ireland | D 8 mg + O 4 mg IV 1 min prior to induction | D 8 mg IV 1 min prior to induction | 4 |
| Fujii13 | 2008 | D 8 mg + P 0.5 mg/kg IV at the end of surgery | P 0.5 mg/kg IV at the end of surgery | 5 | |
| Gautam14 | 2008 | Nepal | D 8 mg + O 4 mg IV just prior to induction | D 8 mg IV just prior to induction; O 4 mg IV just prior to induction | 5 |
| Jo15 | 2012 | Korea | D 8 mg prior to induction + R 0.3 mg IV 15 min prior to the end of surgery | D 8 mg IV prior to induction; R 0.3 mg IV 15 min prior to the end of surgery | 5 |
D = dexamethasone; Do = dolasetron; G = granisetron; IV = intravenous M = metoclopramide; O = ondansetron, P = propofol; R = ramosetron.
| Study | Group | No. of patients | Age (y) | Sex (male/female) | Weight (kg) | Height (cm) | Duration of anesthesia (min) | Duration of surgery (min) |
|---|---|---|---|---|---|---|---|---|
| Fujii4 | D 8 mg + G 40 μg/kg | 60 | 47 ± 10 | 18/42 | 55 ± 8 | 156 ± 6 | 109 ± 32 | 85 ± 30 |
| G 40 μg/kg | 60 | 47 ± 9 | 19/41 | 56 ± 7 | 158 ± 7 | 109 ± 37 | 85 ± 36 | |
| Coloma5 | D 4 mg + Do 12.5 mg | 70 | 34 ± 13 | 16/54 | 78 ± 20 | 158 ± 14 | 112 ± 28 | 91 ± 27 |
| Do 12.5 mg | 70 | 38 ± 12 | 14/56 | 84 ± 25 | 156 ± 17 | 117 ± 33 | 94 ± 27 | |
| Elhakim6 | D 2 mg + O 4 mg | 30 | 42 | 3/22 | 72 ± 8 | 160 ± 5 | — | 106 ± 23 |
| D 4 mg + O 4 mg | 30 | 41 | 3/22 | 70 ± 10 | 162 ± 6 | — | 108 ± 32 | |
| D 8 mg + O 4 mg | 30 | 42 | 4/21 | 71 ± 9 | 164 ± 5 | — | 106 ± 33 | |
| D 16 mg + O 4 mg | 30 | 43 | 3/22 | 72 ± 10 | 160 ± 4 | — | 100 ± 23 | |
| O 4 mg | 30 | 43 | 4/21 | 71 ± 9 | 160 ± 6 | — | 103 ± 37 | |
| Biswas7 | D 8 mg + G 40 μg/kg | 60 | 42.4 | 10/50 | 56 | — | 87 ± 8 | 74 ± 7 |
| G 40 μg/kg | 60 | 41.3 | 14/46 | 54 | — | 90 ± 6 | 75 ± 9 | |
| Nesek-Adam11 | D 8 mg + M 10 mg | 40 | 50.9 ± 14.5 | 14/26 | 68.6 ± 10.9 | 171.3 ± 12.2 | 66 ± 16 | 47 ± 17 |
| D 8 mg | 40 | 49.6 ± 11.7 | 10/30 | 70 ± 12.4 | 170 ± 11.2 | 65 ± 17 | 47 ± 18 | |
| M 10 mg | 40 | 49.4 ± 13.9 | 8/32 | 74.2 ± 14.3 | 173.3 ± 12.7 | 67 ± 28 | 49 ± 25 | |
| Bano12 | D 8 mg + O 4 mg | 49 | 41.4 ± 8.2 | 5/44 | 68.9 ± 5.6 | — | 88.0 ± 5.9 | 61.9 ± 8.1 |
| D 8 mg | 48 | 39.3 ± 8.6 | 7/41 | 69.02 ± 6.3 | — | 88.2 ± 5.5 | 62.9 ± 7.8 | |
| Fujii13 | D 8 mg + P 0.5 mg/kg | 40 | 48 ± 11 | 17/23 | 55 ± 7 | 158 ± 8 | 84 ± 32 | 107 ± 34 |
| P 0.5 mg/kg | 40 | 47 ± 9 | 16/24 | 57 ± 9 | 160 ± 8 | 89 ± 30 | 113 ± 32 | |
| Gautam14 | D 8 mg + O 4 mg | 47 | 38.8 ± 11.2 | 4/43 | 56.6 ± 6.7 | 152 ± 5 | 90.1 ± 20.7 | 72.8 ± 18.6 |
| D 8 mg | 47 | 39.2 ± 8.5 | 5/42 | 61.2 ± 5.7 | 150 ± 6 | 97.9 ± 21.1 | 79.7 ± 19 | |
| O 4 mg | 48 | 38.2 ± 9.2 | 4/44 | 59.9 ± 7.1 | 150 ± 6 | 95.5 ± 20.6 | 77.6 ± 19 | |
| Jo15 | D 8 mg + R 0.3 mg | 40 | 44 ± 10 | 0/40 | 58 ± 8 | 158 ± 5 | 71 ± 15 | 50 ± 15 |
| D 8 mg | 40 | 44 ± 12 | 0/40 | 60 ± 9 | 159 ± 5 | 69 ± 27 | 43 ± 15 | |
| R 0.3 mg | 40 | 48 ± 13 | 0/40 | 58 ± 9 | 158 ± 5 | 67 ± 14 | 44 ± 10 |
D = dexamethasone; Do = dolasetron; G = granisetron; M = metoclopramide; O = ondansetron; P = propofol; R = ramosetron.
3.2. Results of meta-analysis
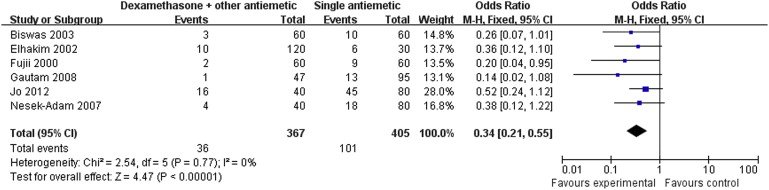
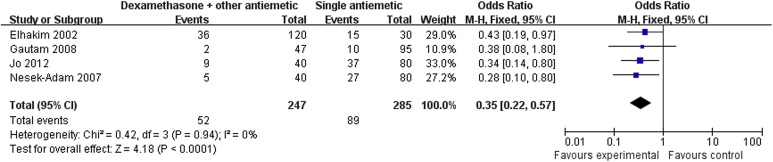
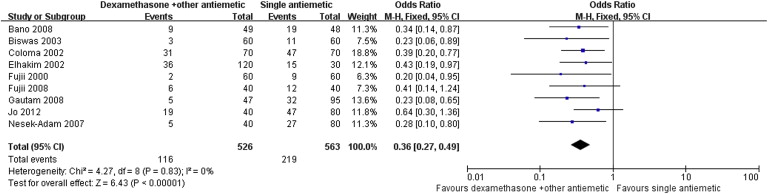
Results of the meta-analysis are presented in Table 3. Pooled analyses showed that combination therapy provided significantly better prophylaxis against PONV after LC than the monotherapy group in the early period (OR: 0.34; 95% CI: 0.21–0.55; p < 0.001; Fig. 1), late period (OR: 0.35; 95% CI: 0.22–0.57; p < 0.001; Fig. 2), and overall period (OR: 0.36; 95% CI: 0.27–0.49; p < 0.001; Fig. 3). Correspondingly, rescue antiemetic usage was significantly less in the combination therapy group (OR: 0.22; 95% CI: 0.12–0.41; p < 0.001). The most frequently reported adverse events were headache, dizziness, and itching. The incidence of adverse events did not differ between the two groups.
| Outcome of interest | No. of studies | No. of patients | Results, % | OR | 95% CI | p | I2 (%) |
|---|---|---|---|---|---|---|---|
| Early period PONV | 6 (4,6,7,11,14,15) | CT = 367, MT = 405 | CT = 9.8, MT = 24.9 | 0.34 | 0.21–0.55 | <0.001 | 0 |
| Late period PONV | 4 (6,11,14,15) | CT = 247, MT = 285 | CT = 21, MT = 31.2 | 0.35 | 0.22–0.57 | <0.001 | 0 |
| Overall period PONV | 9 (4–7,11–15) | CT = 526, MT = 563 | CT = 22, MT = 38.9 | 0.36 | 0.27–0.49 | <0.001 | 0 |
| Rescue | 6 (4,6,7,11,12,14) | CT = 376, MT = 373 | CT = 4.2, MT = 15.8 | 0.22 | 0.12–0.41 | <0.001 | 0 |
| Adverse events | |||||||
| Headache | 6 (4,6,7,11,14,15) | CT = 367, MT = 405 | CT = 7.9, MT = 6.4 | 1.17 | 0.66–2.08 | 0.58 | 0 |
| Dizziness | 6 (4,6,7,11,14,15) | CT = 367, MT = 405 | CT = 3.8, MT = 3.7 | 1.12 | 0.53–2.38 | 0.76 | 0 |
| Itching | 3 (6,12,14) | CT = 216, MT = 173 | CT = 3.2, MT = 4.0 | 0.94 | 0.26–3.38 | 0.92 | 0 |
CI = confidence interval; CT = combination therapy; MT = monotherapy; OR = odds ratio; PONV = postoperative nausea and vomiting.
|
|
|
Figure 1. Forest plot displaying the results of the meta-analysis of postoperative nausea and vomiting in the early period. |
|
|
|
Figure 2. Forest plot displaying the results of the meta-analysis of postoperative nausea and vomiting in the late period. |
|
|
|
Figure 3. Forest plot displaying the results of the meta-analysis of postoperative nausea and vomiting in the overall period. |
3.3. Subgroup analysis
Results of the subgroup analysis are shown in Table 4.
| Outcome of interest | No. of studies | No. of patients | Results, % | OR | 95% CI | p | I2 (%) |
|---|---|---|---|---|---|---|---|
| CT versus dexamethasone alone | |||||||
| Early period PONV | 3 (11,14,15) | CT = 127, MT = 127 | CT = 16.5, MT = 33.8 | 0.30 | 0.15–0.60 | <0.001 | 26 |
| Late period PONV | 4 (6,11,14,15) | CT = 247, MT = 285 | CT = 21.0, MT = 31.2 | 0.35 | 0.22–0.57 | <0.001 | 0 |
| Overall period PONV | 4 (11,12,14,15) | CT = 176, MT = 175 | CT = 21.5, MT = 40.5 | 0.36 | 0.22–0.60 | <0.001 | 0 |
| Rescue | 3 (11,12,14) | CT = 136, MT = 135 | CT = 5.1, MT = 20 | 0.21 | 0.09–0.51 | <0.001 | 0 |
| Adverse events | |||||||
| Headache | 3 (11,14,15) | CT = 127, MT = 127 | CT = 7.8, MT = 4.7 | 1.72 | 0.61–4.90 | 0.31 | 0 |
| Dizziness | 3 (11,14,15) | CT = 127, MT = 127 | CT = 3.1, MT = 3.9 | 0.81 | 0.23–2.90 | 0.75 | 0 |
| Itching | 2 (12,14) | CT = 96, MT = 95 | CT = 2.0, MT = 2.1 | 0.99 | 0.17–5.83 | 0.99 | 0 |
| Dexamethasone plus ondansetron versus ondansetron alone | |||||||
| Early period PONV | 2 (6,14) | CT = 77, MT = 78 | CT = 16.5, MT = 33.8 | 0.20 | 0.04–0.96 | 0.04 | 0 |
| Late period PONV | 2 (6,14) | CT = 77, MT = 78 | CT = 9.0, MT = 29.4 | 0.21 | 0.08–0.55 | <0.001 | 0 |
| Overall period PONV | 2 (6,14) | CT = 77, MT = 78 | CT = 12.9, MT = 39.4 | 0.22 | 0.10–0.50 | <0.001 | 0 |
| Rescue | 2 (6,14) | CT = 77, MT = 78 | CT = 5.1, MT = 24.3 | 0.18 | 0.06–0.54 | 0.002 | 0 |
| Adverse events | |||||||
| Headache | 2 (6,14) | CT = 77, MT = 78 | CT = 7.7, MT = 7.6 | 1.01 | 0.31–3.29 | 0.98 | 0 |
| Dizziness | 2 (6,14) | CT = 77, MT = 78 | CT = 2.5, MT = 2.5 | 1.01 | 0.17–6.00 | 0.99 | 0 |
| Itching | 2 (6,14) | CT = 77, MT = 78 | CT = 2.5, MT = 2.5 | 1.01 | 0.17–6.00 | 0.99 | 0 |
| Dexamethasone plus granisetron versus granisetron alone | |||||||
| Early period PONV | 2 (4,7) | CT = 120, MT = 120 | CT = 4.1, MT = 15.8 | 0.23 | 0.08–0.64 | 0.005 | 0 |
| Overall period PONV | 2 (4,7) | CT = 120, MT = 120 | CT = 4.1, MT = 18.3 | 0.19 | 0.07–0.53 | 0.001 | 0 |
| Rescue | 2 (4,7) | CT = 120, MT = 120 | CT = 0.8, MT = 7.5 | 0.15 | 0.03–0.84 | 0.03 | 0 |
| Adverse events | |||||||
| Headache | 2 (4,7) | CT = 120, MT = 120 | CT = 9.1, MT = 9.1 | 1.00 | 0.42–2.40 | 1.00 | 0 |
| Dizziness | 2 (4,7) | CT = 120, MT = 120 | CT = 6.6, MT = 5.8 | 1.15 | 0.40–2.29 | 0.79 | 0 |
CI = confidence interval; CT = combination therapy; MT = monotherapy; OR = odds ratio; PONV = postoperative nausea and vomiting.
3.3.1. Dexamethasone dose
One study compared four doses of dexamethasone (2 mg, 4 mg, 8 mg, and 16 mg) and found that 8 mg represented the optimal effective dose in combination with ondansetron to prevent PONV after LC.6
3.3.2. Combination therapy versus dexamethasone alone
Dexamethasone 8 mg plus ondansetron 4 mg or metoclopramide 10 mg or ramosetron 0.3 mg was compared with dexamethasone 8 mg alone in four trials.11; 12; 14 ; 15 Analysis of these studies revealed a significantly reduced incidence of PONV and less rescue antiemetic usage in the combination therapy group.
3.3.3. Dexamethasone plus ondansetron versus ondansetron alone
Dexamethasone 8 mg plus ondansetron 4 mg was compared with ondansetron 4 mg alone in two trials.6 ; 14 As Table 4 shows, our results indicate that PONV incidence and rescue antiemetic usage were significantly lower in the dexamethasone plus ondansetron group.
3.3.4. Dexamethasone plus granisetron versus granisetron alone
Pooled analyses of two trials showed that dexamethasone 8 mg plus granisetron 40 μg/kg provided significantly better prophylaxis against PONV than granisetron 40 μg/kg alone.4 ; 7
3.3.5. Dexamethasone plus dolasetron versus dolasetron alone
Dexamethasone 4 mg plus dolasetron 12.5 mg was compared with dolasetron 12.5 mg alone in one trial.5 Although the incidences of nausea (35%) and vomiting (8%) were lower in the combination group, this difference did not achieve statistical significance (p = 0.1 and p = 0.3, respectively).
3.3.6. Dexamethasone plus ramosetron versus ramosetron alone
In one study, the antiemetic efficacy of the combination of ramosetron 0.3 mg and dexamethasone 8 mg was found to be superior to that of ramosetron 0.3 mg alone (p < 0.05). 15
3.3.7. Dexamethasone plus metoclopramide versus metoclopramide alone
Dexamethasone 8 mg plus metoclopramide 10 mg was compared with metoclopramide 10 mg alone in one trial.11 The authors found that patients receiving combination therapy had significantly less PONV than those administered 10 mg metoclopramide alone during 24 hours (p = 0.033).
3.3.8. Dexamethasone plus propofol versus propofol alone
In one study, dexamethasone 8 mg combined with propofol 0.5 mg/kg was found to be superior to propofol 0.5 mg/kg alone for prevention of PONV in patients undergoing LC (p = 0.029). 13 No heterogeneity was detected for any outcome assessed.
3.4. Publication bias
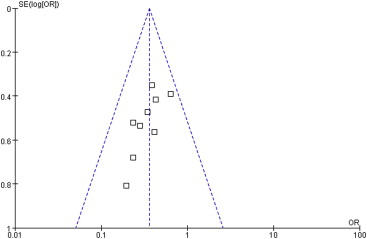
A funnel plot of the studies used in the meta-analysis reporting on PONV in the overall period is shown in Fig. 4. None of the studies lay outside the limits of the 95% CI, indicating no evidence of publication bias.
|
|
|
Figure 4. Funnel plot demonstrating symmetry for postoperative nausea and vomiting in the overall period, indicating no evidence of publication bias. |
4. Discussion
Since its first introduction in 1985, LC has been widely accepted as the standard procedure for gallbladder removal.16 Compared to the open approach,17 the advantages of this procedure included less pain, quicker recovery, shorter hospital stay, and a better cosmetic result.1 However, during the first 24 hours after surgery, 50–70% of LC patients experience PONV,1 which is associated with an increased risk of bleeding, wound dehiscence, aspiration of gastric contents, fluid and electrolyte imbalance, dehydration, delayed hospital discharge, unexpected hospital admission, and decreased satisfaction in surgical patients.18 Several risk factors for PONV have been reported, including female sex, nonsmoking status, a history of motion sickness or previous PONV, and use of postoperative opioids.1
Physiologically, the vomiting reflex is a complex act mediated by the emetic center located in the medulla oblongata. This center receives emetic stimuli from the periphery via afferent neurons of the vagus nerves. Emetic stimuli also come centrally from chemoreceptive trigger zones, vestibular apparatus, solitary tract nucleus, cerebellum, and higher cerebral cortex. These structures are rich in nausea and vomiting related functional receptors (serotonin type 3, dopamine type 2, muscarinic cholinergic type 1, histamine type 1, and opioid).19 There is no single stimulus or cause for PONV, thus, a combination of antiemetics may be more effective than a single antiemetic.
This meta-analysis suggests that dexamethasone combined with other antiemetics provide better prophylaxis against PONV than a single antiemetic drug after LC. The possible mechanisms for increased antiemetic effect are as follows: (1) corticosteroids may affects 5-hydroxytryptamine (5-HT) turnover in the in neural tissue by shunting the metabolism of tryptophan away from 5-HT pathways; (2) corticosteroids may prevent the release of 5-HT in the gut or prevent activation of 5-HT receptors in the gastrointestinal system; and (3) dexamethasone may potentiate the main effect of other antiemetics by sensitizing the pharmacological receptor.20 ; 21
As far as the complication rate is concerned, no trials presented any statistically significant difference among the study groups. The pooled result is also in line with these trials. No corticosteroid-related adverse effects, such as increased risk of infection, delayed wound healing, glucose intolerance, and adrenal suppression, were noted in the combination therapy group. The safety and effectiveness of such prophylaxis was confirmed in this analysis.
Seven RCTs have been undertaken to investigate the antiemetic efficacy of the combination of dexamethasone and 5-HT3 antagonists (ondansetron, granisetron, dolasetron, and ramosetron). Only one trial failed to find that the combination was superior to the single antiemetic drug in reducing the incidence of PONV.5 These results could have been biased by the inefficient dose of dexamethasone (4 mg) used. Currently, the recommended dose of dexamethasone in the prevention of PONV is 8–10 mg. Dexamethasone has been proven to be a less efficacious antiemetic agent at a dose of 4 mg.6
Metoclopramide acts on central dopaminergic receptors, on both central and peripheral 5-HT3 receptors and on peripheral 5-HT4 receptors. It is a prokinetic drug that increases gastric and small intestinal motility.22 One of the trials included in the current analysis showed that patients who received dexamethasone 8 mg after the induction of anesthesia and metoclopramide 10 mg at the end of LC had significantly less PONV than those administered 10 mg metoclopramide along or saline placebo.11 By contrast, Maddali et al23 demonstrated that combination of dexamethasone 8 mg and metoclopramide 10 mg prior to induction of general anaesthesia does not appear to be an effective antiemetic in diagnostic gynecological laparoscopic procedures. This difference must have been attributable to timing of the metoclopramide administration. Metoclopramide is a short-lasting drug, which has a serum half-life of 2.5–6 hours. Therefore, its prophylactic antiemetic effect at the start of surgery may have worn off by the time the patient is fully awake.24
Propofol has an antiemetic effect even though the exact mechanism of action is still unclear. Possible explanations include blocking the 5-HT3 receptor of the serotonergic system, and inhibition of the chemoreceptor trigger zone and vagal nuclei.25 In one study, dexamethasone 8 mg combined with propofol 0.5 mg/kg was found to be superior to propofol 0.5 mg/kg alone for prevention of PONV in patients undergoing LC (p = 0.029). 13
Our study had some limitations. Although combination of dexamethasone and 5-HT3 antagonists is likely to be the most commonly used combination regimen, the high cost of 5-HT3 antagonists remains a major concern in current health care systems. By contrast, metoclopramide and propofol are inexpensive drugs. Further investigation is therefore required to provide an accurate cost-effectiveness comparison between different combination regimens. Another limitation was that studies published in languages other than English were not included in the review, which may have introduced publication bias. However, there was evidence that language restriction of a meta-analysis other than English did not lead to bias estimates of intervention effectiveness.26
In conclusion, this meta-analysis showed the benefits of dexamethasone combined with other antiemetics in the prophylaxis of PONV in patients undergoing LC. All the studies included in this analysis were randomized double-blind design with higher quality. Additionally, there was no evidence of heterogeneity between studies (I2 = 0%) or publication bias. We therefore believe that the results are reliable. It is evident that the use of prophylactic antiemetic therapy in patients at high risk was more effective in preventing PONV and achieved greater patient satisfaction at a lower cost compared with placebo.27 Combination prophylaxis should be considered particularly for patients with high risk factors.
Acknowledgments
The authors thank Yong Seon Choi for kindly providing more details about their reported cases. No financial support was received for this study.
References
- 1 Y. Fujii; Management of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy; Surg Endosc, 25 (2011), pp. 691–695
- 2 I. Henzi, B. Walder, M.R. Tramèr; Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review; Anesth Analg, 90 (2000), pp. 186–194
- 3 A.S. Habib, T.J. Gan; Combination therapy for postoperative nausea and vomiting – a more effective prophylaxis?; Ambul Surg, 9 (2001), pp. 59–71
- 4 Y. Fujii, Y. Saitoh, H. Tanaka, H. Toyooka; Granisetron/dexamethasone combination for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy; Eur J Anaesthesiol, 17 (2000), pp. 64–68
- 5 M. Coloma, P.F. White, S.D. Markowitz, et al.; Dexamethasone in combination with dolasetron for prophylaxis in the ambulatory setting: effect on outcome after laparoscopic cholecystectomy; Anesthesiology, 96 (2002), pp. 1346–1350
- 6 M. Elhakim, M. Nafie, K. Mahmoud, A. Atef; Dexamethasone 8 mg in combination with ondansetron 4 mg appears to be the optimal dose for the prevention of nausea and vomiting after laparoscopic cholecystectomy; Can J Anaesth, 49 (2002), pp. 922–926
- 7 B.N. Biswas, A. Rudra; Comparison of granisetron and granisetron plus dexamethasone for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy; Acta Anaesthesiol Scand, 47 (2003), pp. 79–83
- 8 K. Leksowski, P. Peryga, R. Szyca; Ondansetron, metoclopramide, dexamethasone, and their combinations compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a prospective randomized study; Surg Endosc, 20 (2006), pp. 878–882
- 9 J.C. Bailar 3rd; The practice of meta-analysis; J Clin Epidemiol, 48 (1995), pp. 149–157
- 10 A.R. Jadad, R.A. Moore, D. Carroll, et al.; Assessing the quality of reports of randomized clinical trials: is blinding necessary?; Control Clin Trials, 17 (1996), pp. 1–12
- 11 V. Nesek-Adam, E. Grizelj-Stojcić, Z. Rasić, Z. Cala, V. Mrsić, A. Smiljanić; Comparison of dexamethasone, metoclopramide, and their combination in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy; Surg Endosc, 21 (2007), pp. 607–612
- 12 F. Bano, S. Zafar, S. Aftab, S. Haider; Dexamethasone plus ondansetron for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a comparison with dexamethasone alone; J Coll Physicians Surg Pak, 18 (2008), pp. 265–269
- 13 Y. Fujii, M. Nakayama; Prevention of postoperative nausea and vomiting with a small dose of propofol alone and combined with dexamethasone in patients undergoing laparoscopic cholecystectomy: a prospective, randomized, double-blind study; Surg Endosc, 22 (2008), pp. 1268–1271
- 14 B. Gautam, B.R. Shrestha, P. Lama, S. Rai; Antiemetic prophylaxis against postoperative nausea and vomiting with ondansetron-dexamethasone combination compared to ondansetron or dexamethasone alone for patients undergoing laparoscopic cholecystectomy; Kathmandu Univ Med J (KUMJ), 6 (2008), pp. 319–328
- 15 Y.Y. Jo, J.W. Lee, J.K. Shim, W.K. Lee, Y.S. Choi; Ramosetron, dexamethasone, and their combination for the prevention of postoperative nausea and vomiting in women undergoing laparoscopic cholecystectomy; Surg Endosc, 26 (2012), pp. 2306–2311
- 16 W. Reynolds Jr.; The first laparoscopic cholecystectomy; JSLS, 5 (2001), pp. 89–94
- 17 G. Aprea, E. Coppola Bottazzi, F. Guida, S. Masone, G. Persico; Laparoendoscopic single site (LESS) versus classic video-laparoscopic cholecystectomy: a randomized prospective study; J Surg Res, 166 (2011), pp. e109–e112
- 18 J.B. Rose, M.F. Watcha; Postoperative nausea and vomiting in paediatric patients; Br J Anaesth, 83 (1999), pp. 104–117
- 19 C.M. Ho, H.L. Wu, S.T. Ho, J.J. Wang; Dexamethasone prevents postoperative nausea and vomiting: benefit versus risk; Acta Anaesthesiol Taiwan, 49 (2011), pp. 100–104
- 20 M. Frederikson, T. Mursti, C. Furst, et al.; Nausea in cancer chemotherapy is inversely related to urinary cortisol excretion; Br J Cancer, 65 (1992), pp. 779–780
- 21 S. Sager; The current role of antiemetic drugs in oncology: a recent revolution in patient symptom control; Cancer Treat Rev, 18 (1991), pp. 95–135
- 22 I. Henzi, B. Walder, M.R. Tramèr; Metoclopramide in the prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized, placebo-controlled studies; Br J Anaesth, 83 (1999), pp. 761–771
- 23 M.M. Maddali, J. Mathew, J. Fahr, A.W. Zarroug; Postoperative nausea and vomiting in diagnostic gynaecological procedure: comparison of the efficacy of the combination of dexamethasone and metoclopramide with that of dexamethasone and ondansetron; J Postgrad Med, 49 (2003), pp. 302–306
- 24 H. Quaynor, J.C. Raeder; Incidence and severity of postoperative nausea and vomiting are similar after metoclopramide 20 mg and ondansetron 8 mg given by the end of laparoscopic cholecystectomies; Acta Anaesth Scand, 46 (2002), pp. 109–113
- 25 Y.C. Yoo, S.J. Bai, K.Y. Lee, S. Shin, E.K. Choi, J.W. Lee; Total intravenous anesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot-assisted laparoscopic radical prostatectomy: a prospective randomized trial; Yonsei Med J, 53 (2012), pp. 1197–1202
- 26 D. Moher, B. Pham, T.P. Klassen, et al.; What contributions do languages other than English make on the results of meta-analyses?; J Clin Epidemiol, 53 (2000), pp. 964–972
- 27 R.P. Hill, D.A. Lubarsky, B. Phillips-Bute, et al.; Cost-effectiveness of prophylactic antiemetic therapy with ondansetron, droperidol, or placebo; Anesthesiology, 92 (2000), pp. 958–967
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?