(Created page with "==Summary== ====Background==== This study compared the resistance of glued versus stapled anastomosis of the colon to intraluminal pressures at different times during healin...") |
m (Scipediacontent moved page Draft Content 696573618 to Paral et al 2014a) |
(No difference)
| |
Latest revision as of 12:31, 26 May 2017
Summary
Background
This study compared the resistance of glued versus stapled anastomosis of the colon to intraluminal pressures at different times during healing.
Methods
Forty seven female domestic pigs, mean weight of 30.7 kg, were used. Maximum physiological luminal pressures, i.e. the pressure resisted by a catheter inserted into the intestinal lumen via a puncture without it being released and without injury to the surrounding intestinal wall, was performed in 5 control animals. The remaining 42 animals were divided into 3 groups of 14 animals each based on time from anastomosis construction. Each group was divided into 2 subgroups with stapled or glued anastomoses. Intraluminal pressure was measured on the first, third, and fifth day post-surgery.
Results
The maximum pressures resisted by anastomoses were significantly higher than the physiological pressures in all groups. At all time points, stapled anastomoses resisted higher intraluminal pressures than glued ones. However, glued anastomoses resisted pressures significantly higher than physiological pressure. As healing advanced, glued anastomoses neared the resistance to intraluminal pressures of stapled anastomoses.
Conclusion
Healing with absorbable synthetic glue was as good as with staples. Glued anastomoses resisted pressures that were statistically significantly higher than physiological intraluminal colon pressures but lower than stapled ones.
Keywords
anastomosis;colon;cyanoacrylate;glubran 2;intraluminal pressure;tissue glue
1. Introduction
Correct technical execution is a fundamental prerequisite of any successful surgical procedure. Anastomotic dehiscence is one of the most serious postoperative complications of colon surgery. Anastomotic dehiscence occurs in 2–7% of patients following planned colon resection surgery1; 2; 3 ; 4 and in 7–15% of patients following planned rectal surgery.5; 6 ; 7 These complications cause important morbidity and mortality.1; 3 ; 7 The incidence of dehiscence depends on a range of factors that have long been a subject of research and analysis. Many factors have a potential impact on anastomotic healing, including the surgical technique, tension in the area of the anastomosis, the type of sewing material, previous treatment, and the patients overall health, nutritional status, and comorbidities, as well as the experience and erudition of the surgeon.4; 5; 8; 9; 10 ; 11
Parallel to research on factors with a potentially negative impact on anastomotic healing, new materials, surgical methods, and surgical techniques are being explored that could prevent dehiscence of an anastomosis or could minimize the risk of its occurrence. The main, and seemingly simple, aim of anastomosis construction is to secure good edge-to-edge apposition until healing, i.e., at an optimum distance, free from tension, and enabling appropriate blood perfusion of the connected sections while being of an appropriate strength.11
The use of cyanoacrylate tissue glues could be an alternative to the traditional intestinal suture using surgical sewing materials or staples. Considering their mechanical, physical, and biological properties, tissue glues should facilitate optimal bonding between the connected parts of the intestine with negligible negative effects on intestinal wall perfusion.
The results presented here follow on from our previous experience with cyanoacrylate glues which showed that a suitable glue (a combination of N-butyl-2-cyanoacrylate and metacryloxysulpholan) might be used as an alternative to traditional stapled anastomosis of the colon and provide complete healing of the anastomosis without adhesions, excessive fibrotic tissue in the area of anastomosis, or other signs of pathological healing. 12
This multi-stage experimental study is aimed at comprehensively evaluating all aspects and potential uses of glues in colorectal and gastrointestinal surgery. The aim of the study stage presented here was to compare the resistance to intraluminal pressure of a glued versus a stapled anastomosis of the colon at different time points during the healing process.
2. Methods
2.1. Experimental animals
The study animals were 47 female domestic pigs with a mean weight of 30.7 kg. The experiments were conducted in accordance with the Protection of Animals Against Cruelty Act No. 246/92 Coll., as amended. The experiments were approved by the joint departmental committee of the Faculty of Military Health Sciences and Faculty of Medicine, Charles University in Hradec Kralove, Czech Republic.
The sample consisted of a reference group of five control animals and an experimental group of 42 animals. A control measurement of physiological and maximum luminal pressures, i.e., a pressure resisted by the fastening of the measuring catheter inserted into the intestinal lumen via a puncture incision without it being released and without an injury to the surrounding intestinal wall, was performed on the animals in the reference group (Table 1). An additional 42 animals were used in the study comparing the resistance to rising intraluminal pressure of glued versus stapled anastomoses (Table 2).
| Experiment | Resting intraluminal pressure (mmHg) | Maximum pressure (mmHg)a |
|---|---|---|
| 1 | 7 | 327 |
| 2 | 10 | 347 |
| 3 | 3 | 286 |
| 4 | 9 | 297 |
| 5 | 4 | 301 |
| Mean ± standard deviation | 6.6 ± 3 | 311.6 ± 25 |
a. Pressure at which the measuring catheter fixation became disrupted.
| Experiment | Stapler (group A1) | Glubran 2 (group B1) | Stapler (group A3) | Glubran 2 (group B3) | Stapler (group A5) | Glubran 2 (group B5) |
|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | ||||
| 1 | 225 | 50 | 206 | 108 | 210 | 184 |
| 2 | 207 | 40 | 213 | 45 | 228 | 178 |
| 3 | 185 | 52 | 200 | 141 | 223 | 182 |
| 4 | 180 | 70 | 195 | 137 | 209 | 171 |
| 5 | 178 | 57 | 227 | 95 | 235 | 105 |
| 6 | 215 | 45 | 235 | 112 | 205 | 185 |
| 7 | 222 | 58 | 217 | 97 | 247 | 169 |
| Mean ± standard deviation | 201.71 ± 20 | 53.14 ± 10 | 213.29 ± 14 | 150.00 ± 32 | 222.43 ± 15 | 167.72 ± 28 |
The animals were divided into three groups of 14 animals each based on the time from construction of an anastomosis. Each group was divided into two equal subgroups, one with stapled (groups A1, A3, and A5) and the other with glued (groups B1, B3, and B5) anastomosis. The measurement was performed on the 1st day, 3rd day, and 5th day after the operation (Table 2).
2.2. Anesthesia
The animals were fasted 1 day prior to the operation, but their fluid intake remained unchanged. Feeding and fluid intake were stopped on the day of the operation. Premedication consisted of ketamine 15 mg/kg of body weight intramuscularly (IM; Narkamon, Zentiva, Prague, Czech Republic), azaperon 1.0 mg/kg IM (Stresnil, Janssen, Beerse, Belgium), and atropine 0.02 mg/kg IM (Atropin; Hoechst-Biotika, Martin, Slovakia). A cannula was introduced into an auricular vein to ensure venous entrance. Following orotracheal intubation, the animals were relaxed using pipecuronium 40 mg/kg intravenously (IV; Arduan, Gedeon Richter, Budapest, Hungary) and, following connection to the anesthesia delivery system (Cirrus-Trans, Datex-Ohmeda, GE Company, Fairfield, CT, USA), ventilated using managed volume ventilation.
General sedation was maintained by titration with a combination of midazolam 0.05–0.1 mg/kg IV (Dormicum, Roche, Prague, Czech Republic) and propofol 2–4 mg/kg/hour (Diprivan; Astra Zeneca, Cheshire, Macclesfield, UK). Analgesia was provided by continual administration of metamizole 5 mg/kg/hour (Novalgin; Aventis Pharma, Frankfurt-on-Main, Germany). Muscle relaxation during the surgery was maintained with pipecuronium. Continual volume maintenance treatment was provided using a combination of crystalloids (Infusio Hartmanni; Medicamenta, Vysoke Myto, Czech Republic) and colloids (Hemohes 6%; Braun, Melsungen, Germany). Oxygen saturation, the electrocardiogram, and end-tidal carbon dioxide were monitored throughout the surgery. A single IM bolus dose of Betamox LA 15 mg/kg (amoxicillin; Norbrook Laboratories, Newry, UK) was administered as an antibiotic prophylaxis following the induction of anesthesia.
2.3. Surgical procedure in the control group
A median laparotomy was performed following standard aseptic preparation of the surgical field. A two-way 7.5 F cannula (Vygon, Ecouen, France) was inserted into the anastomosis or other procedure-free sigmoid colon via puncture incision and was watertight-fixed to the intestinal wall with two circular polyester sutures (Mersilene, 2/0 Johnson & Johnson, Division of Ethicon, Somerville, NJ, USA), then attached to the measuring device. The measuring device consisted of a digital manometer (GMH 3111) and a relative pressure sensor (GMSD 1 BRE; Greisinger Electronic, Regenstauf, Germany). These were linked through a computer. The intraluminal pressures in the colon were measured over 60 minutes. With the support of EBS 20M software (Greisinger Electronic), the recording was continuously transferred and saved to a computer.
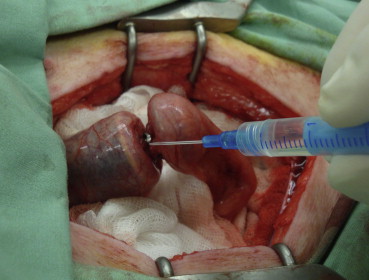
The colon was subsequently closed completely approximately 3.5 cm prior to and 3.5 cm after the anastomosis with circular ligatures, as well as peripherally from the ligatures, with intestinal staples. One cannula outlet was attached to a linear liquid-dosing system (Technic I, AMV Technics, Brno, Czech Republic) via an infusion set and the lumen of the intestine was gradually filled with saline at a rate of 300 mL/hour (Fig. 1). Measurements were repeated up to the point when the physiological solution started to leak around the cannula or the cannula loosened up. The aim of these measurements was to test the strength of the cannula fixation to ensure that the intestine around the cannula fixation was not disturbed sooner than the glued/stapled anastomosis (Table 1).
|
|
|
Figure 1. Measuring device consisting of a computer with software that continuously measured the intraluminal pressure, a digital manometer, a measuring gauge, and a linear liquid dispenser. |
2.4. Surgical procedure: construction of the anastomosis
A median laparotomy was performed following standard aseptic preparation of the surgical field. A catheter was introduced into the urinary bladder through a small incision at the bladder apex and was secured in place with a circular suture to ensure the derivation of urine and management of diuresis throughout the operation. On completion of the procedure, the catheter was withdrawn and the incision closed with absorbable sutures (Vicryl, 3/0; Johnson & Johnson). The sigmoid colon was cut in its entire diameter. Circular monofilament sutures (Prolene 3/0; Johnson & Johnson) were placed on both ends of the disconnected intestine.
Animals in groups A1, A3, and A5 had a standard anastomosis constructed using staples and a transanally inserted original 25 mm circular stapler (Circular Stapler CDH25, Johnson & Johnson). The entire diameter of the anastomosis was checked visually and subsequently tested with a water test.
In animals in groups B1, B3, and B5, the anastomosis was constructed using a modified 25 mm circular stapler (Circular Stapler CDH25; Johnson & Johnson). All staples were ejected and withdrawn from the stapler prior to use. Only the circular blade and mechanical part of the stapler remained intact prior to further use. The stapler cap was placed into the aboral section of the intestine and secured in place with a circular suture. The body of the stapler was inserted transrectally into the sigmoid colon. A metal shaft was ejected and the aboral part of the intestine was fixed on the stapler with a circular suture. A thin layer of the cyanoacrylate tissue glue [Glubran2 (N-butyl-2 cyanoacrylate + methacryloxysulfolane), GEM s.r.l., Viareggio, Italy] was applied on the two ends of the intestine being joined ( Fig. 2). The circular stapler was closed to achieve tight apposition of the glued surfaces. Any excess glue was removed with a gauze swab. After 90 seconds (the time required for glue polymerization), the stapler was fired, partly opened by two turns, and withdrawn from the intestine. The anastomosis was checked visually in its entire diameter and subsequently tested with a water test.
|
|
|
Figure 2. Application of the cyanoacrylate glue on the two sections of the colon being connected. |
After the anastomosis construction was complete, the abdominal cavity of all animals was lavaged with 10% betadine solution (Povidonum iodinatum; Egis Pharmaceuticals LTD, Budapest, Hungary), dried, and closed in one layer, similar to mass closure, with an absorbable monofilament suture, PDS-loop (polydioxanon; Johnson & Johnson). The skin was stapled (Stapler PMR 35; Johnson & Johnson). Following surgery, the experimental animals were extubated and placed in a warm end-of-anesthesia booth. The animals were given liquid feed beginning on the 2nd postoperative day then standard granulated feed beginning on the 3rd postoperative day. Skin staples were left in place until the follow-up operation.
2.5. Measurement of anastomosis resistance to increasing intraluminal pressure
Surgical revision and measurement of anastomosis resistance to an increasing intraluminal pressure was performed 24 hours after the first procedure in groups A1 and B1, and 3 days and 5 days after the operation in groups A3/B3 and A5/B5, respectively (Table 1).
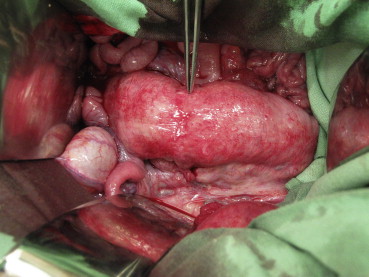
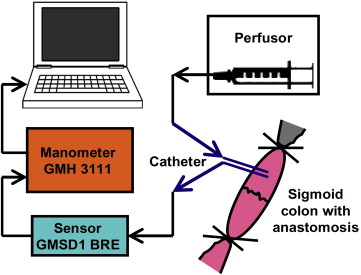
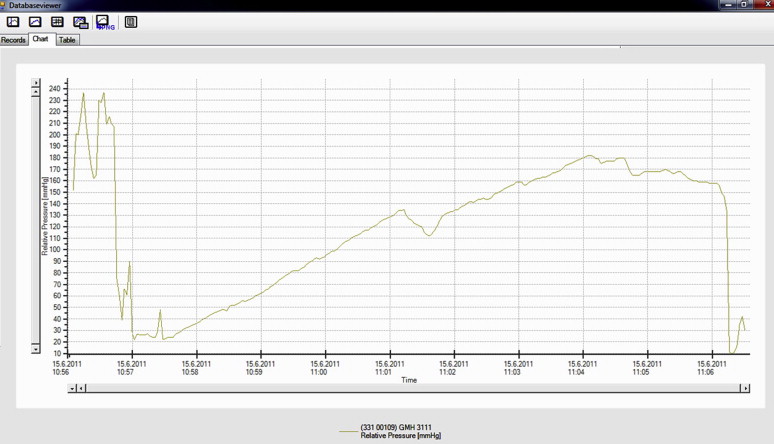
A visual revision of the anastomosis was first performed (Fig. 3). The colon was subsequently completely closed approximately 3.5 cm prior to and 3.5 cm after the anastomosis with circular ligatures. A two-way 7.5-F cannula (Vygon) was inserted into the lumen of the colon via a puncture incision. The cannula was watertight-fixed to the intestinal wall with two circular polyester sutures (Mersilene, 2/0; Johnson & Johnson). One cannula outlet was attached to a linear liquid dosing system via an infusion set, and the lumen of the intestine was gradually filled with saline solution at a rate of 300 mL/hour. The other outlet was attached to a measuring device connected to a computer with pressure-change analysis software (Fig. 4). The manometer continually measured the increasing pressure in the lumen. The measured values were continuously transferred to the computer as described for the control group. The increasing pressure in the intestinal lumen was recorded graphically as a curve and numerically in tables. The monitoring was performed up to the point when the continuity of the anastomosis was disturbed, as evidenced by leakage around the anastomosis and, at the same time, a fall in pressure visible on the graph (Fig. 5). At the end of the operation, the animals were euthanized by IV administration of T61 (Hoechst, Frankfurt-on-Main, Germany).
|
|
|
Figure 3. Healed anastomosis 5 days after it was glued with Glubran 2. The tweezers show the anastomosis. |
|
|
|
Figure 4. Schematic diagram of the measuring device with a two-way cannula inserted into the lumen of the intestine in the area of the anastomosis. |
|
|
|
Figure 5. Graphical record depicting the measurement of resistance of the stapled anastomosis to increasing intraluminal pressure during the gradual filling of the colon with saline. A disruption of the anastomosis is evident as a gradual then sudden fall in pressure. The irregular curves at the beginning resulted from the calibration performed prior to the experimental measurement. |
2.6. Statistical analyses
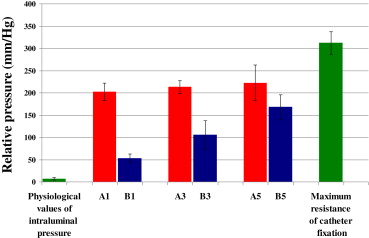
We performed a statistical comparison of the measured maximum pressure in all groups with physiological intraluminal pressures in the control group at rest. The results obtained for the experimental groups at the various postoperative time points were also compared. The analysis comparing the pressures resisted by stapled and glued anastomoses and physiological pressure was performed using the Student t test at a significance level of α = 0.01 with Bonferroni correction. The level of statistical significance was set at p < 0.01. Continuous variables were expressed as mean ± standard deviation (SD). The results were converted into a graphical format ( Fig. 6).
|
|
|
Figure 6. Comparison of the resistance of the stapled (red) and glued (blue) anastomosis to physiological pressures performed using the Student t test at the significance level of α = 0.01 with Bonferroni correction. The data are expressed as mean ± standard deviation. |
3. Results
After the operation, no animal presented with a dehiscence or leak in the area of the anastomosis. In all groups the maximum pressures resisted by anastomoses were significantly higher than the physiological pressures. This was confirmed statistically and is apparent from the graphical expression of the mean of the measured values (Fig. 6). The graph clearly shows that, at all time points evaluated, stapled anastomoses resisted higher intraluminal pressures than glued anastomoses. However, statistical analysis confirmed that the pressures resulting in the destruction of a glued anastomosis were still significantly higher than the physiological pressures. As the healing process advanced after construction of the anastomosis, the strength of a glued anastomosis (resistance to intraluminal pressure) approached that of a stapled anastomosis (Table 2; Fig. 6). As there was no dehiscence of the glued anastomosis and the resistance to pressure increased linearly, no measurement was performed at further time points.
4. Discussion
Tissue glues are biological, semi-synthetic, or synthetic substances, without or with hemocoagulation factors (biological glues), including fibrin and thrombin glues, that adhere strongly to living tissue surfaces. They are composed of cyanoacrylates, polyethylenglycols, albumin with glutaraldehyde, cellulose, and gelatin and collagen.13 ; 14
Fibrin glues were originally used predominantly in abdominal surgery. These adhesives mimic the last step in the hemocoagulation cascade, i.e., the conversion of fibrinogen to fibrin. Concentrated fibrinogen forms the primary component of these glues, combined with fibronectin, factor VIII, and plasminogen.14
Tissue adhesive research currently focuses mostly on cyanoacrylate-based substances. With this type of tissue glue, a tight bond between tissues occurs when monomer cyanoacrylate components (clear colorless liquids) transform into polymer chains. The polymerization process is induced by anions (I–, CH3COO–, OH–), weak organic bases, and amino acids.15 Polymerization induced by amino acids contained in the proteins of living tissues results in the formation of a thin polymer film that binds tightly to the tissue surface. When polymerization occurs between two appositioned tissues, they become firmly attached.16 The polymerized glue film forms a barrier on the tissue surface that maintains the natural environment, enables tissue healing, and simultaneously forms a flexible, water-resistant coating that prevents microbial invasion.15 ; 16 Biodegradation of the polymerized mesh occurs through gradual hydrolysis of the alkyl-group bonds by esterases contained in cellular lysosomes. The products of degradation (polycyanoacrylate acids) are water soluble and are excreted through the kidneys.15 ; 16
It was not until the1990s, when derivatives with long polymer chains became available, that cyanoacrylates were more broadly utilized in experimental and clinical practice. The 2-octyl cyanoacrylate (Dermabond, Nexaband, LiquiBand, SurgiSeal) is the most widely used so far. This derivative is now routinely used to form suture-free closures of skin injuries, predominantly in pediatric surgery and plastic surgery.17; 18; 19 ; 20
2-Octyl cyanoacrylate (Dermabond) was used in experiments with pigs to close a urinary bladder incision.21 ; 22 Two comparative studies showed healing of 7.5 cm incisions made through the entire thickness of the bladder wall. The healed bladder wall resisted a pressure of 200 mmHg in a compression test performed on the 4th postoperative day.21 ; 22
2-Octyl-cyanoacrylate (Dermabond) and N-butyl-cyanoacrylate (Histoacryl) were used in two studies of gastrointestinal surgery experiments in small animal species (laboratory rats) to create a suture-free intestinal juncture. Both studies compared colon closures created with monofilament fiber (polypropylene) and cyanoacrylate tissue glues. Both studies showed comparable levels of intestinal healing by the 3rd postoperative day and 7th postoperative day. Standard anastomoses resisted higher pressures during the compression test conducted on the 4th postoperative day. More intense inflammatory reactions and higher numbers of adhesions were observed in the area surrounding anastomoses created with the glue. This was attributed to irritant effects caused by the glue on the surrounding tissues. Higher yet statistically insignificant incidences of abscess formation were found around the hand-sewn anastomoses; intestinal obstructions were not documented in either group. 23 ; 24 Considering the lower resistance to pressure and higher incidence of inflammatory changes in the area of the anastomosis, it was concluded that the glues tested were not suitable for the construction of colon anastomosis. However, both studies used cyanoacrylates intended primarily for use on the skin, not to bond organs, and this is likely to be the explanation for the more intense reaction and higher number of adhesions around the anastomoses. 23 ; 24
The previous phase of the study reported here showed that 2-octyl-cyanoacrylate (Dermabond), a glue primarily intended for repairing skin defects, is unsuitable for constructing a suture-free anastomosis.12 Anastomoses glued with this adhesive showed a higher degree of organ toxicity that led to more pronounced inflammatory reactions and fibrotization of the intestinal wall tissues. Stenoses and strongly adhering firm perianastomotic adhesions resulting from fibrotization developed around the glued anastomoses.
The combination of N-butyl-2-cyanoacrylate and metacryloxisulfolane (Glubran 2, GEM S.r.l., Viareggio, Italy) is the only commercially manufactured cyanoacrylate intended primarily for surgically bonding organs. The product conforms to the European Directive on Medical Devices 93/42/CEE for surgical use, internally and externally. 25
N-Butyl-2-cyanoacrylate alone is used under various brand names (Indermil, Histoacryl, Xoin, GluStitch) as a tissue glue and is intended for suture-free skin closures or sclerotizations of oesophageal varices. 26; 27 ; 28 The second component of the glue, metacryloxysulpholane monomer, provides the glue with important properties, including reducing the temperature needed for exothermic polymerization (approximately 45 °C), increasing elasticity of the glue after polymerization, decreasing tissue toxicity, and preventing microbial invasion. These properties could crucially affect the process of healing of the glued tissues. The particular ratio of the two substances contained in the glue and the specific biological, physical, and mechanical properties are subject to manufacturers' trade secrets. Experimental use of the glue to create a colon anastomosis in a domestic pig showed that the glue is highly reliable and has minimal effects on the surrounding organs and the abdominal cavity of the experimental animals. We did not identify any significant potentiation of adhesion formation or an excessive fibrotization around the anastomosis.12
The results presented here show that anastomoses constructed with staples resisted higher pressures than anastomoses created with a cyanoacrylate glue. Nevertheless, the pressures leading to the destruction of a glued anastomosis exceeded by several fold the physiological intraluminal pressure in the colon, which ranged from 4 mmHg to 10 mmHg (mean 6.6 mmHg) in the control group. Measurements conducted at different time points from the construction of an anastomosis showed that the glued anastomoses increased in strength over time, probably resulting from continuing healing.
Therefore these results show that an anastomosis constructed using the glue does not have the same original strength as a stapled anastomosis. However, the relevance of resistance to intraluminal pressures is questionable. In other words, a stapled anastomosis is characterized by mechanical resistance, but, despite this, it is associated with dehiscence.2; 3; 4; 5; 6 ; 7
Local ischemia or technical error is the most likely cause of dehiscence associated with stapled anastomosis. The occurrence of microhematomas around staples or local infections should also be considered.29 Unlike staples or sutures, glues do not disturb the intestinal wall. Adhesion and connection occurs on the surface of serosis as an ultrathin film forming an uninterrupted connection between the entire glued areas of both anastomosed ends. In addition, gluing is not associated with the formation of micro-spaces or thin ducts between nearby staples, or sutures that allow for bacterial colonization. Some surgeons consider this an important issue as evident from the availability of surgical sewing materials with surface-bound antibiotics or antiseptics.30; 31 ; 32
The effects of a tissue glue and traditional suture materials on the intestinal wall tissue may be illustrated with a non-medical real-life example presented by the corresponding author of this paper in a discussion with a reviewer of a previous publication when asked: “Why should we test new techniques and materials when currently available staplers and suture materials serve us well?” The author comes from a family of beekeepers and took the liberty of using a parallel with beehives. The authors grandfather used nails to make and repair beehives, whereas the authors father used screws, and the author himself uses, to a large extent, modern technical glues. The advantage of glue is that, unlike nails or screws, it in no way disturbs the structure of the wood. Consequently, the wood does not crack and is not deformed. Gradually, due to climatic factors, the wood around the nails and screws dries more than in other areas and blackens due to corrosion. This compromises the compactness and longevity of the beehive. Furthermore, glues enable full-area interconnection of materials with heterogeneous surfaces and diverse compactness, such as hard wood and soft, fragile insulating materials (polystyrene), and, in the case of the beehives, this minimizes the space where bee pests may subsist.
A glued anastomosis might be characterized analogously, as it does not impair the integrity of the intestinal wall and adhesion occurs on the serous side and through the entire perimeter with no micro-spaces predisposing the intestine to bacterial infection. Furthermore, there is no risk of ischemia due to excessive tightness of the sutures or from stapler compression.
In conclusion, the results of this study suggest that the use of suitable absorbable synthetic glues is associated with healing that is equally good as that of a stapled anastomosis. Glued anastomoses were resistant to lower intraluminal pressures than stapled anastomoses, but resisted statistically significantly higher pressures than the physiological intraluminal pressures in the colon.
The present stage of experiments does not yet warrant the clinical use of synthetic glues to construct gastrointestinal tract anastomoses. However, these results suggest that research of this use of the glues should continue. We believe that, in the near future, using a combination of a classical, sparsely sutured (i.e., less traumatic for the tissue) anastomosis and a tissue adhesive, or a stapler with fewer staples and a tissue glue, could offer the most rational options for anastomoses. It may also be hypothesized that glues with a higher mechanical resistance against external effects could be developed.
The use of glues on an unbound intestine, i.e., when it is technically impossible to use a modified circular staple-free stapler, as in the case of the sigmoid colon, is unclear. This establishes the need to develop a simple technique to enable a firm connection of the serosis of both anastomosed ends of the intestine throughout their entire perimeter. Further research by our team is aimed at offering solutions to these issues.
Acknowledgments
This study was supported by the Project of Ministry of Defence of the Czech Republic: A Long-term Organization Development Plan 1011.
References
- 1 T.P. Kingham, H.L. Pachter; Colonic anastomotic leak: risk factors, diagnosis, and treatment; J Am Coll Surg, 208 (2009), pp. 1152–1153
- 2 C. Platell, N. Barwood, G. Dorfmann, G. Makin; The incidence of anastomotic leaks in patients undergoing colorectal surgery; Colorectal Dis, 9 (2007), pp. 71–79
- 3 N.C. Buchs, P. Gervaz, M. Secic, P. Bucher, B. Mugnier-Konrad, P. Morel; Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study; Int J Colorectal Dis, 23 (2008), pp. 265–270
- 4 T. Konishi, T. Watanabe, J. Kishimoto, H. Nagawa; Risk factors for anastomotic leakage after surgery for colorectal cancer: results of prospective surveillance; J Am Coll Surg, 202 (2006), pp. 439–444
- 5 P. Matthiessen, O. Hallböök, M. Andersson, J. Rutegård, R. Sjödahl; Risk factors for anastomotic leakage after anterior resection of the rectum; Colorectal Dis, 6 (2004), pp. 462–469
- 6 I. Kanellos, K. Vasiliadis, S. Angelopoulos, et al.; Anastomotic leakage following anterior resection for rectal cancer; Tech Coloproctol, 8 (suppl 1) (2004), pp. S79–S81
- 7 T. Eberl, M. Jagoditsch, A. Klingler, J. Tschmelitsch; Risk factors for anastomotic leakage after resection for rectal cancer; Am J Surg, 196 (2008), pp. 592–598
- 8 H.K. Choi, W.L. Law, J.W. Ho; Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors; Dis Colon Rectum, 49 (2006), pp. 1719–1725
- 9 M.A. Lipska, I.P. Bissett, B.R. Parry, A.E. Merrie; Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk; A N Z J Surg, 76 (2006), pp. 579–585
- 10 A. Alves, Y. Panis, D. Trancart, J.M. Regimbeau, M. Pocard, P. Valleur; Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients; World J Surg, 26 (2002), pp. 499–502
- 11 J. Waninger, G.W. Kauffmann, I.A. Shah, E.H. Farthmann; Influence of the distance between interrupted sutures and the tension of sutures on the healing of experimental colonic anastomoses; Am J Surg, 163 (1992), pp. 319–323
- 12 J. Paral, Z. Subrt, P. Lochman, et al.; Suture-free anastomosis of the colon. Experimental comparison of two cyanoacrylate adhesives; J Gastrointest Surg, 15 (2011), pp. 451–459
- 13 B. Shekarriz, M.L. Stoller; The use of fibrin sealant in urology; J Urol, 167 (2002), pp. 1218–1225
- 14 K. Jönsson, H. Jiborn, B. Zederfeldt; Comparison of healing in the left colon and ileum. Changes in collagen content and collagen synthesis in the intestinal wall after ileal and colonic anastomoses in the rat; Acta Chir Scand, 151 (1985), pp. 537–541
- 15 F. Leonard, R.K. Kulkarni, G. Brandes, J. Nelson, I.J. Cameron; Synthesis and degradation of poly (alkyl-cyanoacrylate); J Appl Polym Sci, 10 (1966), pp. 259–272
- 16 C. Vauthier, C. Dubernet, E. Fattal, H. Pinto-Alphandary, P. Couvreur; Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications; Adv Drug Deliv Rev, 55 (2003), pp. 519–548
- 17 L. Bernard, J. Doyle, S.F. Friedlander, L.F. Eichenfield, N.F. Gibbs, B.B. Cunningham; A prospective comparison of octyl cyanoacrylate tissue adhesive (dermabond) and suture for the closure of excisional wounds in children and adolescents; Arch Dermatol, 137 (2001), pp. 1177–1180
- 18 M.D. Nipshagen, J.J. Hage, W.H. Beekman; Use of 2-octyl-cyanoacrylate skin adhesive (Dermabond) for wound closure following reduction mammaplasty: a prospective, randomized intervention study; Plast Reconstr Surg, 122 (2008), pp. 10–18
- 19 G.R. Scott, C.L. Carson, G.L. Borah; Dermabond skin closures for bilateral reduction mammaplasties: a review of 255 consecutive cases; Plast Reconstr Surg, 120 (2007), pp. 1460–1465
- 20 J.M. Cooper, K.T. Paige; Primary and revision cleft lip repairs using octyl-2-cyanoacrylate; J Craniofac Surg, 17 (2006), pp. 340–343
- 21 R. Marcovich, A.L. Williams, M.A. Rubin, J.S. Wolf Jr.; Comparison of 2-octyl cyanoacrylate adhesive, fibrin glue, and suturing for wound closure in the porcine urinary tract; Urology, 57 (2001), pp. 806–810
- 22 B.D. Seifman, M.A. Rubin, A.L. Williams, J.S. Wolf; Use of absorbable cyanoacrylate glue to repair an open cystotomy; J Urol, 167 (2002), pp. 1872–1875
- 23 M.M. Ozmen, N. Ozalp, B. Zulfikaroglu, et al.; Histoacryl blue versus sutured left colonic anastomosis: experimental study; A N Z J Surg, 74 (2004), pp. 1107–1110
- 24 T.Z. Nursal, R. Anarat, S. Bircan, S. Yildirim, A. Tarim, M. Haberal; The effect of tissue adhesive, octyl-cyanoacrylate, on the healing of experimental high-risk and normal colonic anastomoses; Am J Surg, 187 (2004), pp. 28–32
- 25 Glubran 2. http://www.gemitaly.it/web/en/glubran2.html. Accessed 15.03.14.
- 26 A. Charters; Wound glue: a comparative study of tissue adhesives; Accid Emerg Nurs, 8 (2000), pp. 223–227
- 27 Y.M. Wang, L.F. Cheng, N. Li, K. Wu, J.S. Zhai, Y.W. Wang; Study of glue extrusion after endoscopic N-butyl-2-cyanoacrylate injection on gastric variceal bleeding; World J Gastroenterol, 15 (2009), pp. 4945–4951
- 28 S. Seewald, T.L. Ang, H. Imazu, et al.; A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos); Gastrointest Endosc, 68 (2008), pp. 447–454
- 29 C.E. Edmiston, G.R. Seabrook, M.P. Goheen, et al.; Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination?; J Am Coll Surg, 203 (2006), pp. 481–489
- 30 A. Gómez-Alonso, F.J. García-Criado, F.C. Parreño-Manchado, et al.; Study of the efficacy of Coated VICRYL Plus antibacterial suture (coated Polyglactin 910 suture with Triclosan) in two animal models of general surgery; J Infect, 54 (2007), pp. 82–88
- 31 H.R. Ford, P. Jones, B. Gaines, K. Reblock, D.L. Simpkins; Intraoperative handling and wound healing: controlled clinical trial comparing coated VICRYL plus antibacterial suture (coated polyglactin 910 suture with triclosan) with coated VICRYL suture (coated polyglactin 910 suture); Surg Infect (Larchmt), 6 (2005), pp. 313–321
- 32 X. Ming, M. Nichols, S. Rothenburger; In vivo antibacterial efficacy of MONOCRYL plus antibacterial suture (Poliglecaprone 25 with triclosan); Surg Infect (Larchmt), 8 (2007), pp. 209–214
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?