Summary
Inflammatory myofibroblastic tumors (IMTs) are rare soft-tissue tumors that can occur at virtually any anatomical site. We report the case of a 58-year-old male with an IMT of the fourth part of the duodenum who presented with signs and symptoms of high intestinal obstruction and bilious vomiting. The patient underwent a surgical resection of the fourth part of the duodenum with end-to-end duodenojejunal anastomosis. The follow-up period of 6 months was uneventful with no evidence of recurrence. According to our knowledge, only six cases of duodenal IMTs have been reported in the literature thus far, and this is the first report of a duodenal IMT sited at the fourth part of the duodenum. The duodenum is among the rarest sites of IMTs. Signs and symptoms resulting from diagnostic imaging investigations are nonspecific and inadequate to obtain diagnosis accurately. In most cases, surgical treatment is considered a cure for IMTs. There is no evidence of deaths caused by duodenal IMT. IMT of the duodenum is a possible diagnosis in differential diagnosis of tumor-like lesions of the duodenum.
Keywords
duodenal neoplasms;inflammatory pseudotumor;neoplasm;soft tissue
1. Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare tumors histologically composed of spindle myofibroblasts and an inflammatory infiltrate dominated by plasma cells, lymphocytes, and eosinophils.1 According to the World Health Organization, IMTs belong to a group of soft-tissue tumors, a subset of fibroblastic/myofibroblastic tumors.2 These principally occur in soft tissues and visceral organs at possibly any anatomical location. Nevertheless, duodenal IMTs remain an extremely rare condition. We report a case of IMT of the fourth part of the duodenum. Only six cases of duodenal IMTs have been reported in literature thus far. We present a review of these cases.3; 4; 5; 6; 7 ; 8
2. Case report
A 58-year-old male who presented with a 1-week history of an intermittent epigastric pain, bilious vomiting, and unintentional weight loss of 10 kg in 4 weeks was admitted to the Department of Surgery of Clinical University Hospital Center. His medical history was significant for gastroesophageal reflux disease (GERD), Helicobacter pylori gastritis, Gilberts syndrome, and acute pancreatitis of unknown exact cause 2 months ago, which was successfully treated conservatively. Multiple abdominal ultrasonographies (USs) and multislice computed tomography (MSCT) scans excluded cholelithiasis and there was no evidence of alcohol abuse in the patients history (gamma glutamyl transferase: 17 U/L). Other causes of acute pancreatitis could neither be proven with certainty nor excluded.
A physical examination demonstrated abdominal bloating and visible distension with pain on palpation of the epigastric area of the abdomen. There was no jaundice, fever, or anemia. Laboratory tests revealed slightly elevated levels of aspartate transaminase (46 U/L), alanine transaminase (72 U/L), total bilirubin (58 μmol/L), and direct bilirubin (10 μmol/L). Other laboratory examinations analyzing the levels of C-reactive protein, erythrocyte sedimentation rate, complete blood count, coagulation factors, urea, creatinine, lipidogram, serum amylase, electrolytes including calcium, phosphorus, and magnesium were all within normal limits. Tumor markers such as cancer antigen 19-9, alpha-fetoprotein, and cancer antigen 125 were all negative.
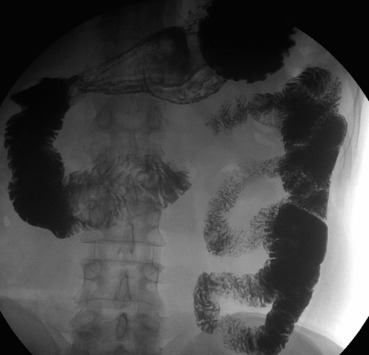
Abdominal X-ray, abdominal US, and esophagogastroduodenoscopy showed no significant findings. Contrast imaging of the small intestine displayed a severe stricture of the ascending duodenal portion (D4) measuring 0.8 cm in diameter with normal duodenal mucosal folds and normal morphology of the horizontal and descending portions of the duodenum (Fig. 1).
|
|
|
Figure 1. Contrast imaging of the small intestine displays the narrowing of the ascending duodenum. |
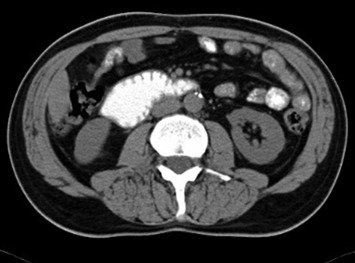
The MSCT scan of the abdomen revealed a filling defect in the fourth part of the duodenum measuring 4.3 × 3.6 cm2 and dilatation of the second and third part of the duodenum. Triangle-shaped infiltration area of the adipose tissue measuring 2.1 × 1.9 cm2 was identified cranial to the fourth part of the duodenum (Fig. 2). No regional lymphadenopathy or focal lesions of parenchymatous organs of the abdomen suggestive of metastatic lesions were identified.
|
|
|
Figure 2. Multislice computed tomography demonstrates a filling defect in the fourth part of the duodenum. |
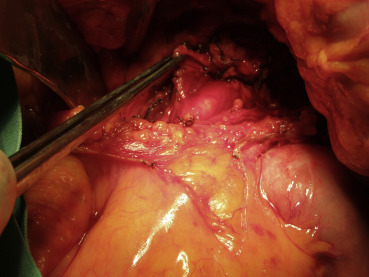
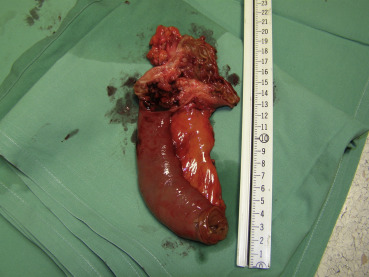
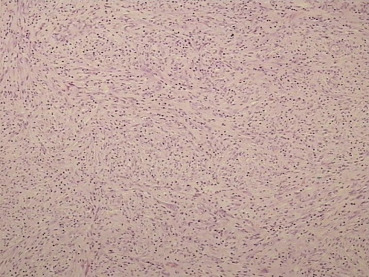
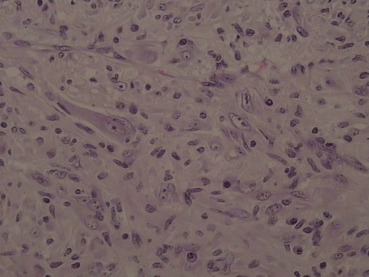
Because imaging findings could not exclude malignancy and the mass appeared to be resectable, a surgical exploration was performed through an upper midline laparotomy. A firm, elastic tumor measuring 4 cm was found in the fourth part of the duodenum, adjacent to the paraduodenal adipose tissue and peritoneum surrounding the duodenojejunal flexure. The head of the pancreas and the hepatoduodenal ligament were intact. After the right colon and the mesenteric root mobilization, superior mesenteric artery 5 cm in length was exposed, and resection of the duodenojejunal flexure measuring 15 cm in length with 2-cm surgical resection margin and clearance of loco-regional lymph nodes were performed (Fig. 3). End-to-end duodenojejunal anastomosis with single-layer continuous suture was made for reconstruction. Macroscopic pathology demonstrated a segment of the duodenum 15.5 cm in length and 3.2 cm in diameter with exophytic polypoid tumor measuring 2.1 × 1.6 cm2 arising from the duodenal wall (Fig. 4). Histologically, the tumor was composed of spindle-shaped interspersed myofibroblasts separated with fibrous stroma infiltrated by predominantly mononuclear inflammatory cells (Figs. 5 and 6). The tumor cells were immunohistochemically positive for actin, vimentin, AE1/AE3, and negative for nonspecific esterase, CD34, CD117, S-100, and DOG1. The resection margins were clear of malignancy. In the surrounding adipose tissue, seven lymph nodes were found, which had a diameter from 0.4 to 1.2 cm, uninvolved with tumor tissue. A postoperative course was uneventful with complete resolution of patients symptoms. Six months after the surgery the patient remained asymptomatic with no evidence of recurrence detected by the MSCT scans.
|
|
|
Figure 3. Surgical exploration of the tumor and the demonstration of the superior mesenteric artery. |
|
|
|
Figure 4. Segment of the duodenum 15.5 cm in length and 3.2 cm in diameter with exophytic polypoid tumor measuring 2.1 × 1.6 cm arising from the duodenal wall. |
|
|
|
Figure 5. Photomicrograph demonstrating an admixture of spindle-shaped and ovoid cells with a prominent inflammatory infiltrate (hematoxylin–eosin; low-power view; magnification 10×). |
|
|
|
Figure 6. Photomicrograph showing a conspicuous admixture of lymphocytes and plasma cells (high-power view; magnification 40×). |
3. Discussion
Different terms for IMT have been used—inflammatory pseudotumor,9 fibrous xanthoma, plasma cell granuloma,10 pseudosarcoma, lymphoid hamartoma, myxoid hamartoma,11 inflammatory myofibrohistiocytic proliferation, pseudosarcomatous myofibroblastic proliferation.12 The term “inflammatory pseudotumor” has for many years been used for any clinically, macroscopic, or microscopic tumor-like lesion caused by inflammatory or reactive process.13 Later, the term has been applied only to neoplastic lesions microscopically characterized by the proliferation of spindle cells of mesenchymal origin with morphological characteristics of myofibroblasts and large inflammatory infiltration of different types of cells, usually with predominance of mature lymphocytes and plasma cells, regardless of etiology. However, during the last two decades, due to a greater understanding of the importance of myofibroblastic component in relation to inflammatory component1 ; 14 and additional electron microscopic and immunohistochemical findings, this term has entirely replaced other terms.15
IMTs occur mainly in children and young adults, with more reports published on IMTs in adults recently.16 ; 17 However, their etiology is unknown. Two basic hypotheses, reactive and neoplastic, are complemented with presumably infectious and autoimmune hypotheses. However, the exact infectious agents are not known. Some relate IMTs to Epstein–Barr virus, human herpesvirus-8, the bacterium Eikenella corrodens, and schistosomiasis. 18; 19; 20 ; 21 Others claim that previous surgical manipulation and chemotherapy or radiotherapy are potential causes. 22; 23 ; 24 Some studies suggest the possible autoimmune origin of IMTs.25 According to these hypotheses, a medical history of acute pancreatitis, GERD, and H. pylori gastritis could be contributing factors in our case.
Based on the anatomical site, IMTs can be pulmonary and extrapulmonary.17 Extrapulmonary IMTs are most frequent in the abdomen including the mesentery and omentum.16 Other sites of the gastrointestinal tract are all visceral organs such as the appendix, Meckels diverticulum, and Vaters papilla.3; 22; 24; 26 ; 27 Duodenal IMTs are among the rarest. To our knowledge, only six cases of duodenal IMTs have been reported, five of which had anatomical sites at the first and second parts of the duodenum, and one, particularly interesting, multiple IMT arising from the first part of the duodenum and extending up to the first part of the jejunum.3; 4; 5; 6; 7 ; 8 According to the available literature, our patient is the first with IMT localization in the fourth part of the duodenum (Table 13–8). Specific risk factors are not defined and potential risk factors of duodenal IMT are provided in Table 1. Considering these cases, there seems to be no gender or age predilection (Table 1). Signs and symptoms of abdominal IMTs often mimic malignant tumors.22 ; 28 Duodenal IMTs mostly present with upper gastrointestinal-tract obstructive symptoms, nausea, vomiting, pain in the epigastric area, anorexia, weight loss, malaise and fatigue, while in one of the cases night sweats were referred to as one of the significant symptoms. None of the cases presented with jaundice.3; 4; 5; 6; 7 ; 8 Bilious vomiting, present in our patient, was not described previously (Table 1).
| Author | Segment of duodenum | Age (y)/sex | Symptoms and signs | Treatment | Recurrence (treatment) | Potential risk factors |
|---|---|---|---|---|---|---|
| Fong et al3 | Second/third | 57/F | Gastric outlet obstruction | Whipple operation | None | No report |
| Stringer et al4 | Second | 5/F | Nonbilious vomiting; significant weight loss | Whipple operation with standard reconstruction | None | None |
| Mattei and Barnaby5 | First/second | 13/M | Asymptomatic (incidentally diagnosed IMT) | Intravenous NSAID application (ketorolac); laparotomy (tumor mass regression)—Roux-en-Y gastrojejunostomy | None | Paratesticular rhabdomyosarcoma |
| Wynn et al6 | First | 16/M | Abdominal pain; night sweats; malaise | En-bloc excision with primary closure of the duodenum | Yes (perioral corticosteroids; azathioprine) | No report |
| Mirshemirani et al7 | First | 13/M | Abdominal pain; weight loss; epigastric mass | Surgical resection of the tumor; duodeno–duodenostomy | None | No report |
| Xiang et al8 | Multiple IMT (from the first part of the duodenum to starting part of the jejunum) | 20/M | Intermittent right epigastric pain; nausea; vomiting | Whipple operation | None | Calculous colecystits |
| Current study | Fourth | 58/M | Bilious vomiting; epigastric pain; significant weight loss | Resection of the duodenojejunal flexure with termino-terminal anastomosis | None | Acute pancreatitis; GERD; H. pylori gastritis |
GERD = gastroesophageal reflux disease; IMT = inflammatory myofibroblastic tumor; NSAID = nonsteroidal anti-inflammatory drug.
Diagnostic imaging techniques demonstrate tumor mass with its extent without specific features necessary for obtaining accurate diagnosis. Stricture of the duodenum, impossible passage, and irregular masses arising from different parts of the duodenum are most usual findings on radiological examination. It can be difficult to distinguish duodenal IMT from other groups of soft-tissue tumors or even pure (post)inflammatory strictures due to long-standing ulcer or diverticulitis. Stenosis due to duodenal ulcer can be divided into acute/active and chronic ulceration. Acute ulceration results in inflammatory edema and pylorospasm. Signs and symptoms of stenosis may regress if the ulcer heals under medical treatment, which is not noticed in IMT cases. Long-standing ulceration can present with radiological demonstration of scarring and deformities of the duodenum. In most cases, there are no signs of tumorous mass. Past medical history of peptic ulcer-type pain for 10 or more years strongly suggests the precise diagnosis29 ; 30 contrary to duodenal diverticula, which are the uncommon site of inflammation due to their large size and mostly sterile content of the duodenum. The incidence of duodenal diverticula is approximately 22% with the incidence of complicated duodenal diverticulum estimated from 0.03% to 5%. Inflammation of the diverticulum can be distinguished before surgery by meticulous CT scan analysis. The CT findings vary between saccular and tubular configuration, diverticular content (homogenous liquid, liquid debris, and hydroaeric levels), wall (thickened, enhanced, and thin), retroperitoneal infiltration, free air, and signs of common bile duct dilatation.31 ; 32
Definitive diagnosis of IMTs is confirmed by histopathological analysis and immunohistochemical tests of surgically removed specimen. Some histopathological IMT patterns such as compact fascicular spindle cells with myxoid or collagenized regions intermingled with inflammatory cells may resemble fibromatoses. Fibromatosis colli, juvenile hyaline fibromatosis, superficial fibromatosis, desmoid-type fibromatosis, and lipofibromatosis are well-defined soft-tissue tumors histopathologically differentiated from IMT. These benign neoplasms can extend into adjacent tissues, and sometimes have a tendency toward local recurrence, but do not metastasize. Except superficial fibromatosis and desmoid-type fibromatosis, other entities of this group occur mostly in infants or early childhood with no sex predilection. Superficial and desmoid-type fibromatosis occur mostly in adults with slight predominance in men of first entity and in women of second entity. To the best of our knowledge and according to available literature, no cases of fibromatoses of duodenum were reported so far.2
The basic principle for IMTs treatment is complete surgical resection.16 In nonresectable tumors, a palliative treatment regimen with radiotherapy and nonsteroidal anti-inflammatory drugs (NSAIDs) can be applied. However, guidelines for this treatment approach do not exist. Some studies recommend the use of NSAIDs, corticosteroids, and immunosuppressive drugs after the surgery. One case of the duodenal IMT recurrence was successfully treated by the application of NSAIDs and immunosuppressive drugs.5; 6 ; 33 IMTs are among the increasing number of diseases in which aberrant anaplastic lymphoma kinase gene (ALK) has been implicated in their pathogenesis.34 Considering ALK expression in approximately 50% of IMTs, abrogation of ALK signaling through direct kinase inhibition has become an attractive therapeutic intervention point. So far, IMTs are among particularly promising tumors for targeted therapy using crizotinib, a small-molecule inhibitor.35
Surgical excision of the tumor is considered to cure IMTs; however, due to its potential recurrence capability, a long-term follow-up is recommended. Relapse predictors are not known. Combination of atypia, ganglion-like cells, ALK positivity, and aneuploidy suggests more aggressive potential. The main prognostic indicator is the adequacy of the primary excision.2 According to available literature, there are no general IMT survival statistics. These previous studies are mainly related to low mortality rate due to IMT, rare distant metastases, and rare recurrence of the disease. In Coffins review of 84 cases of extrapulmonary IMTs after clinical follow-up in 53 cases, 44 had no evidence of disease, four had IMT, and five died due to complications related to the location of the lesion or the treatment of the disease.17 Moreover, Coffin et al define IMT as a nonmetastasizing proliferation of myofibroblasts. Only a few cases described in the literature specify IMT as a cause of death, mostly due to the localization of the tumor (e.g., heart).36
A review of the treatment methods for duodenal IMTs is provided in Table 1. There were no complications reported during postoperative recovery. One patient developed a recurrence 6 months after the surgery, which was successfully treated by immunosuppressive drugs (Table 1). During the follow-up of 2 months to 2 years, all patients including ours, were alive with no evidence of recurrence. According to these reports, IMT of the duodenum can be considered as a tumor with good prognosis, low mortality rate, and low recurrence potential.
In conclusion, the duodenum is among the rarest sites of IMTs. Signs and symptoms as results of diagnostic imaging investigations are nonspecific and inadequate to accurately obtain diagnosis. The diagnosis can be confirmed only by a histopathological analysis and immunohistochemical tests of the surgically removed specimens. In most cases of duodenal IMTs, surgical treatment is considered to cure the disease. There is no evidence of deaths caused by duodenal IMTs. All reported patients with duodenal IMTs had an uneventful recovery. Duodenal IMT should be considered in differential diagnosis of tumor-like lesions of the duodenum.
References
- 1 G. Pettinato, J.C. Manivel, N. De Rosa, L.P. Dehner; Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations; Am J Clin Pathol, 94 (1990), pp. 538–546
- 2 C.D.M. Fletcher, K. Unni, F. Mertens (Eds.), World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone (vol. 2), IARC Press, Lyon (2002), pp. 91–93
- 3 S.S. Fong, C. Zhao, W.M. Yap, S.C. Loke, K.H. Lim; Inflammatory myofibroblastic tumour of the duodenum; Singapore Med J, 53 (2012), pp. e28–e31
- 4 M.D. Stringer, P. Ramani, C.K. Yeung, S.N. Capps, E.M. Kiely, L. Spitz; Abdominal inflammatory myofibroblastic tumours in children; Br J Surg, 79 (1992), pp. 1357–1360
- 5 P. Mattei, K. Barnaby; Rapid regression of duodenal inflammatory myofibroblastic tumor after intravenous ketorolac: case report and review of the literature; J Pediatr Surg, 43 (2008), pp. 1196–1199
- 6 G.R. Wynn, A. Giles, A.S. Steger, M.L. Wilkinson; Case report: inflammatory myofibroblastic tumor of the duodenum; J Gastrointest Cancer, 39 (2008), pp. 79–81
- 7 A. Mirshemirani, A.K. Tabari, N. Sadeghian, S. Shariat-Torbaghan, M. Pourafkari, L. Mohajerzadeh; Abdominal inflammatory myofibroblastic tumor: report on four cases and review of literature; Iran J Pediatr, 21 (2011), pp. 543–548
- 8 J. Xiang, X. Liu, S. Wu, Y. Lv, H. Wang; Multiple inflammatory myofibroblastic tumor of the duodenum: case report and literature review; J Gastrointest Surg, 16 (2012), pp. 1442–1445
- 9 J.P. Wu, E.J. Yunis, G. Fetterman, W.F. Jaeschke, E.F. Gilbert; Inflammatory pseudo-tumours of the abdomen: plasma cell granulomas; J Clin Pathol, 26 (1973), pp. 943–948
- 10 H. Spencer; The pulmonary plasma cell/histiocytoma complex; Histopathology, 8 (1984), pp. 903–916
- 11 F. Gonzalez-Crussi, D.E. deMello, C. Sotelo-Avila; Omental-mesenteric myxoid hamartomas. Infantile lesions simulating malignant tumors; Am J Surg Pathol, 7 (1983), pp. 567–784
- 12 T.T. Tang, A.D. Segura, H.W. Oechler, et al.; Inflammatory myofibrohistiocytic proliferation simulating sarcoma in children; Cancer, 65 (1990), pp. 1626–1634
- 13 W.O. Umiker, L. Iverson; Postinflammatory tumors of the lung; report of four cases simulating xanthoma, fibroma, or plasma cell tumor; J Thorac Surg, 28 (1954), pp. 55–63
- 14 B.C. Gleason, J.L. Hornick; Inflammatory myofibroblastic tumours: where are we now?; J Clin Pathol, 61 (2008), pp. 428–437
- 15 P. Czauderna, K. Schaarschmidt, L. Komasara, et al.; Abdominal inflammatory masses mimicking neoplasia in children—experience of two centers; Pediatr Surg Int, 21 (2005), pp. 346–350
- 16 C.M. Coffin, P.A. Humphrey, L.P. Dehner; Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey; Semin Diagn Pathol, 15 (1998), pp. 85–101
- 17 C.M. Coffin, J. Watterson, J.R. Priest, L.P. Dehner; Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases; Am J Surg Pathol, 19 (1995), pp. 859–872
- 18 T.S. Neuhauser, G.A. Derringer, L.D. Thompson, et al.; Splenic inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunophenotypic study of 12 cases; Arch Pathol Lab Med, 125 (2001), pp. 379–385
- 19 S.H. Lee, Y.C. Fang, J.P. Luo, H.I. Kuo, H.C. Chen; Inflammatory pseudotumour associated with chronic persistent Eikenella corrodens infection: a case report and brief review; J Clin Pathol, 56 (2003), pp. 868–870
- 20 J.J. Gómez-Román, G. Ocejo-Vinyals, P. Sánchez-Velasco, E.H. Nieto, F. Leyva-Cobián, J.F. Val-Bernal; Presence of human herpesvirus-8 DNA sequences and overexpression of human IL-6 and cyclin D1 in inflammatory myofibroblastic tumor (inflammatory pseudotumor); Lab Invest, 80 (2000), pp. 1121–1126
- 21 V.L. Pannain, J.V. Passos, A. Rocha Filho, C. Villela-Nogueira, A. Caroli-Bottino; Aggressive inflammatory myofibroblastic tumor of the liver with underlying schistosomiasis: a case report; World J Gastroenterol, 16 (2010), pp. 4233–4236
- 22 S.J. Kim, W.S. Kim, J.E. Cheon, et al.; Inflammatory myofibroblastic tumors of the abdomen as mimickers of malignancy: imaging features in nine children; AJR Am J Roentgenol, 193 (2009), pp. 1419–1424
- 23 G.M. Vujanić, D. Milovanović, S. Aleksandrović; Aggressive inflammatory pseudotumor of the abdomen 9 years after therapy for Wilms tumor. A complication, coincidence, or association?; Cancer, 70 (1992), pp. 2362–2366
- 24 C.J. Leon, J. Castillo, J. Mebold, L. Cortez, R. Felmer; Inflammatory myofibroblastic tumor of the stomach: an unusual complication after gastrectomy; Gastrointest Endosc, 63 (2006), pp. 347–349
- 25 G. Klöppel, B. Sipos, G. Zamboni, M. Kojima, T. Morohoshi; Autoimmune pancreatitis: histo- and immunopathological features; J Gastroenterol, 42 (2007), pp. 28–31
- 26 E.M. Lopez-Tomassetti Fernandez, H.D. Luis, A.M. Malagon, I.A. Gonzalez, A.C. Pallares; Recurrence of inflammatory pseudotumor in the distal bile duct: lessons learned from a single case and reported cases; World J Gastroenterol, 12 (2006), pp. 3938–3943
- 27 S. Pungpapong, X.J. Geiger, M. Raimondo; Inflammatory myofibroblastic tumor presenting as a pancreatic mass: a case report and review of the literature; JOP, 5 (2004), pp. 360–367
- 28 C.J. Sambhaji, A. Chauhan, C. Kakkar; Inflammatory myofibroblastic tumor mimics an abdominal neoplasm; Gastrointest Cancer Res, 3 (2009), pp. 254–255
- 29 H. Ellis; Stenosis due to duodenal ulceration; Postgrad Med J, 42 (1966), pp. 778–782
- 30 V.H. Low, L.E. Heyneman, D.M. Hough; Inflammatory pseudotumour of the post-bulbar duodenum; Australas Radiol, 42 (1998), pp. 370–373
- 31 T. de Perrot, P.A. Poletti, C.D. Becker, A. Platon; The complicated duodenal diverticulum: retrospective analysis of 11 cases; Clin Imaging, 36 (2012), pp. 287–294
- 32 T.Y. Ming, H.K. Feng, Y.J. Cherng, C.D. Chuan, L.T. Pai, L.Y. Chi; Clinical challenge: diverticulitis of third and fourth portion of the duodenum with perforation; Rev Esp Enferm Dig, 104 (2012), pp. 156–157
- 33 W. Su, A. Ko, T. O'Connell, H. Applebaum; Treatment of pseudotumors with nonsteroidal antiinflammatory drugs; J Pediatr Surg, 35 (2000), pp. 1635–1637
- 34 C.M. Coffin, A. Patel, S. Perkins, K.S. Elenitoba-Johnson, E. Perlman, C.A. Griffin; ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor; Mod Pathol, 14 (2001), pp. 569–576
- 35 J.E. Butrynski, D.R. D'Adamo, J.L. Hornick, et al.; Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor; N Engl J Med, 363 (2010), pp. 1727–1733
- 36 L. Li, A. Burke, J. He, L. Chang, H.R. Zielke, D.R. Fowler; Sudden unexpected death due to inflammatory myofibroblastic tumor of the heart: a case report and review of the literature; Int J Legal Med, 125 (2011), pp. 81–85
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?