Abstract
Background
Chronic inflammation, the fundamental pathogenetic process of atherosclerosis, can be modified by pharmacological and non-pharmacological measures as a part of secondary prevention after acute myocardial infarction (AMI). The aim of our study was to determine the effect of diet, rich with natural antioxidants, added to physical activity (as a part of cardiac rehabilitation (CR) program) on inflammatory markers and ox-LDL, a marker of oxidative stress, closely involved in the process of chronic inflammation.
Methods
41 male patients after AMI undergoing CR were divided into a diet group (supervised cardioprotective diet throughout the CR), and control group (CR without diet). We measured hsCRP, leucocytes, neutrophils, IL-6, oxLDL, exercise capacity and classic risk factors before and after CR program.
Results
Patients from the diet group presented with a significant decline in classic risk factors (BMI, waist circumference, waist to hip ratio, systolic blood pressure, heart rate, blood glucose, total cholesterol, LDL, TAG) and inflammatory markers (hsCRP, leucocytes, neutrophils) compared to control group. Furthermore, when studying nonsmokers, we observed significant decline of oxLDL in the diet group.
Conclusions
The addition of cardioprotective diet, rich with natural antioxidants, to physical activity as a part of a CR program, positively modifies not just classic risk factors and exercise capacity, but also diminishes chronic inflammation markers. These effects, and oxLDL decline were most prominent in nonsmoking patients.
Abbreviations
AMI, acute myocardial infarction;CAD, coronary artery disease;CR, cardiac rehabilitation;LDL, low density lipoprotein;HDL, high density lipoprotein;TAG, triglycerides;hsCRP, high sensitivity CRP;oxLDL, oxidized LDL;IL-6, interleukin 6;BMI, body mass index;BP, blood pressure
Keywords
Cardiac rehabilitation;Acute myocardial infarction;Cardioprotective diet;Inflammation;Oxidized LDL;Smoking
1. Background
Acute myocardial infarction (AMI) is a common manifestation of atherosclerotic coronary artery disease (CAD), which is one of the most important causes of mortality in the developed world [1] ; [2]. Although improved management has significantly lowered CAD mortality [3], we are constantly searching for new strategies to influence the cardinal pathological process of atherosclerosis; to diminish inflammation [4]. On the contrary, oxidized low density lipoprotein (oxLDL), a useful marker of coronary artery disease [5], exhibits a marked influence on chronic inflammation, since it stimulates the release of chemotactic factors, cytokines and growth factors from artery wall cells, which play an important role in the development and progression of atherosclerotic plaque [6]. Furthermore, it was shown in vivo that host response to infection and inflammation itself induces LDL oxidation [7], so not only oxLDL stimulates inflammation but also the opposite happens. This confirms the existence of a strong connection between inflammatory response and oxLDL.
Regarding pharmacological therapy, statins and acetylsalicylic acid have already been proven to effectively reduce low-grade inflammation in AMI survivors [8]; [9] ; [10] and also to protect LDL against oxidation [11] ; [12]. Non-pharmacological measures such as physical activity and diet are also gaining recognition as non-classic risk factor modifiers. Physical activity is associated with diminished inflammation in adults with or without CAD, independently of body composition and weight loss [13] ; [14]. Furthermore, cardioprotective diet also improves survival in patients after AMI [15] ; [16]. In addition, it has been shown that diet can also reduce the degree of inflammatory markers such as hsCRP and IL-6, not only in patients with developed coronary heart disease [17], but also in healthy adults at high risk for developing CAD [18]. Whereas many research works have proven favorable effects of coupled diet and physical exercise on inflammation in adults with high risk for CAD [19] ; [20], we did not find any studies describing the effects of cardioprotective diet added to exercise training in patients after acute AMI (fully developed CAD).
The aim of our study was to explore how the addition of a cardioprotective diet (rich with natural antioxidants) to physical activity, which is as an integral part of a rehabilitation program, influences not just exercise capacity and classic risk factors for CAD, but also inflammatory markers including high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6) and oxLDL. The secondary aim of the study was introduced subsequently since the results were less pronounced as we expected at the commencement of the study. In search of confounding factors that could influence inflammation and oxidation markers we further divided the patients into smoker and nonsmoker groups.
2. Materials and methods
2.1. Patients
Forty-one male patients with the median age of 59 years (minimum 36 years, maximum 73 years), discharged from the hospital at least 2 and not more than 9 weeks after AMI were included in our study.
Patients were successively recruited from the standard short-term (two-week) cardiac rehabilitation (CR) program. In Slovenia the national insurance company funds such CR programs for all patients after AMI. Their aims are to improve patients' physical performance and educate them about prevention of further disease progression, emphasizing the importance of drug therapy and lifestyle changes. The exercise program is supervised and all patients participate in lectures about healthy, cardioprotective diet, however they are allowed to choose their meals by themselves.
All patients after AMI were optimally treated according to current guidelines [2]. All patients were receiving acetylsalicylic acid 100 mg daily (combined with clopidogrel in 9 patients, prasugrel in 18 or ticagrelor in 13 patients for a year after AMI), angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers and statins. All patients were treated with appropriate statin doses (31 of them were receiving rosuvastatin, 9 atorvastatin and one simvastatin), so that the target values recommended by ECS were met. All but three patients were taking beta-blockers and one patient was on ivabradine. The patient group consisted of 21 smokers and 20 nonsmokers. We defined nonsmokers as patients who never smoked or stopped smoking at least a year before AMI occurred (most of them had quit smoking many years before AMI occurred).
After we acquired informed consent each patient was sorted to either a diet (21 patients, 10 nonsmokers and 11 smokers) or a control group of patients (20 patients, 10 nonsmokers and 10 smokers). The patients from the diet group consumed cardioprotective diet rich with natural antioxidants throughout the CR, individually prepared by a clinical dietitian (explained in detail in the chapter Diet). All patients were advised to immediately report to us about possible side effects of the diet, and all the patients had the right to cease from the study at any time due personal or other reasons (such as allergic reactions and changes in digestion).
To minimize the influence of smoking and statin treatment on the effects of diet intervention during CR, we sorted out the patients to the diet or the control group according to smoking status and treatment with statins. In that way, we obtained groups which did not differ in percentage of smokers (p = 0.78) and were also balanced regarding statin doses (no statistically significant differences were seen in equivalent statin doses between diets and controls, p = 0.380). Patients from the diet group proved to be significantly younger than patients included in the control group (52 vs 62.5 years, p = 0.013), what can be explained by higher motivation of younger patients, who agreed to participate in the intervention group.
Exclusion criteria for the studied group were: female gender, symptoms, signs and laboratory indications of acute infection, known diseases with ongoing chronic inflammation, and daily intake of dietary supplements and/or vitamins, AMI treated with urgent coronary bypass surgery, complications of AMI involving the need for urgent surgical intervention and other larger surgical procedures needed during hospitalization in the coronary unit.
The study was approved by the Slovenian Board for Medical Ethics No. 159/06/10 and all subjects gave their written informed consent.
2.2. Study design
2.2.1. CR protocol
The CR program was carried out in a local health resort. The rehabilitation comprised a fourteen-day long, gradual training regimen in accordance with the Position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Rehabilitation and Prevention [21]. The program consisted of supervised physical activity, which included daily interval ergometric training (25–30 min), during which we gradually increased the load with intervals of low intensity (25–40 W) workout inbetween. Patients also participated in daily stretching and respiratory exercises (30 min), exercises in the pool (15 min) and nordic walk (60 min). Stress relief program including massages and baths was also at patients' disposal. All patients took part in the educational program, which provided information about the importance of pharmacological therapy, changes in lifestyle and healthy diet, since mandatory cardioprotective diet is usually not a part of CR programs in Slovenia.
2.2.2. Cardiac stress testing
We performed a cardiac stress test on a stationary exercise bicycle ergometer (cycloergometry) for each patient before entering and at the end of CR program. During the cycloergometry, we raised the load for 25 W every 3 min. The testing was terminated when the patients reached the sub-maximal value for their heart rate (85% of the maximal heart rate), complained of dyspnea or pain (in the chest or other parts of the body), due to exhaustion, if we found ischemic changes in the ECG, bundle branch block, hypertensive reaction or if arrhythmias appeared. Almost all patients from our study had undergone exercise testing already before the discharge from the hospital or during previous course of the disease, thus at the inclusion to CR the exercise testing could not be effected by learning bias.
2.2.3. Cardioprotective diet rich on natural antioxidants
Of all 41 patients, 21 were included in the diet group. They agreed and were highly motivated to strictly follow diet recommendations and not to eat anything else but the food advised and supervised by a clinical dietitian, which was individually prepared for them. The diet group was initially larger but during the course of study 6 patients withdraw: 2 because of acute enterocolitis, 1 because of acute gout attack, one because of adverse reactions of the diet (digestion problems and allergic reactions to nuts) and 2 because of personal reasons. None of the patients from control group withdrew from the study. Each patient from the diet group had a meeting with a clinical dietitian, who assembled an individual menu according to patients weight, body composition, presence of diabetes and personal preferences. Since we wanted to compose a cardioprotective diet, which would include our national healthy foods, we not only balanced the diet according to caloric intake and percentage of macronutrients, but also enriched it with 300 g of apples and 30 g of raw nuts daily from integrated, high quality Slovenian production. Both apples and nuts are namely part of our seasonal harvest and are traditionally eaten throughout the year either raw or in traditional Slovenian pastries. Furthermore, they are among the most inexpensive fruits. Apples and walnuts were provided for this study by our colleagues from Biotechnical University, who have confirmed in previous trials that both apples and walnuts are rich source of antioxidants and that the apples do not importantly lose antioxidant potential during shelf life [22] ; [23]. The dietitian calculated the total amount of desired caloric intake for each individual patient, with the aim to target his or her ideal weight. The majority of patients were on a reduction diet with a daily intake of approximately 1800 kcal. The percentage of macronutrients depended on individual needs, but in general the diet was composed of 25% fat, 60% carbohydrates and 15% proteins. Patients with diabetes had a lower percentage of carbohydrates and higher percentage of proteins.
Breakfast, lunch and dinner were served specifically for the individual patient, considering the dietitians recommendations. Two unpeeled apples and nuts were consumed as snacks between meals.
2.3. Clinical parameters
Clinical examination, measurements of blood pressure (RR), body weight, height, waist and hip circumference, calculation of body mass index (BMI), waist to hip ratio and blood sampling were performed for each patient before and at the end of the CR program. All measurements were carried out in the morning, in the fasting state, data from both groups were collected in the same time of the year.
2.4. Specimen characteristics
Blood samples were drawn from the antecubital vein of patients in the morning, after 15 min of rest before entering and at the end of CR program.
Laboratory tests for complete blood count, glucose, lipids and hsCRP were performed on the day of the blood collection. For other tests performed later on, plasma or serum was stored immediately after retrieval at − 70 °C.
2.4.1. Assay methods
The metabolic variables: fasting glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol were determined from serum by routine biochemical methods (all Cobas, Roche, Germany). Leucocytes were determined from whole blood by an automated analyzer (Sysmex, Clin Lab Products, USA).
HsCRP was determined using polystyrene particles coated with monoclonal antibodies specific to human CRP (Siemens, CardioPhase hsCRP). In this procedure, the sample is mixed with the coated particles and these aggregates scatter a beam of light passed through the sample. The result is evaluated by comparison with a standard known concentration [24] ; [25].
Interleukin 6 (IL-6) was quantitatively determined by enzyme immunoassay method for the quantitative determination of human IL-6 in serum, plasma and cell culture supernatants (Interleukin-6 ELISA®, Hamburg, Germany). In this method anti-human IL-6 coating antibody is adsorbed onto microwells and human IL-6 binds to the antibodies on the microwells. Biotin-conjugated anti-human IL-6 antibody is added, which attaches to the human IL-6. Following incubation, unbound anti-human IL-6 antibody is removed by a wash step and Streptavidin HRP is added, which binds to the biotin conjugated anti-human IL-6 antibody and consequently a colored product is formed in proportion to the amount of human IL-6 present in the sample. The reaction is terminated by addition of acid and absorbance is measured at 450 nm.
We used Mercodia Oxidized LDL ELISA for the determination of oxLDL, which is a solid phase two-side enzyme immunoassay. Two monoclonal antibodies are directed against separate antigenic determinants on the oxLDL apolipoprotein B molecule. During incubation oxLDL reacts with antibodies bound to microtitration well and after washing anti-human apolipoprotein B recognizes oxLDL and the bound conjugate is detected by reaction with 3,3′,5,5′-tetramethylbenzidine (TMB). The reaction is stopped by adding acid to give colorimetric endpoint, which is then read spectrophotometrically. This method has been used in many preceding studies [26].
2.5. Statistical analysis methods
Considering that the distribution was not normal, we used non-parametrical tests for statistical analysis. Our data is presented as medians with ranges between the first and the third quartile for continuous variables and as numbers and percentages for categorical variables. Differences in data in independent samples were compared using the Mann–Whitney U test. Differences in medians of measured variables before and after the CR program in the whole group of patients were calculated using the Wilcoxons rank-sum test for continuous measures. Depending on the number of cases included in the analysis, the χ2 or Fisher exact test was used for comparison of differences for discrete variables. Non-parametric Spearmans correlation coefficients were calculated to test associations between inflammatory and other parameters. To check the impact of smoking on these results, the whole group of patients was divided into smokers (N = 20) and nonsmokers (N = 21). Differences in measured variables before and after the CR program between the subgroups of smokers and nonsmokers were determined using Wilcoxons rank-sum test for continuous measures and Fishers exact test for categorical variables. Differences between the group of smokers and nonsmokers were then compared using the Mann–Whitney U test.
The significant effects were indicated when the p value was less than 0.05. We used the SPSS statistic program version 16.0 for statistic analysis and the graphic presentation of data.
3. Results
3.1. Comparison of exercise capacity, classic risk factors, inflammatory markers and oxLDL between the diet group and the control group of patients before and after CR
We confirmed that no significant differences in exercise testing, classic risk factors, and inflammatory markers were present at the inclusion to the study (before CR) between the diet group and the control group of patients (Table 1).
| Diet group (N = 21) | Control group (N = 20) | |||
|---|---|---|---|---|
| Before CR (N = 30) | After CR | Before CR (N = 30) | After CR | |
| Maximal systolic BP (mm Hg) | 170 (160; 200) | 185 (176; 210)⁎ | 165 (159; 204) | 186 (171; 210)⁎⁎ |
| Maximal HR (bpm) | 114 (102; 128) | 119 (108; 131)⁎ | 112 (104; 118) | 118 (108; 129)⁎⁎ |
| Double product (mm Hg ∗ bpm) | 20,230 (16,271; 23,898) | 22,785 (19,669; 25,860)⁎ | 18,450 (14,644; 23,981) | 22,489 (17,562; 26,701)⁎⁎ ; ⁎ |
| Maximal load (W) | 112 (83; 128) | 125 (93; 154)⁎⁎ ; ⁎ | 100 (82; 120) | 106 (100; 149)⁎⁎ ; ⁎ |
| Maximal load (MET) | 5.0 (4.5; 6.2) | 5.8 (4.7; 6.9)⁎⁎ ; ⁎ | 5.4 (4.6; 5.9) | 5.7 (5.4; 6.3)⁎⁎ ; ⁎ |
| Time of load (min) | 11.0 (10.0; 13.0) | 12.0 (10.0; 14.5)⁎ | 10.5 (9.0; 12,8) | 12.5 (10.3; 14.0)⁎⁎ ; ⁎ |
| BMI (kg/m2) | 29.3 (27.5; 30.9) | 28.0 (26.9; 29.8)⁎⁎ | 27.5 (25.2; 29.8) | 27.5 (25.2; 29.7) |
| Waist circumference (cm) | 102 (98.5; 107.5) | 100 (94.5; 104.3)⁎⁎ ; ⁎ | 102 (94.3; 109.0) | 102 (93.5; 108.5) |
| Waist/hip ratio | 0.98 (0.95; 1.0) | 0.97 (0.93; 0.98)⁎⁎ | 0.99 (0.90; 1.04) | 0.98 (0.91; 1.02) |
| Resting systolic BP (mm Hg) | 130 (121; 140) | 120 (110; 130)⁎⁎ ; ⁎ | 125 (118; 136) | 124 (106; 141) |
| Resting diastolic BP (mm Hg) | 77 (70; 86) | 76 (72; 83) | 77 (68; 83) | 77 (73; 83) |
| Resting HR (bpm) | 68 (63; 77) | 61 (59; 68)⁎⁎ | 71 (60; 78) | 70 (65; 77) |
| Glucose (mmol/l) | 5.2 (4.6; 8.4) | 4.6 (4.5; 6.1)⁎⁎ ; ⁎ | 4.9 (4.7; 5.8) | 5.2 (4.6; 5.7) |
| Total Cholesterol (mmol/l) | 3.2 (3.0; 3.7) | 2.9 (2.3; 3.3)⁎⁎ | 3.6 (3.0; 4.1) | 3.5 (3.1; 3.9) |
| LDL (mmol/l) | 1.8 (1.5; 2.0) | 1.6 (1.0; 1.8)⁎⁎ | 1.8 (1.7; 2.2) | 2.0 (1.5; 2.1) |
| HDL (mmol/l) | 0.9 (0.9; 1.0) | 0.9 (0.8; 1.0) | 0.9 (0.8; 1.1) | 1.0 (0.9; 1.2) |
| TAG (mmol/l) | 1.3 (1.0; 2.0) | 1.2 (0.9; 1.6)⁎⁎ | 1.3 (1.0; 1.6) | 1.2 (1.0; 2.0) |
| oxLDL (U/l) | 106.7 (74.5; 149.3) | 82.8 (57.6; 146.8) | 120.8 (73.7; 141.2) | 123.2 (90.9; 142.3) |
| oxLDL/LDL ratio | 59.9 (43.4; 73.2) | 63.0 (46.1; 80.3) | 54.4 (41.7; 72.3) | 67.3 (50.9; 78.1) |
| hsCPR (mg/l) | 1.34 (0.63; 4.71) | 0.94 (0.60; 1.69)⁎⁎ | 1.76 (1.16; 3.25) | 1.12 (0.75; 4.90) |
| IL-6 (ng/l) | 4.0 (2.3; 5.9) | 3.1 (2.4; 6.3) | 5.4 (3.6; 9.3) | 4.4 (3.1; 7.9) |
| Leucocytes (× 109/l) | 7.3 (6.3; 9.0) | 7.1 (6.1; 8.8)⁎ | 7.9 (6.6; 9.8) | 7.9 (6.1; 8.9) |
| Neutrophils (× 109/l) | 4.4 (3.5; 5.4) | 3.7 (3.2; 4.8)⁎ | 4.8 (3.5; 6.3) | 4.7 (3.8; 5.3) |
All values are presented as medians with 1st and 3rd quartile in parenthesis.
BP — blood pressure, HR — heart rate, BMI — body mass index, LDL — low density lipoprotein, HDL — high density lipoprotein, TAG — triglycerides, oxLDL — oxidized low density lipoprotein, hsCRP — high sensitivity C reactive protein, IL-6 — interleukin 6.
⁎. p < 0.05 both when compared to the parameter before CR.
⁎⁎. p ≤ 0.01 both when compared to the parameter before CR.
During CR we observed important and comparable improvement of exercise capacity in both the diet group and the control group of patients. Significant decline in classic risk factors including all measured parameters of metabolic syndrome, except HDL, was however observed only in the diet group. Such benefits were absent in the control group. No significant changes in inflammatory markers were observed in the control group of patients in contrast to the diet group of patients, where we demonstrated a decline in leucocytes, neutrophils and hsCRP, but no significant decline of IL-6. No significant decline in ox-LDL was observed in either the diet group or the control group of patients (Table 1).
3.2. Comparison of inflammatory markers and oxLDL between smokers and nonsmokers in the diet group and the control group of patients before and after CR
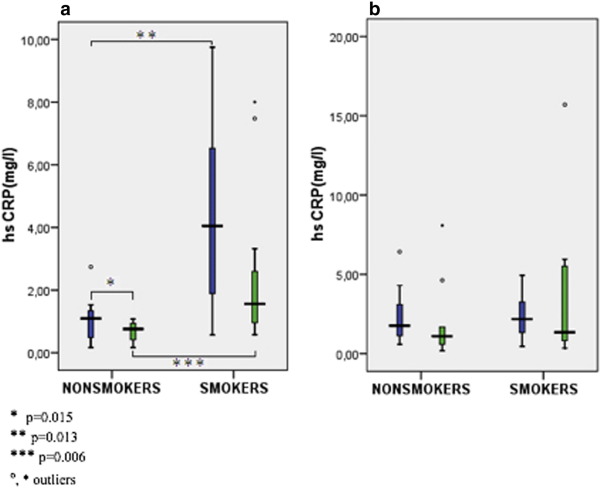
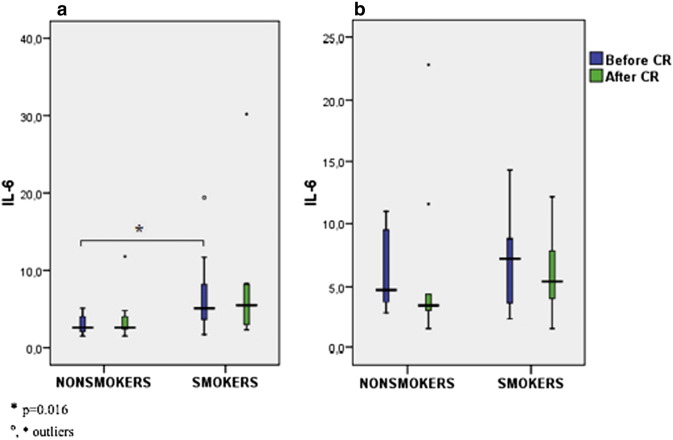
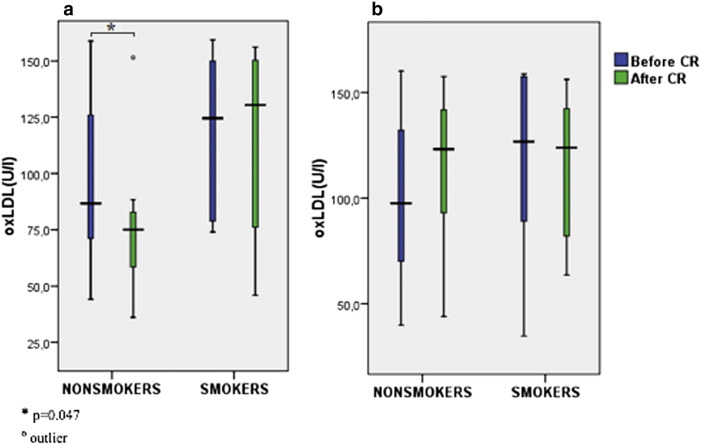
We found significant decline of hsCRP, leucocytes and neutrophils during CR in nonsmoking diets, and a trend towards the decline of hsCRP in smoking diets. On the other hand, differences between pre- and post-CR values of hsCRP in nonsmoking and smoking controls were not significant (Table 2A ; Table 2B, Fig. 1). OxLDL significantly declined in nonsmoking diet group, and it showed a strong trend towards increment in nonsmoking control group (Table 2A ; Table 2B, Fig. 3). While no significant changes in IL-6 were observed during intervention in all subgroups of patients (Fig. 2), smokers presented with a higher concentration of IL-6 in the diet (significant) group and in the control group (nonsignificant) when compared to nonsmokers (Table 2A ; Table 2B).
| Non-smoking diets | Smoking diets | Diets: smokers vs. nonsmokers before CR | Diets: smokers vs. nonsmokers after CR | |||
|---|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | Sig. | Sig. | |
| hsCPR (mg/l) | 1.10 (0.48; 1.39) | 0.76 (0.42; 0.95)⁎ | 4.05 (1.13; 6.79) | 1.56 (0.71; 3.32)# | 0.013 | 0.006 |
| IL-6 (ng/l) | 2.6 (2.1; 4.1) | 2.6 (2.4; 4.4) | 5.1 (3.3; 9.2) | 5.5 (2.9; 8.2) | 0.016 | NS (0.079) |
| Leucocytes (× 109/l) | 6.9 (5.7; 7.3) | 6.1 (5.1; 6.8)⁎ | 8.7 (7.4; 11.2) | 7.1 (7.8; 10.4) | 0.004 | 0.000 |
| Neutrophils (× 109/l) | 3.7 (3.2; 4.3) | 3.2 (2.7; 3.8)⁎⁎ | 5.2 (4.4; 6.2) | 4.6 (3.6; 6.1) | 0.013 | 0.001 |
| oxLDL (U/l) | 86.7 (69.7; 131.7) | 75.1 (54.3; 84.2)⁎ | 124.5 (77.9; 150.2) | 130.4 (56.7;150.9) | NS (0.197) | NS (0.085) |
| oxLDL/LDL ratio | 57.1 (40.8; 73.0) | 54.4 (43.4; 75.4) | 61.3 (45.8; 74.0) | 68.6 (55.8; 88.0) | NS | NS |
| Max. systolic BP (mm Hg) | 176 (157; 204) | 184 (179; 209) | 167 (159; 197) | 188 (150; 210) | NS | NS |
| Max. HR (bpm) | 113 (98; 122) | 121 (109; 138)⁎ | 116 (106; 128) | 119 (105; 121) | NS | NS |
| Double product (mm Hg ∗ bpm) | 20,408 (14,695; 24,018) | 24,028 (19,094; 27,308)⁎ | 20,230 (16,960; 24,426) | 22,372 (20,760; 25,410) | NS | NS |
| Max. load (W) | 111 (99; 153) | 133 (114; 180)⁎ | 112 (75; 125) | 120 (84; 150)⁎ | NS | NS |
| Max. load (MET) | 5.7 (4.8; 6.4) | 6.7 (5.6; 7.3)⁎ | 5.0 (3.2; 5.8) | 4.9 (4.3; 6.4)⁎ | NS | NS (0.051) |
| Time of load (min) | 12.5 (10.8; 13.5) | 13.5 (11.8; 17.3) | 10.0 (8.0; 12.0) | 11.0 (10.0; 13.0) | 0.029 | NS (0.072) |
All values are presented as medians with 1st and 3rd quartile in parenthesis; all when compared to the parameter before CR; hsCRP — high sensitivity C reactive protein, IL-6 — interleukin 6, oxLDL — oxidized low density lipoprotein, BP — blood pressure, HR — heart rate.
⁎. p < 0.05.
⁎⁎. p ≤ 0.01.
- . p ≤ 0.09.
| Non-smoking controls | Smoking controls | Controls: smokers vs. nonsmokers before CR | Controls: smokers vs. nonsmokers after CR | |||
|---|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | Sig. | Sig. | |
| hsCPR (mg/l) | 1.76 (1.12; 3.39) | 1.10 (0.51; 2.42) | 2.18 (1.12; 3.31) | 1.35 (0.80; 5.62) | NS | NS |
| IL-6 (ng/l) | 4.8 (3.6; 9.6) | 3.4 (3.0; 8.0) | 7.3 (3.4; 9.3) | 5.5 (3.6; 8.0) | NS | NS |
| Leucocytes (× 109/l) | 6.1 (5.8; 7.7) | 6.3 (5.8; 7.9) | 9.2 (8.0; 10.6) | 8.5 (8.0; 9.1) | 0.003 | 0.015 |
| Neutrophils (× 109/l) | 3.9 (3.4; 5.1) | 3.9 (3.0; 5.2) | 5.7 (4.3; 7.1) | 4.8 (4.5; 5.8) | 0.023 | 0.029 |
| oxLDL (U/l) | 97.5 (66.0; 134.8) | 123.2 (92.0; 142.4)# | 126.8 (86.3; 157.7) | 123.9 (77.6; 142.5) | NS | NS |
| oxLDL/LDL ratio | 44.3 (32.9; 59.6) | 58.2 (44.7; 67.8) | 63.5 (51.7; 81.5) | 74.8 (52.5; 80.2) | 0.043 | NS (0.095) |
| Max. systolic BP (mm Hg) | 165 (160; 207) | 197 (171; 213)⁎ | 170 (153; 200) | 184 (170; 200) | NS | NS |
| Max. HR (bpm) | 107 (92; 112) | 113 (101; 127)⁎ | 118 (110; 120) | 123 (114; 132)# | NS (0.063) | NS |
| Double product (mm Hg ∗ bpm) | 17,970 (14,080; 24,231) | 23,478 (17,385; 26,911)⁎ | 19,078 (15,257; 23,702) | 22,489 (19,816; 26,669)⁎ | NS | NS |
| Max. load (W) | 101 (79; 121) | 103 (100; 134)⁎ | 100 (84; 120) | 114 (93; 150)⁎ | NS | NS |
| Max. load (MET) | 5.5 (4.6; 6.0) | 5.9 (5.4; 7.0)⁎ | 5.1 (4.5; 6.7) | 5.6 (5.4; 6.2)⁎ | NS | NS |
| Time of load (min) | 9.5 (9.0; 13.5) | 12.0 (10.8; 14.3)⁎ | 11.0 (9.0; 12.3) | 13.0 (10.0; 14.0)⁎ | NS | NS |
All values are presented as medians with 1st and 3rd quartile in parenthesis; all when compared to the parameter before CR; hsCRP — high sensitivity C reactive protein, IL-6 — interleukin 6, oxLDL — oxidized low density lipoprotein, BP — blood pressure, HR — heart rate.
⁎. p < 0.05.
- . p ≤ 0.09.
|
|
|
Fig. 1. Comparison of hsCRP in smokers and non-smokers before and after CR in the diet group (a) and the control (b) group of patients. |
|
|
|
Fig. 2. Comparison IL-6 in smokers and nonsmokers before and after CR in the diet group (a) and the control (b) group of patients. |
|
|
|
Fig. 3. Comparison ox-LDL in smokers and nonsmokers before and after CR in the diet group (a) and the control (b) group of patients. |
3.3. Correlations
Correlations between hsCRP, IL-6 and oxLDL, other inflammatory markers, classic risk factors and exercise testing parameters in the whole group of patients before and after CR are presented in the Table 3.
| hsCRP (mg/l) | IL-6 (ng/l) | oxLDL (U/l) | |||||
|---|---|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | Before CR | After CR | ||
| Leucocytes (× 109/l) | Before CR | 0.440⁎⁎ | 0.428⁎⁎ | 0.422⁎⁎ | NS | NS | NS |
| After CR | 0.346⁎ | 0.543⁎⁎⁎ | 0.388⁎ | NS | NS | NS | |
| Neutrophils (× 109/l) | Before CR | 0.496⁎⁎⁎ | 0.423⁎⁎ | 0.491⁎⁎⁎ | NS | NS | NS |
| After CR | 0.346⁎⁎ | 0.591⁎⁎⁎ | 0.517⁎⁎⁎ | NS | NS | NS | |
| Lymphocites (× 109/l) | Before CR | NS | NS | NS | NS | NS | NS |
| After CR | NS | NS | NS | NS | NS | NS | |
| hsCRP (mg/l) | Before CR | / | 0.348⁎ | 0.549⁎⁎⁎ | NS | 0.341⁎ | 0.302# |
| After CR | 0.348⁎ | / | 0.388⁎ | 0.310# | NS | NS | |
| IL-6 (ng/l) | Before CR | 0.549⁎⁎⁎ | 0.388⁎ | / | NS | 0.352⁎ | 0.350⁎ |
| After CR | NS | NS | NS | / | NS | NS | |
| oxLDL (U/l) | Before CR | 0.341⁎ | NS | 0.352⁎ | NS | / | 0.643⁎⁎⁎ |
| After CR | 0.302# | NS | 0.350⁎ | NS | 0.643⁎⁎⁎ | / | |
| Total cholesterol (mmol/l) | Before CR | 0.307# | NS | 0.329⁎ | NS | 0.516⁎⁎⁎ | 0.527⁎⁎⁎ |
| After CR | 0.301# | NS | 0.306# | NS | 0.461⁎⁎ | 0.725⁎⁎⁎ | |
| LDL (mmol/l) | Before CR | 0.376⁎ | NS | 0.398⁎⁎ | NS | 0.598⁎⁎⁎ | 0.576⁎⁎⁎ |
| After CR | 0.331⁎ | NS | 0.343⁎ | NS | 0.516⁎⁎⁎ | 0.721⁎⁎⁎ | |
| BMI (kg/m2) | Before CR | 0.363⁎ | NS | NS | NS | NS | NS |
| After CR | 0.371⁎ | NS | NS | NS | NS | NS | |
| Resting HR (bpm) | Before CR | 0.286# | NS | NS | NS | NS | NS |
| After CR | 0.397⁎⁎ | 0.350⁎ | 0.448⁎⁎ | NS | 0.329⁎ | NS | |
| Maximal systolic BP (mm Hg) | Before CR | NS | NS | NS | − 0.348⁎ | NS | NS |
| After CR | NS | NS | NS | − 0.441⁎⁎ | NS | NS | |
| Maximal load (MET) | Before CR | − 0.325⁎ | NS | NS | − 0.334⁎ | NS | NS |
| After CR | − 0.358⁎ | NS | NS | NS | NS | NS | |
| Maximal load (W) | Before CR | NS | − 0.412⁎⁎ | NS | − 0.326⁎ | NS | NS |
| After CR | − 0.273# | − 0.364⁎ | NS | NS | NS | NS | |
| Double product | Before CR | NS | NS | NS | − 0.382⁎ | NS | NS |
| After CR | NS | NS | NS | − 0.420⁎⁎ | NS | NS | |
| Time of load (min) | Before CR | NS | − 0.333⁎ | NS | − 0.407⁎ | NS | NS |
| After CR | NS | − 0.347⁎ | NS | NS | NS | NS | |
All values are presented as correlation coefficient; hsCRP — high sensitivity C reactive protein, IL-6 — interleukin 6, oxLDL — oxidized low density lipoprotein, BMI — body mass index, HR — heart rate, BP — blood pressure.
⁎. p < 0.05.
⁎⁎. p ≤ 0.01.
⁎⁎⁎. p ≤ 0.001.
- . p ≤ 0.09
In complete group of patients hsCRP is the parameter which correlated with the most other inflammatory markers (leucocytes, neutrophils, IL-6), oxLDL and BMI. IL-6 is the second and the last is oxLDL, which correlates with the least of inflammatory markers but correlates with blood lipids (Table 3). When we calculated the correlations of patients' subgroups (smokers and nonsmokers) we indeed found similar but less significant correlations (probably due to low number of patients and high variability of parameters) as in the complete group of patients (data not shown). Regarding correlations with parameters of exercise capacity we found in the complete group of patients significant negative correlations between hsCRP, IL-6 and parameters of exercise capacity (Table 3). In smoker subgroup hsCRP before CR correlated negatively with maximal load before CR (p = 0.043, r = − 0.445) and with maximal load after CR (p = 0.007, r = − 0.573), hsCRP after CR correlated negatively with max. load before CR (p = 0.025, r = − 0.488). No similar correlations were found in nonsmokers. In nonsmokers oxLDL after CR negatively correlated with double product before CR (p = 0.030, r = − 0.498) and with double product after CR (p = 0.031, r = − 0.496). No similar correlations were found in smokers.
4. Discussion
The results of our study show that relatively short (two week long) CR program for patients suffering from AMI effectively and appropriately improves patients' exercise capacity. Further, it favorably modifies classic risk factors and also beneficially interferes with the pathological processes of the underlying disease — atherosclerosis. It diminishes observed markers of chronic inflammation and reduces oxidative modification of LDL. However, these positive consequences, as well as significant decline in oxLDL, become pronounced only if we add a supervised, balanced cardioprotective diet rich with natural antioxidants to the CR program and if the patient does not smoke. Thus we could extrapolate that the addition of diet importantly amplifies the beneficial effects of exercise training on both inflammation and oxidized LDL already in relatively short (two week long) CR program in patients suffering from AMI.
It has already been shown that coupled physical activity and diet lead to positive consequences in patients with metabolic syndrome; they not only improve lipid and metabolic profile, but also diminish inflammation and oxidative stress and therefore stabilize lipid plaques [20]. Firstly, as expected, our two week CR program, which includes supervised physical activity and counseling about healthy diet, improved parameters of exercise testing. Improvement of classic risk factors, inflammatory markers and oxLDL levels on the other hand was not significant in the control group of patients, without a supervised diet. Many studies emphasize the importance of a cardioprotective diet as a non-pharmacological measure in secondary prevention of coronary heart disease [27] ; [28]. Contrary to the control group of patients, we observed noteworthy benefits of diet intervention: reduction of classic risk factors, including markers of metabolic syndrome (BMI, waist to hip ratio, LDL, TAG, blood glucose, systolic blood pressure), and reduction of inflammatory marker levels (leucocytes, neutrophils, hsCRP). It has been known that dietary regimens decrease concentration of inflammatory markers such as hsCRP, IL-6, white blood cell count and homocysteine in healthy adults [29] and patients with metabolic syndrome [18], but very few studies described the effect of such diets in patients with fully developed CAD [17]. Our cardioprotective diet, with the addition of walnuts and apples rich in antioxidants, already proved to be effective in a very short time period (2 weeks) in patients after AMI undergoing CR. Thus, cardioprotective diet should become an obligatory part of regular CR programs in health resorts and a strong recommendation for secondary prevention of CAD not only in Slovenia but also in other countries.
Our diet group consisted of significantly younger patients than the control group, which might be a source of bias in our results. It is known that higher age is associated with higher levels of inflammatory and oxidative stress markers [30]; [31] ; [32], but we must keep in mind that no differences in risk factors, inflammatory markers or oxLDL were proved at the starting point of our study. Additionally, most of the studies describing the differences in inflammatory and oxidative stress profile between young people and adults studied groups with a much larger age difference than demonstrated in our study, as our patients from both the control group and the diet group belong to a group of middle aged adults. On the other hand, we believe that patients from the diet group had more severe disease, since they had no significant differences in risk factors than patients from the control group at the inclusion to the study, but suffered from AMI at a younger age. These patients were also highly motivated to participate in the diet intervention, which attested effective. Thus, cardioprotective diet should be advised to all patients after AMI, especially to younger patients with early atherosclerosis.
When trying to interpret the lack of influence of the diet on oxLDL levels and IL-6, we searched for the presence of confounding factors that could have affected our results. It is known that smokers have elevated levels of oxLDL [33] ; [34], as cigarette smoke contains large amounts of oxidants and free radicals, which promote oxidative modification of lipids and proteins [35] ; [36]. Moreover, Hovolet and coworkers demonstrated that current smoking presents one of the strongest risk factor correlates of oxLDL [37]. Only a few studies have mentioned the influence of smoking on IL-6 and have shown that apparently healthy smoking man may have significantly higher IL-6 levels than nonsmoking man [38]. Therefore, we divided patients into smokers and nonsmokers in both the diet group and the control group of patients. We are aware of the difficulty to interpret our results, since some significant associations might be found only by chance, due to measurements of large number of variables in small groups of patients, yet we found some truly interesting results. We observed that nonsmokers in the diet group of patients altogether gained the most benefits from CR: we observed significant decline in inflammatory markers (hsCRP, leucocytes, neutrophils) except IL-6 and significant decline in oxLDL during CR. Thus, positive results of the entire diet group can be regarded mainly to nonsmokers, while we could observe only a trend towards hsCRP decline in smokers from the diet group. Effect of CR was rather similar in smokers and nonsmokers from the control group. This result could imply the importance of diet intervention during CR and smoking cessation before CR to reach the optimal results regarding both inflammatory markers and oxLDL. The two week period of rehabilitation is probably too short to annotate the lack of improvement of certain inflammatory and oxidative markers in smokers only to higher compliance of nonsmokers. It might be that smokers, due to already known higher baseline level of oxidative stress and inflammation [33]; [34]; [39] ; [40], need longer periods of time than nonsmokers to reach the level, where the desired effects become clinically shown. Since physical activity and diet may have synergistic effects, it is possible that smokers, who usually present with lower exercise capacity, were therefore less able to exert the beneficial effects of coupled diet and exercise program. Our study did not demonstrate lower exercise capacity of smokers, but a study performed by Hoad and Clay showed impeded performance adaption of British Army Officer cadets, who were smokers. Performance improvement in 6 month training program proved to be significantly greater in nonsmokers [41]. The underlying mechanisms induced by smoking that mediate impaired adaption to training remain unclear, but several mechanisms such as oxidative stress, inflammation and circulatory hormone levels have been suggested [42]; [43] ; [44]. To add up, we anticipate even better results if patients would have been included in long term supervision after CR, since 2 week rehabilitation program is unfortunately too short for patients to adopt major long term lifestyle changes.
When we calculated correlations, we observed many positive correlations between hsCRP and other inflammatory markers and between hsCRP, IL-6 and oxLDL before CR. OxLDL positively correlated with hsCRP before CR, which is a known fact. Data from the literature even suggest that elevated levels of oxLDL and hsCRP may serve as markers of the severity of coronary artery disease and are related to classical risk factors such as dyslipidemia, obesity and smoking, what was similarly demonstrated also in our study [37] ; [45]. Furthermore, we observed that oxLDL positively correlated with IL-6 before CR, which is in accordance with a previous research, which showed clear correlation and also predictive value of IL-6 and oxLDL in development of mobility limitations in older adults [46]. We discovered that in our complete group of patients, the ones with higher hsCRP presented with lower physical fitness and gained less physical capacity during CR (negative correlations with maximal load before and after CR). Similarly the oxLDL negatively correlated with double product in nonsmokers after CR, which also implicates that higher oxidative stress presents an impediment for exercise capacity gain. The most interesting result regarding correlations between hsCRP, IL-6 and oxLDL was that significant correlations between them demonstrated before CR in the complete group of patients, which were related to lipids and obesity, disappeared after CR. This result further supports our observations that CR has a diverse impact on inflammatory and oxidative stress markers in different subgroups of patients (diet/controls, smokers/nonsmokers). Subsequent trials studying relationships between oxidative stress and inflammatory markers are necessary.
To sum up, our results indicate the importance of diet intervention during CR program. Furthermore, diet positively modifies the inflammatory state and oxLDL mainly in nonsmokers. Smoking showed to be an important unfavorable confounding factor regarding the improvement of exercise capacity, reduction of oxLDL and inflammatory markers. Thus, it is crucial that patients stop smoking before entering the CR program and that diet intervention becomes an integral part of every CR program for patients after AMI. Positive effects of CR might be only transient if patients discontinue to eat healthy, be physically active and start smoking again when coming home from CR program. For long-term benefit of CR program it would be crucial, if patients had support from special associations and also health system, which would enable them to continue with healthy lifestyle.
5. Limitations
This study was an interventional, non-randomized, pilot study on a rather small number of patients and therefore some expected differences between groups might not be significant only due to this fact. On the contrary, some observed significant differences and correlations might be result of measuring several parameters in groups with small number of patients. Further, the results of the study regarding control group of patients might be partly subject to spontaneous disease processes after AMI including reduction markers of inflammation and oxLDL, since we did not include a group of patients with no CR during the same period of time, what we thought would not be ethical. The diet group proved to be significantly younger than the control group, which could result in biased results, however no significant differences in classical risk factors, inflammation markers or oxLDL existed at the start of the CR. Multiple other confounding factors besides smoking, such as motivation and compliance, which might be lower in smokers and could also influence the outcome of the CR, were not studied.
Disclosure statement
The study was completely supported by the Slovenian project Target Research Programs until 2013, grant No. CRP V4-0513. All subjects gave their written informed consent and the approval from the Slovenian board for Medical Ethics No 159/06/10 was obtained before the commencement of the study. The paper or parts of the paper have not been published yet and have not been submitted to any other journal. We are not aware of any financial or other relations that might lead to a conflict of interests. The manuscript has been read and approved by all authors. No competing financial interests exist.
Acknowledgments
Our special thanks go to: head nurse Marinka Sečnik and all medical and nurse staff from Terme Krka Šmarješke Toplice Health resort, Slovenia, who helped with the clinical part of the study; to our colleagues from the Biotechnical faculty Anita Solar, Maja Mikulič Petkovšek, Robert Veberič, Štampar Franci for providing high quality walnuts and apples; and last but not least, to laboratory technicians Marjana Prah and Vera Troha working at Clinical Institute of Clinical Chemistry and Biochemistry, University Medical Center Ljubljana, Slovenia, for the excellent performance of the laboratory investigations.
References
- [1] Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation 125:188-197
- [2] Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Int J Behav Med 19:403-488
- [3] D. Lloyd-Jones, R. Adams, M. Carnethon, G. De Simone, T.B. Ferguson, K. Flegal, et al.; Heart disease and stroke statistics — 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee; Circulation, 119 (2009), pp. 480–486
- [4] R.R. Packard, P. Libby; Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction; Clin Chem, 54 (2008), pp. 24–38
- [5] P. Holvoet, A. Mertens, P. Verhamme, K. Bogaerts, G. Beyens, R. Verhaeghe, et al.; Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease; Arterioscler Thromb Vasc Biol, 21 (2001), pp. 844–848
- [6] J.L. Witztum, D. Steinberg; Role of oxidized low density lipoprotein in atherogenesis; J Clin Invest, 88 (1991), pp. 1785–1792
- [7] R.A. Memon, I. Staprans, M. Noor, W.M. Holleran, Y. Uchida, A.H. Moser, et al.; Infection and inflammation induce LDL oxidation in vivo; Arterioscler Thromb Vasc Biol, 20 (2000), pp. 1536–1542
- [8] H. Yu, N. Rifai; High-sensitivity C-reactive protein and atherosclerosis: from theory to therapy; Clin Biochem, 33 (2000), pp. 601–610
- [9] G.J. Blake, P.M. Ridker; Are statins anti-inflammatory?; Curr Control Trials Cardiovasc Med, 1 (2000), pp. 161–165
- [10] P.M. Ridker, M. Cushman, M.J. Stampfer, R.P. Tracy, C.H. Hennekens; Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men; N Engl J Med, 336 (1997), pp. 973–979
- [11] K.A. Steer, T.M. Wallace, C.H. Bolton, M. Hartog; Aspirin protects low density lipoprotein from oxidative modification; Heart, 77 (1997), pp. 333–337
- [12] M. Aviram, M. Rosenblat, C.L. Bisgaier, R.S. Newton; Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation; Atherosclerosis, 138 (1998), pp. 271–280
- [13] E.P. Plaisance, P.W. Grandjean; Physical activity and high-sensitivity C-reactive protein; Sports Med, 36 (2006), pp. 443–458
- [14] S.G. Wannamethee, G.D. Lowe, P.H. Whincup, A. Rumley, M. Walker, L. Lennon; Physical activity and hemostatic and inflammatory variables in elderly men; Circulation, 105 (2002), pp. 1785–1790
- [15] K.R. Tuttle, L.A. Shuler, D.P. Packard, J.E. Milton, K.B. Daratha, D.M. Bibus, et al.; Comparison of low-fat versus Mediterranean-style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial); Am J Cardiol, 101 (2008), pp. 1523–1530
- [16] A. Trichopoulou, T. Costacou, C. Bamia, D. Trichopoulos; Adherence to a Mediterranean diet and survival in a Greek population; N Engl J Med, 348 (2003), pp. 2599–2608
- [17] D.B. Panagiotakos, K. Dimakopoulou, K. Katsouyanni, T. Bellander, M. Grau, W. Koenig, et al.; Mediterranean diet and inflammatory response in myocardial infarction survivors; Int J Epidemiol, 38 (2009), pp. 856–866
- [18] K. Esposito, R. Marfella, M. Ciotola, C. Di Palo, F. Giugliano, G. Giugliano, et al.; Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial; JAMA, 292 (2004), pp. 1440–1446
- [19] J.K. Wegge, C.K. Roberts, T.H. Ngo, R.J. Barnard; Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease; Metabolism, 53 (2004), pp. 377–381
- [20] C.K. Roberts, D. Won, S. Pruthi, S. Kurtovic, R.K. Sindhu, N.D. Vaziri, et al.; Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors; J Appl Physiol, 100 (2006), pp. 1657–1665 (1985)
- [21] Corra U, Piepoli MF, Carre F, Heuschmann P, Hoffmann U, Verschuren M, Halcox J, Giannuzzi P, Saner H, Wood D, Benzer W, Bjarnason-Wehrens B, Dendale P, Gaita D, McGee H, Mendes M, Niebauer J, Zwisler AD, Schmid JP Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J 31:1967-1974
- [22] Veberic R, Schmitzer V, Petkovsek MM, Stampar F Impact of shelf life on content of primary and secondary metabolites in apple (Malus domestica Borkh.). J Food Sci 75:S461-468
- [23] A. Solar, M. Mikulič Petkovšek, R. Veberič, F. Štampar; Walnuts and apples as a rich source of phenolic compounds; Ann Nutr Metab, 62 (2013), p. 78
- [24] E.M. Macy, T.E. Hayes, R.P. Tracy; Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications; Clin Chem, 43 (1997), pp. 52–58
- [25] O. Chenillot, J. Henny, J. Steinmetz, B. Herbeth, C. Wagner, G. Siest; High sensitivity C-reactive protein: biological variations and reference limits; Clin Chem Lab Med, 38 (2000), pp. 1003–1011
- [26] P. Holvoet, J. Donck, M. Landeloos, E. Brouwers, K. Luijtens, J. Arnout, et al.; Correlation between oxidized low density lipoproteins and von Willebrand factor in chronic renal failure; Thromb Haemost, 76 (1996), pp. 663–669
- [27] de Lorgeril M, Salen P Mediterranean diet in secondary prevention of CHD. Public Health Nutr 14:2333-2337
- [28] Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, Avezum A, Probstfield JL, Dans T, Yusuf S Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 126:2705-2712
- [29] C. Chrysohoou, D.B. Panagiotakos, C. Pitsavos, U.N. Das, C. Stefanadis; Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA Study; J Am Coll Cardiol, 44 (2004), pp. 152–158
- [30] T. Singh, A.B. Newman; Inflammatory markers in population studies od ageing; Ageing Res Rev, 10 (2011), pp. 319–329
- [31] A.L. Nuttall, F. Dunne, M.J. Kendall, U. Martin; Age-independent oxidative stress in elderly patients with non-insulin-dependent diabetes mellitus; QMJ, 92 (1999), pp. 33–38
- [32] T. Finkel, N.J. Holbrook; Oxidants, oxidative stress and the biology of ageing; Nature, 408 (2000), pp. 239–247
- [33] H. Yoshida, K. Sasaki, Y. Hirowatari, H. Kurosawa, N. Sato, N. Furutani, et al.; Increased serum iron may contribute to enhanced oxidation of low-density lipoprotein in smokers in part through changes in lipoxygenase and catalase; Clin Chim Acta, 345 (2004), pp. 161–170
- [34] E. Kassi, M. Dalamaga, E. Faviou, G. Hroussalas, K. Kazanis, C. Nounopoulos, et al.; Circulating oxidized LDL levels, current smoking and obesity in postmenopausal women; Atherosclerosis, 205 (2009), pp. 279–283
- [35] B. Frei, T.M. Forte, B.N. Ames, C.E. Cross; Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid; Biochem J, 277 (1991), pp. 133–138
- [36] M. Reilly, N. Delanty, J.A. Lawson, G.A. FitzGerald; Modulation of oxidant stress in vivo in chronic cigarette smokers; Circulation, 94 (1996), pp. 19–25
- [37] P. Holvoet, N.S. Jenny, P.J. Schreiner, R.P. Tracy, D.R. Jacobs; The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA); Atherosclerosis, 194 (2007), pp. 245–252
- [38] J.M. Fernandez-Real, M. Vayreda, C. Richart, C. Gutierrez, M. Broch, J. Vendrell, et al.; Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women; J Clin Endocrinol Metab, 86 (2001), pp. 1154–1159
- [39] H. Yasue, N. Hirai, Y. Miyuno, E. Harada, T. Itoh, M. Yoshimura, et al.; Low-grade inflammation, trombogenicity, and atherogenic lipid profile in cigarette smokers; Circ J, 70 (2006), pp. 8–13
- [40] C. Madsen, P. Nafstad, L. Eikvar, P.E. Schwarze, K.S. Ronningen, L.L. Haaheim; Association between tobacco smoke exposure and levels of C-reactive protein in the Oslo II Study; Eur J Epidemiol, 22 (2007), pp. 311–317
- [41] N.A. Hoad, D.N. Clay; Smoking impairs the response to a physical training regime: a study of officer cadets; J R Army Med Corps, 138 (1992), pp. 115–117
- [42] S. Basu, J. Helmersson, D. Jarosinska, G. Sällsten, B. Mazzolai, L. Barregård; Regulatory factors of basal F(2)-isoprostane formation: population, age, gender and smoking habits in humans; Free Radic Res, 43 (2009), pp. 85–91
- [43] J. Helmersson, A. Larsson, B. Vessby, S. Basu; Active smoking and history of smoking are associated with enhanced prostaglandin F(2 alpha), interleukin-6 and F2-isoprostane formation in elderly men; Atherosclerosis, 181 (2005), pp. 201–207
- [44] D. Kapoor, T.H. Jones; Smoking and hormones in health and endocrine disorders; Eur J Endocrinol, 152 (2005), pp. 491–499
- [45] Zhang YC, Wei JJ, Wang F, Chen MT, Zhang MZ Elevated levels of oxidized low-density lipoprotein correlate positively with C-reactive protein in patients with acute coronary syndrome. Cell Biochem Biophys 62:365-372
- [46] M. Cesari, S.B. Kritchevsky, B.J. Nicklas, B.W. Penninx, P. Holvoet, P. Koh-Banerjee, et al.; Lipoprotein peroxidation and mobility limitation: results from the Health, Aging, and Body Composition Study; Arch Intern Med, 165 (2005), pp. 2148–2154
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?