Summary
Adrenal tumors with more than one cellular component are uncommon. Furthermore, an adrenal tumor composed of a pheochromocytoma and a malignant peripheral nerve sheath tumor is extremely rare. A composite pheochromocytoma with malignant peripheral nerve sheath tumor in a 42-year-old man is reported here. After adequate preoperative control, left adrenalectomy was performed simultaneously with resection of the ipsilateral kidney for spontaneous rupture of the left adrenal tumor. Pathological findings demonstrated pheochromocytoma and malignant peripheral nerve sheath tumor in a ruptured adrenal tumor. To date, there have been only four reported cases of composite pheochromocytoma with malignant peripheral nerve sheath tumor, so the present case is only the fifth case in the world. Despite the very poor prognosis of patients with pheochromocytoma and malignant peripheral nerve sheath tumors reported in the literature, the patient remains well without evidence of recurrence or new metastatic lesions at 36 months postoperatively.
Keywords
adrenalectomy;adrenal tumor;malignant peripheral nerve sheath tumor;pheochromocytoma;spontaneous rupture
1. Introduction
The rare coexistence of a pheochromocytoma with neuroblastoma, ganglioneuroblastoma, ganglioneuroma, or malignant peripheral nerve sheath tumor (MPNST) is termed a composite pheochromocytoma.1 Bolande2 designated this pathologic mechanism as neurocristopathy, based on the concept that various kinds of tumors originate from developmental arrest and maldevelopment of totipotential neural crest cells. An adrenal tumor composed of pheochromocytoma and MPNST is extremely rare.1; 3; 4 ; 5 To date, only four cases of composite pheochromocytoma with MPNST have been reported.1; 3; 4 ; 5 An unusual case of composite pheochromocytoma with MPNST in a 42-year-old man who was treated surgically is reported here.
2. Case report
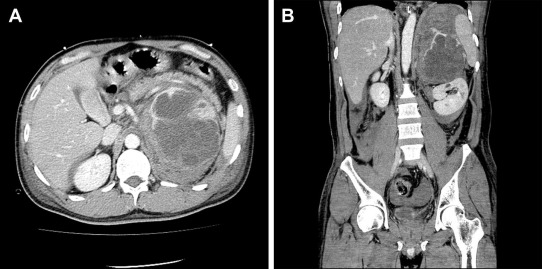
In August 2009, a 42-year-old man presented with acute left low back pain. Abdominal and pelvic computed tomography (CT) scan revealed an elliptic, 112 mm × 88 mm2, adrenal tumor with massive retroperitoneal hemorrhage (Fig. 1A and B). Magnetic resonance imaging suggested an adrenal lesion with T1 hypointensity and T2 hyperintensity. Preoperative serum and urinary concentrations of catecholamines and catecholamine metabolites were as follows: serum epinephrine 2883 pg/mL [normal range (NR) ≤100 pg/mL], serum norepinephrine 36,829 pg/mL (NR 100–450 pg/mL), serum dopamine 761 pg/mL (NR ≤20 pg/mL), serum vanillylmandelic acid 43.4 ng/mL (NR 4–5 ng/mL), urinary epinephrine 536.4 μg/d (NR 3.4–26.9 μg/d), urinary norepinephrine 9234.0 μg/d (NR 48.6–168.4 μg/d), urinary dopamine 289.6 μg/d (NR 365.0–961.5 μg/d), and urinary vanillylmandelic acid 27.4 mg/d (NR 1.5–4.3 mg/d). Other laboratory data demonstrated that this adrenal tumor was not hormonally active except for catecholamines. On physical examination, no evidence of neurofibromatosis type 1, such as neurofibromas over his entire skin and café au lait spots, was found.
|
|
|
Figure 1. Preoperative computed tomography findings showed the left adrenal lesion with enhancement and large hemorrhage: (A) transverse plane and (B) coronal plane. |
Finally, a definite diagnosis of a pheochromocytoma was made based on the abovementioned results. After adequate preoperative control, in September 2009, open left adrenalectomy with simultaneous ipsilateral nephrectomy was performed because of posthemorrhagic adhesions.
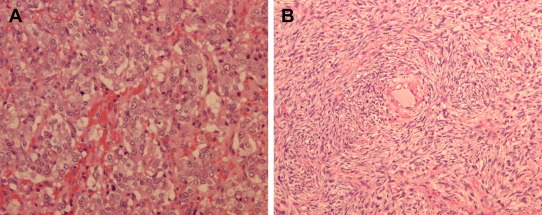
Macroscopically, the adrenal tumor appeared as a yellow–brown myxoid tumor with hemorrhage and necrosis on cross-section, measuring 90 × 70 mm2. Histologically, this tumor consisted of two different components. The main tumor cells forming predominantly zellballen-type nests were typical of a pheochromocytoma (Fig. 2A). These tumor cells were immunoreactive for chromogranin, synaptophysin, CD56, CD57, and, focally, S100 protein. Furthermore, separated from the domain of pheochromocytoma, oval to spindle cells arranged in a streamy fasciculated pattern (Fig. 2B). These spindle cells were positively stained for S100 protein and were positive for Ki-67 within the range of 10–40%. These histological findings were consistent with the features of MPNST. Thus, we concluded a composite pheochromocytoma with MPNST in a ruptured adrenal tumor. Microscopic positive margins were absent.
|
|
|
Figure 2. Histopathological examination of the adrenal specimen shows two different components: (A) pheochromocytoma (hematoxylin–eosin stain, magnification 200×), and (B) malignant peripheral nerve sheath tumor (hematoxylin–eosin stain, magnification 200×). |
This patient has been followed regularly and has not undergone any adjuvant therapy. There is no evidence of recurrence or new metastatic lesions on CT and bone scan at 36 months postoperatively.
3. Discussion
During embryogenesis, certain cells in the neural crest migrate ventrally and give rise to the essential cellular components of sympathetic ganglia and adrenal medulla.3 Migration disturbance and developmental arrest or maldevelopment of neural crest-derived cells may result in various types of neoplasms.5 Bolande2 first described the concept of neurocristopathies. Tumors composed of various combinations of varying stages of differentiation have been reported as part of neurocristopathies.3; 4 ; 5 Furthermore, in a pheochromocytoma and MPNST tumor, sustentacular cells in the stroma of the pheochromocytoma have been suggested as the origin of MPNST recently.1; 4 ; 5 Ch'ng et al1 suggested that the morphological transition from the pheochromocytoma component to the MPNST component, coupled with immunohistochemistry for chromogranin in the pheochromocytoma and S100 protein in the MPNST, indicated that the MPNST was a derivative of the former.
The MPNST component shows spindly tumor cells arranged in a streamy fasciculated pattern with conspicuous compact nodules of less differentiated spindle cells.1; 3; 4 ; 5 The presence of S100 protein immunoreactivity in some spindle tumor cells provides further support to the diagnosis of MPNST.1; 3; 4 ; 5 This case met the histological findings that were typical of an MPNST.
Spontaneous rupture of an adrenal pheochromocytoma has a high possibility of a lethal outcome.6; 7 ; 8 Kobayashi et al6 analyzed the clinical outcomes in 50 patients with a ruptured pheochromocytoma. Although the mortality rate was very high in patients who could not undergo an adequately prepared surgery, there was no mortality in patients who underwent an elective surgery after control of blood pressure and total circulating blood volume using α-adrenergic blockers and fluid infusion therapy.6 In the present case, the surgery was performed uneventfully after adequate preoperative control, and there were no complications in the perioperative period. We also conclude that adequate preparation prior to surgery is the most important aspect of the treatment of a ruptured pheochromocytoma.
To the best of our knowledge, four cases of composite pheochromocytoma with MPNST have been reported.1; 3; 4 ; 5Table 1 demonstrates the patients' characteristics reported in the literature and those of the present patient. Patients with pheochromocytoma and MPNST showed a very poor prognosis, such as cancer death or survival with cancer. An MPNST itself is a markedly metastatic and aggressive, poor-prognosis tumor.9 However, in the present case, the tumor was completely removed despite a ruptured pheochromocytoma with MPNST, and the patient survives well without any evidence of tumor recurrence at the present time. Zou et al9 suggested that a predictor for local recurrence in surgically resected patients with MPNST is positive margin status. They also showed that prognostic factors for the development of distant metastasis and MPNST-specific mortality in the same patients were tumor size >10 cm and negative S100 staining.9 The present patient can be classified as having a low risk of local recurrence, development of distant metastasis, and disease-specific mortality from the viewpoint of MPNST,9 although the pathological diagnosis of a malignant pheochromocytoma is difficult at the present time.
| Age (y) | Sex | Side | NF-1 | MP | Metastasis | Pathological confirmation | Follow-up | Report | |
|---|---|---|---|---|---|---|---|---|---|
| Years | Status | ||||||||
| 39 | F | L | − | − | None | Operation | 0.67 | Cancer death | Min et al3 |
| 38 | F | L | − | − | Liver | Operation | 1.5 | Survival with cancer | Miettinen and Saari4 |
| Retro | |||||||||
| 48 | M | L | + | + | Lung | Autopsy | 0.25 | Cancer death | Sakaguchi et al5 |
| Para IVC | |||||||||
| 37 | F | L | − | + | Bone, liver | Autopsy | 28 | Cancer death | Ch'ng et al1 |
| Lung, LN | |||||||||
| 42 | M | L | − | − | None | Operation | 2.5 | NED | Present patient |
F = female; IVC = inferior vena cava; L = left; LN = lymph node; M = male; MP = malignant pheochromocytoma; MPNST = malignant peripheral nerve sheath tumor; NED = no evidence of disease; NF-1 = neurofibromatosis type 1; Retro = retroperitoneal tumor.
Acknowledgments
None
References
- 1 E.S. Ch'ng, Y. Hoshisa, N. Iizuka, et al.; Composite malignant pheochromocytoma with malignant peripheral nerve sheath tumor: a case with 28 years of tumor-bearing history; Histopathology, 51 (2007), pp. 420–422
- 2 R.P. Bolande; The neurocristopathies: a unifying concept of disease arising in neural crest maldevelopment; Hum Pathol, 5 (1974), pp. 409–429
- 3 K.W. Min, A. Clemens, J. Bell, H. Dick; MPNST and pheochromocytoma. A composite tumor of the adrenal; Arch Pathol Lab Med, 112 (1988), pp. 266–270
- 4 M. Miettinen, A. Saari; Pheochromocytoma combined with malignant schwannoma: unusual neoplasm of the adrenal medulla; Ultrastruct Pathol, 12 (1988), pp. 513–527
- 5 N. Sakaguchi, K. Sano, M. Ito, T. Baba, M. Fukuzawa, M. Hotchi; A case of von Recklinghausens disease with bilateral pheochromocytoma—MPNSTs of the adrenal gastrointestinal autonomic nerve tumors; Am J Surg Pathol, 2 (1996), pp. 889–897
- 6 T. Kobayashi, A. Iwai, R. Takahashi, Y. Ide, K. Nishizawa, K. Mitsumori; Spontaneous rupture of adrenal pheochromocytoma: review and analysis of prognostic factors; J Surg Oncol, 90 (2005), pp. 31–35
- 7 Y. Naya, T. Ichikawa, H. Suzuki, et al.; Efficacy and safety of laparoscopic surgery for pheochromocytoma; Int J Urol, 12 (2005), pp. 128–133
- 8 K. Orikasa, T. Namima, T. Ohnuma, M. Munakata, N. Kimura, Y. Arai; Spontaneous rupture of adrenal pheochromocytoma with capsular invasion; Int J Urol, 11 (2004), pp. 1013–1015
- 9 C. Zou, K.D. Smith, J. Liu, et al.; Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome; Ann Surg, 249 (2009), pp. 1014–1022
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?