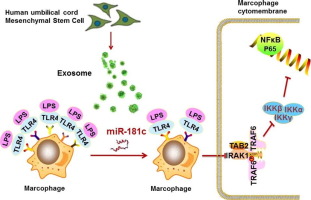
Highlights
- hUCMSC-exosome administration attenuated burn-induced inflammation
- miR-181c in hUCMSC-exosome served an pivotal role in regulating inflammation
- miR-181c repressed burn-induced inflammation via targeting TLR4
This study wants to address the potential function of exosomal miRNA on regulating burn-induced inflammation, and our results indicated that exosome derived from stem cells administration attenuated burn-induced inflammation of rats. Further study elucidated that miR-181c might serve an essential role in regulating inflammation. miR-181c suppressed toll-like receptor 4 expression, and subsequently reduced the expression of pro-inflammatory factors TNF-α, IL-1β. Forced expression of miR-181c in exosome further alleviated burn-induced inflammation. So our study provided a complete understanding of exosomal miR-181c in regulating burn-induced inflammation and its potentials as clinical therapeutic target of burn patients.
Abstract
Mesenchymal stem cell (MSC)-derived exosomes have diverse functions in regulating wound healing and inflammation; however, the molecular mechanism of human umbilical cord MSC (hUCMSC)-derived exosomes in regulating burn-induced inflammation is not well understood. We found that burn injury significantly increased the inflammatory reaction of rats or macrophages exposed to lipopolysaccharide (LPS), increased tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) levels and decreased IL-10 levels. hUCMSC-exosome administration successfully reversed this reaction. Further studies showed that miR-181c in the exosomes played a pivotal role in regulating inflammation. Compared to control hUCMSC-exosomes, hUCMSC-exosomes overexpressing miR-181c more effectively suppressed the TLR4 signaling pathway and alleviated inflammation in burned rats. Administration of miR-181c-expressing hUCMSC-exosomes or TLR4 knockdown significantly reduced LPS-induced TLR4 expression by macrophages and the inflammatory reaction. In summary, miR-181c expression in hUCMSC-exosomes reduces burn-induced inflammation by downregulating the TLR4 signaling pathway.
Graphical Abstract
Keywords
Exosome ; miR-181c ; Burn ; Inflammation
1. Introduction
Various source-derived mesenchymal stem cells (MSCs) show promise as cell-based therapies. They have emerged as a productive, feasible, accepted, and universal source by various methods of MSC isolation (Karahuseyinoglu et al., 2007 and Frausin et al., 2015 ). Previous studies have shown that human umbilical cord mesenchymal stem cells (hUCMSCs) can be applied to alleviate inflammatory diseases, such as colitis, pancreatitis, sepsis and focal cerebral ischemia (Liang et al., 2011 and Li et al., 2012 ; Lin et al., 2012 ).
Recent studies have shown that the majority of the therapeutic benefits of MSCs result from the release of paracrine soluble factors (Liang et al., 2014 ). Exosomes are microvesicles secreted from the endosomal membrane and have been shown to act as regulators of cell-cell communication (E.L. et al., 2013 and Gangoda et al., 2015 ). A rapidly increasing number of reports have suggested that MSC-derived exosomes are a very attractive candidate for cell therapy applications in several diseases (Katsuda et al., 2013 , Akyurekli et al., 2015 and Rani et al., 2015 ). Exosomes range in size from 10 to 100 nm and contain proteins, mRNA and miRNA molecules, which can be functionally transferred between cells and affect protein expression in target cells (Tetta et al., 2013 , Colombo et al., 2014 and Turturici et al., 2014 ).
MiRNAs are a class of endogenous small noncoding RNAs responsible for post-transcriptional regulation of gene expression that have been implicated in the pathogenesis of many diseases (Lee et al., 1993 and Rebane and Akdis, 2013 ). Emerging evidence has suggested that miRNAs reduce inflammation by regulating target proteins in inflammatory signaling pathways (Cardoso et al., 2015 and Chiu and Wu, 2015 ). miR-181c has been suggested as an essential therapeutic in anti-inflammation treatment (Takahashi et al., 2012 ). Previous studies have demonstrated that miR-181c plays a potential role in regulating TNF-a expression after ischemia-mediated neuronal injury and hypoxia microglial activation-induced neuroinflammation (Zhang et al., 2012 and Hutchison et al., 2013 ). We previously compared miRNA expression between severe burn rats and sham rats by miRNA array and found that miR-181c was deregulated in early stage burn rats (Haijun et al., 2015 ).
Reports from our laboratory have demonstrated that hUCMSCs remarkably decreased the number of inflammatory cells and TNF-a, IL-1, IL-6 levels and increased IL-10 levels in wounds of severe burn rats (Liu et al., 2014 ). However, the mechanisms underlying the therapeutic effects of hUCMSCs in excessive inflammation of severe burns are unclear. We hypothesized that miRNAs transferred by hUCMSC-exosomes suppress the severe burn-induced inflammatory response via specific gene expression regulated by miRNAs.
In this study, we performed miRNA profiling of hUCMSC-exosomes by miRNA array to identify miRNAs significantly enriched in hUCMSC-exosomes between severe burn rat and sham rat. We focused on the role of hUCMSC-exosomes in severe burn-induced excessive inflammation in vitro and in vivo, and we performed miR-181c gain of function experiments to increase the miR-181c level in hUCMSC-exosomes to test whether miR-181c suppressed NF-κB activation and downstream proinflammatory factors by downregulating the TLR4 signaling pathway.
2. Materials and Methods
2.1. Exosome Isolation (Salama et al., 2014 )
Exosomes were isolated and purified from the supernatant of hUCMSCs and hSFCs. Briefly, hUCMSCs (catalog #7530) and hSFCs (catalog #2320) were purchased from Sciencell company (Carlsbad, CA, USA) and the cells at passages 3–8 were cultured in serum-free medium, supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2 for 48 h before harvesting the medium. Conditioned medium was centrifuged at 2000g for 10 min at 4 °C to remove cell debris and then passed through a 0.22 μm filter. The cleared supernatant was transferred to a new glass tube and kept on ice. The supernatant was mixed with A/B/C solution (101Bio company, CA, USA) (2 ml supernatant with 0.75 ml A/B/C solution) in a new tube, vigorously vortexed for 30 s, and incubated at 4 °C for 30 min. The mixture separated into 2 layers, and the top layer was removed and discarded. The bottom layer was transferred to a microcentrifuge tube and spun at 5000g for 3 min. The middle fluff layer was transferred to a new microcentrifuge tube and was spun at 5000g for 3 min. The cap was left open to air dry for 10 min at room temperature. A total of 4 × volume of 1 × PBS was added to the tube and pipetted vigorously. The tube was placed on a horizontal shaker for 15 min at high speed, then spun at 5000g for 5 min. The supernatant was carefully transferred to a PureExo® Column (101Bio company, CA, USA) and spun at 1000g for 5 min. The flow-through contained the isolated pure exosome fraction suspended in PBS (Thery et al., 2006 ).
2.2. Transmission Electron Microscopy Analysis of hUCMSC-exosomes
Three drops of fresh exosome suspension (10 μl each) were deposited on clean parafilm. Formvar-carbon coated EM grids were floated onto the drop with the coated side facing the suspension. The grid membranes were allowed to absorb exosomes for 15 min in a dry environment. Wash buffer was used to clean the grid twice on parafilm, and the grids were transferred (coated side down) to wash buffer drops. The grid was incubated in wash buffer drops for 1 min. A 10 μl drop of EM solution was deposited onto clean parafilm. The grids were transferred (coated side down) to the drop and incubated for 15 min, then washed twice with wash buffer. The grids were transferred to filter paper with the coated side up and allowed to air dry at room temperature for 10 min. Exosomes were examined on a HITACHI H-7650 transmission electron microscope (HITACHI company, Japan) at 80 kv.
2.3. NanoSight Dynamic Light Scattering Analysis of hUCMSC-exosomes
A total of 10 ml supernatant from cells cultured for 48 h was used to isolate hUCMSC-exosomes using the PureExo® Exosome Isolation kit (101Bio, California, USA). The exosome pellet was re-suspended in 100 μl PBS and further analyzed by NanoSight dynamic light scattering (632.8 nm laser) using a BI 200SM Research Goniometer System (Brookhaven Instruments Corporation, Holtsville, NY, USA).
2.4. MicroRNA Array Analysis of hUCMSC-exosomes
Total RNA was extracted from hUCMSC-exosomes using an exosomal RNA extraction kit (101Bio, CA, USA) according to the manufacturers instructions. This protocol effectively recovers both mRNA and miRNA. Array experiments were performed by Genechem Corp. (Shanghai, China) as described on the companys website (http://www.genechem.com.cn ) and in previous reports using an miRNA array (GeneChip miRNA Array; Affymetrix Inc., Santa Clara, CA, USA), which contains 6153 probe sets from the miRNAs registered in the Sanger miRBase miRNA database (release 21.0; http://www.mirbase.org/; accessed Jun 2014). miRNA expression levels (normalized total reads) in hUCMSC-exosomes and fold-change enrichment were calculated.
2.5. miR-181c Pre-treatment of hUCMSC-exosomes
hUCMSCs were cultured in six-well plates and were pretreated with siPORT NeoFX containing 100 nM miR-181c mimics or Negative Control (Genechem Corp., Shanghai, China) at 80% cell confluence for 24 h. The medium was replaced, and the cells were cultured for an additional 48 h. The medium was collected for exosome isolation.
2.6. Severe Burn Rat Model and hUCMSC-exosome Administration
All rat studies were performed in accordance with the National Institutes of Health guide for the care and use of guidelines and approved by the Institutional Animal Care and Use Committee at the First Affiliated Hospital of PLA General Hospital. Six-week-old adult male SD rats (weighing 200 ± 20 g) purchased from Academy of Military Medical Sciences Laboratory Animal Center were used for all experiments. A rat model of third degree 30% total body surface area (TBSA) and full-thickness burn wound was established as described previously. Briefly, rats were anesthetized by intraperitoneal injection of 300 mg/kg Avertin (20 mg/ml) (2,2,2-tribro-moethanol, Sigma, USA), and the dorsal hair was completely removed. The backside of the rats was placed in hot water (94 °C) for 12 s, which caused a 30% TBSA full-thickness burn. Balanced salt solution (40 mg/kg) was immediately administered to prevent shock. The wound was treated with a 1% tincture of iodine and kept dry to prevent infection.
Burn rats were randomly assigned to different treatment groups via tail vein injection: hUCMSC-exosome group, injection with 800 μg (RNA concentration) hUCMSC-exosomes suspended in 1 ml PBS; hSFC-exosome group, 800 μg injection of hSFC-exosomes suspended in 1 ml PBS; miR-181c-pretreated hUCMSC-exosome group, 800 μg hUCMSC-exosomes transfected with miR-181c suspended in 1 ml PBS; negative control pretreated hUCMSC-exosomes, 800 μg hUCMSC-exosomes transfected with negative control suspended in 1 ml PBS. Sham group rats were placed in water at 37 °C for 12 s and subjected to the same processes as those applied to the burned rats. Wounds were left open and animals were sacrificed at 24 or 48 h after administration.
2.7. Administration of Macrophages with hUCMSC-exosomes or siRNA Transfection
To knockdown TLR4 gene expression, oligonucleotides (5′-CTGCTGGATGGTAAATCAT-3′) specific to mouse TLR4 mRNA (siTLR4) or nonsense oligonucleotides (5′-TTCTCCGAACGTGTCACGT-3′) were inserted into the multiple cloning sites of the GV248 vector using restriction endonuclease AngI and EcoRI. Mouse RAW264.7 cells were purchased from Peking Union Medical College and seeded in 6-well plate with serum-free medium. Upon reaching 60–70% confluence, cells were transfected with siRNA specific to mouse TLR4 or nonsense plasmid using Lipofectamine™ 2000 reagent as previously described (Yu et al., 2014 ), or administered with 500 μg (RNA concentration) hUCMSC-exosomes suspended in 500 μl PBS, 500 μg hUCMSC-miR-181c-exosomes suspended in 500 μl PBS or 500 μg hUCMSC-NC-exosomes suspended in 500 μl PBS. After treatment for 6 h, cells were stimulated with LPS (100 ng/ml) for 24 h, and cells and media were collected for further analysis.
2.8. Measurement of Inflammatory Factors
Plasma samples were obtained from rats at different time points after burn injury. Cell culture medium samples were collected from RAW264.7 cells at 24 or 48 h after treatment. The levels of TNF-a (ab46070 for rat, ab100474 for mouse), IL-1β (ab100768 for rat, ab100704 for mouse), and IL-10 (ab100765 for rat, ab46103 for mouse) were detected by a multidetection microplate reader using a double-antibody sandwich ELISA kit (Abcam, Cambridge, MA, USA) according to the manufacturers protocols.
2.9. Histological Analyses
Histological analyses were performed as described previously (Liu et al., 2014 ). The specimens were embedded in paraffin after fixation with 4% paraformaldehyde. Preparative sections were stained with hematoxylin-eosin (HE) staining for light microscopy. Other sections were incubated with specific antibodies against CD-68, or MPO (Santa Cruz Biotechnology, Santa Cruz, CA), CD-68- and MPO-positive inflammatory cells in the wounds were counted in 5 randomly selected fields on each slide.
2.10. Western Blotting Analysis
Western blotting was performed as described previously. Briefly, total protein was extracted from RAW264.7 cells and skin tissue samples. Protein was quantified using a commercial bicinchoninic acid (BCA) kit (BCA Protein Assay Kit; Pierce Biotechnology Inc., Rockford, IL, USA). Equal amounts of protein were separated by SDS-PAGE gel. Antibodies specific to TLR4 (ab22048), NF-κB/P65 (ab16502), phospho-P65 (ab86299), and GAPDH (ab9485) were purchased from Abcam (Cambridge, MA, USA). Target proteins were detected using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Uppsala, Sweden).
2.11. Real-time PCR Detection
Total RNA was extracted from RAW264.7 cells and rat skin tissues. Real-time PCR was used to compare the relative expression levels of TLR4 mRNA by SYBR Green I system. TaqMan miRNA assays (Applied Biosystems Inc., Carlsbad, CA, USA) were used to quantify miR-181c expression levels according to the manufacturers protocol. Primer sequences were as follows: rat TLR4 (forward: 5′-GATGATGCCTCTCTTGCATC-3′; reverse: 5′-GGATTCAAGCTTCCTGGTCT-3′), mouse TLR4 (forward: 5′- TCATGGCACTGTTCTTCTCC-3′; reverse: 5′- TCATCAGGGACTTTGCTGAG-3′), rat GAPDH (forward: 5′-CAACTCCCTCAAGATTGTCAGCAA-3′; reverse: 5′-GGCATGGACTGTGGTCATGA-3′), mouse GAPDH (forward: 5′-GGGTGTGAACCATGAGAAGT-3′; reverse: 5′-GACTGTGGTCATGAGTCCT-3′), miR-181c (forward: 5′-ATGGTTCGTGCGAACATTCAACCTGTCGG-3′; reverse: 5′-GCAGGGTCCGAGGTATTC-3′) and U6 (forward: 5′-GCTTCGGCAGCACATATACTAAAAT-3′; reverse: 5′-CGCTTCACGAATTTGCGTGTCAT-3′). All data measurements were performed to obtain a mean CT value for each sample. The CT values of the different samples were compared using the 2−ΔΔCT method.
2.12. Flow Cytometry for hUCMSCs Identification.
The hUCMSCs were seeded to 6-well plate and cultured for 48 h. After trypsinization, the cells were collected and incubated with antibodies against FITC-CD44 (ab6124), FITC-CD90 (ab23894), FITC-CD105 (ab44967), FITC-HLA-I (ab23840), FITC-CD31 (ab27333), FITC-CD34 (ab18227), FITC-CD45 (ab10559), and PE-HLA-DR (ab95830) at 37 °C for 30 min from light, respectively. Then the cells were collected after centrifugation and washed twice using PBS. The fluorescence was assessed by flow cytometry (FC500; Beckman Coulter, Brea, CA, USA).
2.13. Statistical Analysis
All data were analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterization of hUCMSC-exosomes
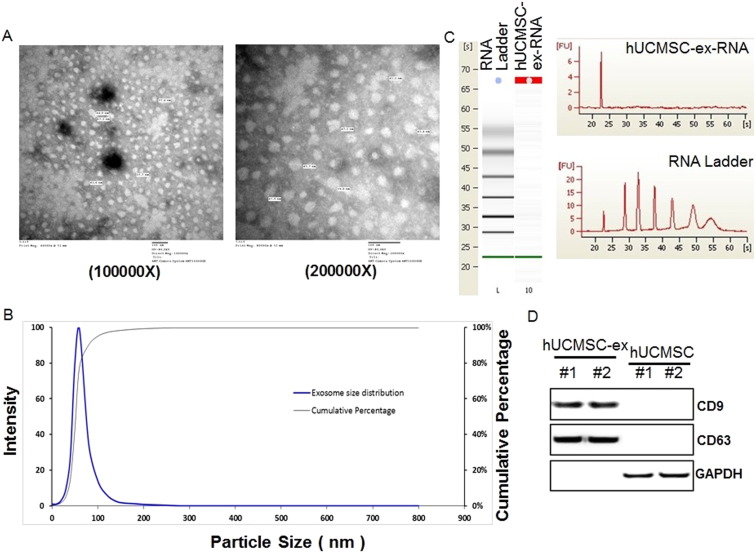
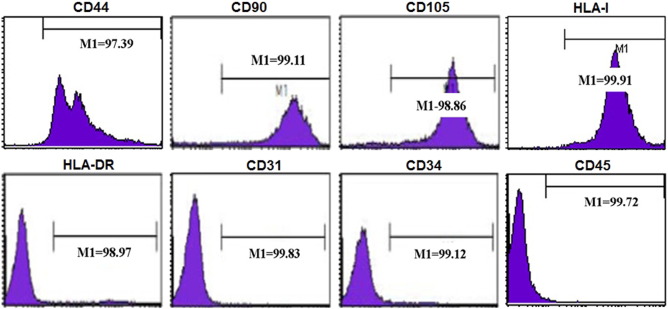
Exosomes are small phospholipid bilayer vesicles ranging in size from 30–100 nm, and they exert various biological functions. hUCMSCs were cultured and identified using flow cytometry assay. As shown in Fig. S1 , the surface markers, CD44, CD90, CD105, and HLA-I, were observed. And the expressions of CD34, CD45, CD31, and HLA-DR were negative. To explore the effect of hUCMSC-exosomes on the burn-induced inflammatory response, we isolated exosomes from hUCMSC supernatant. We confirmed that we indeed isolated exosomes using several methods. Firstly, we used transmission electron microscopy to measure the size of hUCMSC-exosomes. hUCMSC-derived exosomes were round vesicles and ranged in size from 30 to 100 nm (Fig. 1 A). We next used dynamic light scattering to accurately measure the diameter distribution of hUCMSC-exosomes. As shown in Fig. 1 B, the diameter of isolated exosomes ranged from 30 to 100 nm, with a single peak at approximately 60 nm. Finally, we subjected an RNA ladder and exosomal RNA to agarose gel electrophoresis. Ribosomal RNA, which was abundant in mammalian cells, was not present in hUCMSC-exosomes, though they were rich in small RNAs (Fig. 1 C, left panel). We confirmed these results by Agilent 2100 Bioanalyzer analysis (Fig. 1 C, right panel). And the markers of exosomes, CD9 and CD63, were also detected by western blot, the results showed that CD9 and CD63 were rich in exosome samples (Fig. 1 D). Taken together, the results indicated that the isolated hUCMSC-exosome fraction was pure and could be used for subsequent experiments.
|
|
|
Fig. 1. Characterization of exosomes derived from human umbilical cord mesenchymal stem cells (hUCMSCs). hUCMSC-exosomes were isolated and purified from hUCMSC supernatant using a 101Bio Exosome Kit. (A). Identification of the main morphological characteristics of hUCMSC-exosomes by transmission electron microscopy images, Scale bar = 100 nm/200 nm. (B). Dynamic light scattering (DLS) number distribution measurement of hUCMSC-exosomes have a single peak (~ 60 nm) diameter. (C). RNA expression profiles of hUCMSC-exosomes by Genechip analysis. (D). The markers of exosomes are detected using western blot assay. |
3.2. hUCMSC-exosomes Alleviated the Inflammatory Response of Rats after Burn Injury
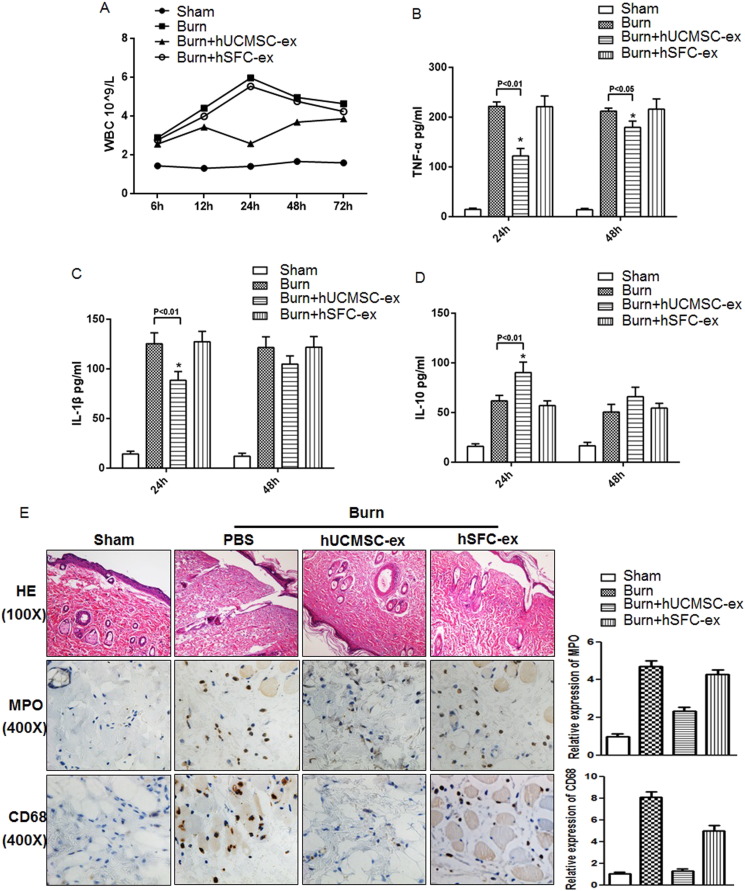
To meet the strict diagnosis criteria and to serve as a tool to investigate the potential of therapeutics, we established a stable and optimized rat model with 30% TBSA full-thickness burn injury, which induces an excessive inflammatory response. Compared to the sham group, the total white blood cell count was significantly higher in burn rats (Fig. 2 A). Interestingly, hUCMSC-exosome administration dramatically reduced the number of total white blood cells induced by burn injury, especially after 24 h (Fig. 2 A). hSFC-exosomes were used as a negative control, as their administration alone had no effect on the amount of white blood cells after burn injury (Fig. 2 A). We examined the inflammatory factors present in rat serum using ELISA assay and found that TNF-a (Fig. 2 B) and IL-1β levels (Fig. 2 C) remarkably increased after burn injury compared to the sham group. Treatment with hUCMSC-exosomes successfully reduced their levels. Interestingly, the anti-inflammatory factor IL-10 was significantly increased in the hUCMSC-exosome administration groups at 24 h (Fig. 2 D). Histological evaluation of cutaneous wounds collected from different groups at 24 h revealed that the number of inflammatory cells in burn rats, such as neutrophils (MPO) and macrophages (CD68) were markedly higher than the sham group. hUCMSC-exosome administration significantly reduced the number of neutrophils (MPO) and macrophages (CD68) compared to the burn group (Fig. 2 E, left panel). And quantative analysis was also done using Image Pro Plus software (Fig. 2 E, right panel). In general, hUCMSC-exosome administration significantly suppressed inflammation after burn injury.
|
|
|
Fig. 2. hUCMSC-exosomes alleviate the inflammatory response in severe burn rats. 30% TBSA full-thickness burn and sham rats were used, and burn rats were treated with hUCMSC-exosomes, hSFC-exosomes or PBS via tail vein injection. Rats were sacrificed, and serum and skin tissues were collected at different time points after treatment. (A). Total WBCs were detected at the indicated times in sham and burn rats treated with hUCMSC-exosomes, hSFC-exosomes or PBS. Data are shown as mean ± SD, n = 6 for each treatment. (B–D). ELISA analysis of serum TNF-α, IL-1β and IL-10 levels in sham and burn rats treated with hUCMSC-exosomes, hSFC-exosomes or PBS at the indicated times. Data are shown as mean ± SD, n = 6 for hUCMSC-exosome treated, n = 6 for hSFC-exosome treated, and n = 5 for PBS treated. (E). Representative histological micrographs analysis (HE stain) and positive MPO, CD68 staining in cutaneous wounds from different groups were examined by immunohistochemistry at 24 h. And the quantitive assay is done using Image Pro Plus software. |
3.3. hUCMSC-exosomes Inhibited TLR4 Signaling Pathway Activation in the Cutaneous Wound of Severe Burn Rats
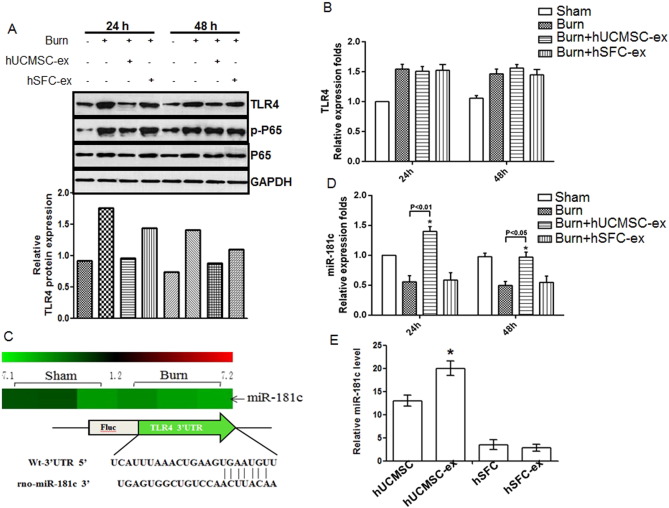
The TLR4 signaling pathway is one of the most important regulators of the inflammatory reaction. To confirm the role of hUCMSC-exosomes in inhibiting inflammation, we examined TLR4 expression and its downstream target proteins NF-κB/P65, and p-P65 in the cutaneous wound of rats after different treatments. Compared with the sham group, TLR4, NF-κB/P65 and p-P65 protein levels were significantly increased in burn rats, and hUCMSC-exosome administration significantly downregulated TLR4, NF-κB/P65 and p-P65 protein expression, especially at 24 h. hSFC-exosomes had no effect on TLR4, NF-κB/P65 and p-P65 protein expression after burn injury (Fig. 3 A, upper panel). And the consistent result was gained after quantification of relative TLR4 protein level (Fig. 3 A, lower panel). We also examined TLR4 mRNA levels in cutaneous wound of rats from different groups by RT-PCR and found that TLR4 mRNA levels (Fig. 3 B) increased after burn injury compared to the sham group, but hUCMSC-exosome administration did not affect TLR4 mRNA expression. Because hUCMSC-exosomes regulated TLR4 protein levels but not mRNA levels, we examined potential mediators of this effect. We compared miRNA expression between severe burn rats and sham rats by miRNA array and found that miR-181c expression was remarkably lower in burn rats compared to sham rats (Fig. 3 C). Sequence alignment using TargetScan software indicated that miR-181c could bind to the TLR4 mRNA 3′-untranslational region (Fig. 3 C). The report also demonstrated that miR-181c could bind to 3′-UTR of TLR4 mRNA, and downregulation its protein expression (Zhang et al., 2015 ). Furthermore, miR-181c was upregulated in the cutaneous wound of hUCMSC-exosome administration group, especially at 24 h (Fig. 3 D). To confirm the active component of hUCMSC-exosomes in anti-inflammation, we compared miR-181c expression in hUCMSCs, hUCMSC-exosomes, hSFCs and hSFC-exosomes. miR-181c expression was remarkably higher in the hUCMSC-exosome group compared to the hSFC-exosome group (Fig. 3 E). Taken together, these data suggest that hUCMSC-exosome administration significantly increased miR-181c expression in cutaneous wounds, which might contribute to regulation of TLR4 protein translation.
|
|
|
Fig. 3. hUCMSC-exosomes decreased TLR4 protein level and increased relative miR-181c expression in severe burn rats. Samples collected from rats from different groups or cultured cells were used to further analyze the potential molecular mechanism. (A). Western blot assay for TLR4 expression and its downstream target proteins NF-κB/P65, p-P65 in cutaneous wounds of rats from different groups. And the relative protein level of TLR4 is measured by Image J software. (B). Quantitative analysis of relative TLR4 mRNA expression in cutaneous wounds of rats from different groups at the indicated times. Data are shown as mean ± SD, n = 6 for each treatment. (C). MiRNA array comparing the relative miR-181c level in cutaneous wounds from severe burn rats and normal skin of sham rats. (D). Quantitative analysis of relative miR-181c expression in cutaneous wounds of sham and severe burn rats treated with hUCMSC-exosomes, hSFC-exosomes or PBS at the indicated times. Data are shown as mean ± SD, n = 6 for each treatment. (E). miR-181c levels in hUCMSCs, hUCMSC-exosomes, hSFCs and hSFC-exosomes were detected by quantitative RT-PCR. Data are shown as mean ± SD, n = 5 for each treatment. |
3.4. hUCMSC-exosomes Suppressed LPS-induced Macrophage Inflammation
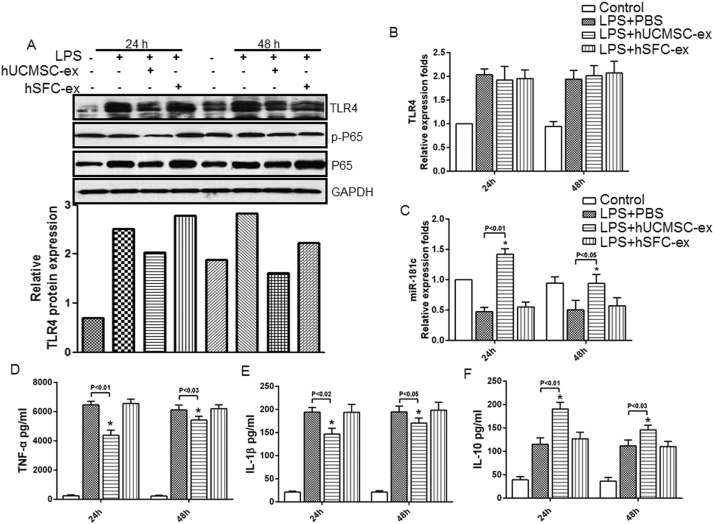
To identify the mechanisms by which hUCMSC-exosomes exerted anti-inflammatory effects, we used LPS-stimulated macrophages as an inflammatory cell model. Compared to the in control group, TLR4, NF-κB/P65 and p-P65 protein levels were significantly higher in LPS-stimulated macrophages, and hUCMSC-exosome administration significantly downregulated LPS-induced TLR4, NF-κB/P65 and p-P65 protein expression, especially at 24 h (Fig. 4 A, upper panel). Compared to hUCMSC-exosomes, hSFC-exosomes had no effect on inflammatory protein levels after LPS stimulation (Fig. 4 A, upper panel). The quantative data also showed that hUCMSC-exosome administration significantly reduced the protein level of TLR4 (Fig. 4 A, lower panel). As in vivo, RT-PCR analysis also showed that TLR4 mRNA levels (Fig. 4 B) increased after LPS stimulation compared to the control group, while hUCMSC-exosome administration had no effect on TLR4 mRNA expression. Similarly, miR-181c levels were upregulated in LPS-stimulated macrophages after hUCMSC-exosome administration, especially at 24 h (Fig. 4 C). We performed ELISA assays to examine the inflammatory factors in the supernatant of cultured macrophages. The results demonstrated that TNF-a (Fig. 4 D) and IL-1β levels (Fig. 4 E) significantly increased after LPS stimulation compared to the control group, and hUCMSC-exosomes successfully decreased their levels. Consistent with the in vivo results, IL-10 levels notably increased in the hUCMSC-exosome administration groups after 24 h (Fig. 4 F). The above results demonstrated that hUCMSC-exosomes suppressed LPS-induced macrophage inflammation.
|
|
|
Fig. 4. hUCMSC-exosomes suppressed the LPS-stimulated inflammatory response in macrophages. Mouse RAW264.7 macrophages were cocultured in normal medium or medium containing hUCMSC-exosomes, hSFC-exosomes, or PBS for 6 h, and the cells were then treated with or without 100 ng/ml LPS for the indicated times. Cell supernatant and cells were collected after LPS stimulation. (A). Total protein was extracted from cell samples, and after quantitative analysis, equal protein samples were subjected to western blot assay. And the relative protein level of TLR4 is quantified using Image J software. (B–C). Quantitative analysis of relative TLR4 levels (B) and miR-181c levels (C) in macrophages after the indicated treatment for 24 or 48 h. Data are shown as mean ± SD, n = 5 for each treatment. (D–E). ELISA analysis for TNF-α, IL-1β and IL-10 levels in macrophage supernatants after the indicated treatment for 24 h. Data are shown as mean ± SD, n = 5 for each treatment. |
3.5. MiR-181c Inhibited TLR4 Expression and Suppressed the Inflammatory Reaction
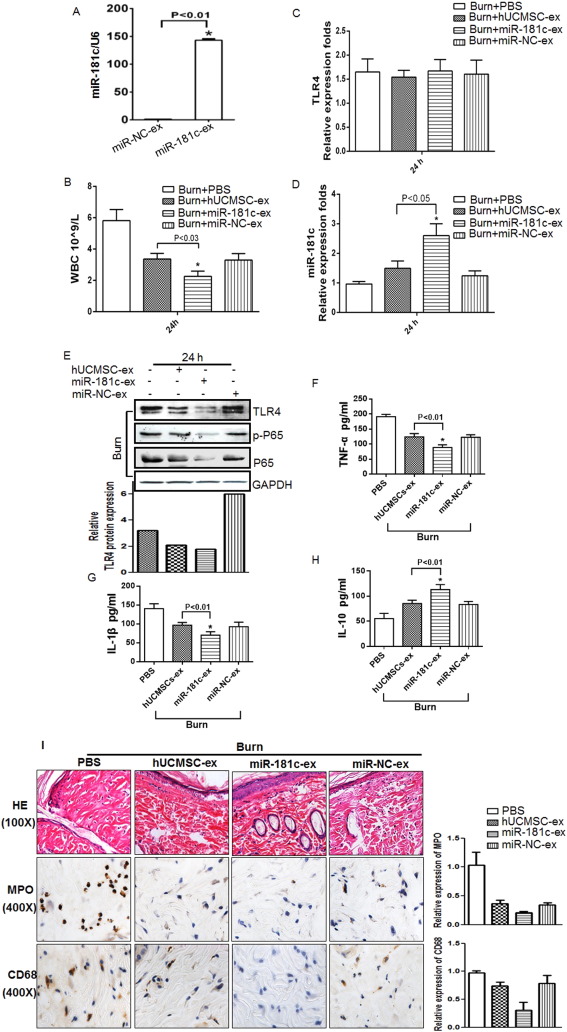
The above results suggested that miR-181c is involved in the regulation of burn-induced inflammation. To verify the relationship between the inflammatory response and miR-181c expression, we injected severe burn rats with exosomes derived from miR-181c- or miR-NC-transfected hUCMSCs. We transfected hUCMSCs with miR-181c or miR-NC and confirmed that miR-181c levels increased upon miR-181c transfection (Fig. 5 A). We then compared the anti-inflammatory function of the miR-181c-exosome administration group and the hUCMSC-exosome administration group and found that miR-181c-exosome administration dramatically decreased total white blood cell count 24 h after severe burn injury compared to the hUCMSC-exosome group (Fig. 5 B). RT-PCR analysis showed that TLR4 mRNA levels were not significantly different between the groups (Fig. 5 C), but miR-181c levels were obviously increased in the cutaneous wound of the miR-181c-exosome administration group at 24 h (Fig. 5 D). TLR4, NF-κB/P65 and p-P65 protein levels were significantly decreased in the miR-181c-exosome administration group at 24 h compared to the hUCMSC-exosome administration group (Fig. 5 E, upper panel), and quantative analysis of TLR4 protein level was also done using Image J software (Fig. 5 E, lower panel). We also examined the inflammatory factors in the serum from the indicated groups by ELISA assay and found that TNF-a (Fig. 5 F) and IL-1β levels (Fig. 5 G) significantly decreased in the miR-181c-exosome administration group. Furthermore, IL-10 levels were higher in the miR-181c-exosome administration group at 24 h (Fig. 5 H). We also performed histological evaluation of cutaneous wounds from different groups at 24 h. The number of inflammatory cells in the miR-181c-exosome administration groups, including neutrophils (MPO) and macrophages (CD68), were markedly lower than that in the hUCMSC-exosome administration group (Fig. 5 I, left panel). And quantative analysis was also done using Image Pro Plus software (Fig. 5 I, right panel). In general, miR-181c-exosome administration notably inhibited inflammation after burn injury. These data suggest that miR-181c plays a critical role in regulating burn-induced inflammation by downregulating TLR4 protein expression.
|
|
|
Fig. 5. MiR-181c in hUCMSC-exosomes mediated the alleviation of TLR4 signaling in severe burn rats. 30% TBSA full-thickness burn rats were intravenously injected with hUCMSC-exosomes, miR-181C-exosomes, miR-NC-exosomes or PBS. Rats were sacrificed, and serum and skin wound tissues were collected 24 h after administration. (A). Quantitative analysis for relative miR-181c expression in hUCMSCs transfected with miR-181c. Data are shown as mean ± SD, n = 3. (B). Total WBCs in severe burn rats were detected 24 h after treatment with hUCMSC-exosomes, miR-181C-exosomes, miR-NC-exosomes or PBS. Data are shown as mean ± SD, n = 6 for each treatment. (C–D). Quantitative analysis for relative TLR4 levels (C) and miR-181c levels (D) in cutaneous wounds from severe burn rats after intravenous injection of hUCMSC-exosomes, miR-181C-exosomes, miR-NC-exosomes or PBS at 24 h. Data are shown as mean ± SD, n = 6 for each treatment. (E). Western blot assay for TLR4 expression and downstream target proteins NF-κB/P65, and p-P65 in cutaneous wounds from severe burn rats after the indicated treatment for 24 h. And the relative protein level of TLR4 is quantified using Image J software. (F–H). ELISA analysis of TNF-α (F), IL-1β (G) and IL-10 (H) serum levels in severe burn rats after 24 h. Data are shown as mean ± SD, n = 6 for each treatment. (I). Representative histological micrograph analysis (HE stain) and positive MPO and CD68 staining of cutaneous wounds after the indicated treatment for 24 h. And the quantitive assay is done using Image Pro Plus software. |
3.6. hUCMSC-exosome-derived miR-181c Restricted LPS-induced Inflammation by Targeting TLR4
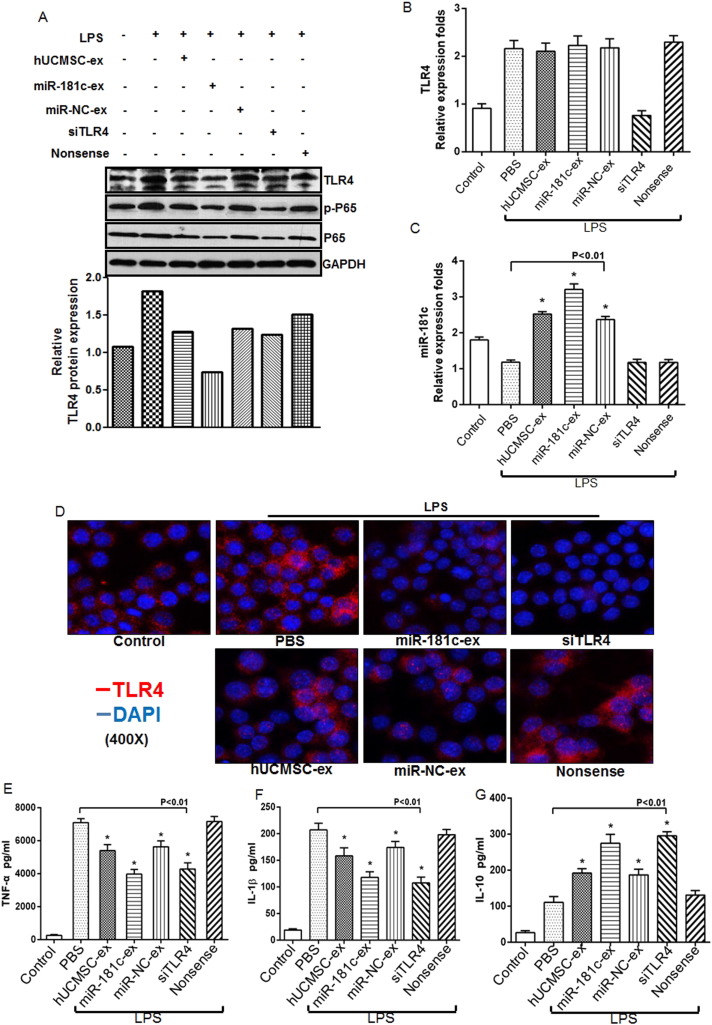
To explore the mechanism by which hUCMSC-exosome-derived miR-181c suppressed inflammation, we treated macrophages with exosomes derived from miR-181- or miR-NC-transfected hUCMSCs, or transfected macrophages with TLR4 or nonsense siRNA. After 6 h, we treated cells with LPS. Consistent with our in vivo results, TLR4, NF-κB/P65 and p-P65 protein expression was significantly downregulated in the miR-181c-exosome group and TLR4 siRNA group at 24 h compared to hUCMSC-exosome administration (Fig. 6 A, upper panel). And the consistent result was gained after quantification of relative TLR4 protein level (Fig. 6 A, lower panel). RT-PCR analysis also showed that miR-181c-exosome administration had no effect on TLR4 mRNA expression, which was significantly decreased in TLR4 siRNA-transfected macrophages (Fig. 6 B). miR-181c levels were notably upregulated in LPS-stimulated macrophages in the miR-181c-exosome administration group and TLR4 siRNA group at 24 h (Fig. 6 C). To observe the influence of exosomes and siRNA on TLR4 expression, we performed immunofluorescent staining to detect surface TLR4 expression by macrophages after multiple treatments. We found that compared to the control group, TLR4 expression significantly increased after LPS exposure, and miR-181c-exosome administration or TLR4 siRNA transfection dramatically reduced TLR4 expression ELISA assay revealed that macrophages secreted significantly lower levels of TNF-a (Fig. 6 E) and IL-1β (Fig. 6 F) after miR-181c-exosome or TLR4 siRNA administration compared to the other groups at 24 h. miR-181c-exosome or siRNA TLR4 administration successfully increased IL-10 levels at 24 h (Fig. 6 G). These results suggested that miR-181c inhibited LPS-induced inflammation by downregulating TLR4 pathway activation.
|
|
|
Fig. 6. hUCMSC-exosome-derived miRNA-181c targets TLR4 signaling to inhibit LPS-induced inflammation. Mouse RAW264.7 macrophages were cocultured with hUCMSC-exosomes, miR-181c-exosomes, miR-NC-exosomes and PBS or transfected with siTLR4 or nonsense. Cells were treated with or without 100 ng/ml LPS for 24 h, and the cell supernatant and cells were collected. (A). Western blot assay for TLR4 expression and downstream target proteins NF-κB/P65 and p-P65 after the indicated treatment for 24 h. And the relative protein level of TLR4 is quantified using Image J software. (B–C). Quantitative analysis for relative TLR4 levels (B) and miR-181c levels (C) after the indicated treatment for 24 h. Data are shown as mean ± SD, n = 5 for each treatment. (D). Representative immunofluorescence images of TLR4 expression in macrophages after the indicated treatment for 24 h. (E–G). ELISA analysis for TNF-α (E), IL-1β (F) and IL-10 (G) in the supernatant of macrophages after the indicated treatment for 24 h. Data are shown as mean ± SD, n = 5 for each treatment. |
4. Discussion
Recent studies have demonstrated that hUCMSC administration can reduce inflammation in many disease models. hUCMSCs significantly reduced systemic inflammation and attenuated LPS-induced acute lung injury in rats (Li et al., 2012 ), reduced inflammatory cytokine production and regulated the balance of the coagulation and anticoagulation system to cure immune-related thrombophilia (Lin et al., 2012 ), and inhibited IFN-γ expression and IL-17 production by lamina propria mononuclear cells (LPMCs) in colitic mice (Liang et al., 2011 ).
Interestingly, MSCs have been found to secrete exosomes, which are enriched in the extracellular environment. Exosomes have been considered vital mediators of cellular communication and may regulate various physiological and pathological processes by transferring membrane proteins, mRNAs, and miRNAs to recipient cells (Hu et al., 2012 , Hoshino et al., 2013 and Kowal et al., 2014 ). Recent studies have demonstrated that exosomes can act at different stages of the inflammatory response by transporting bioactive factors, and MSC-exosomes have reported to suppress inflammation in different animal models (Choi et al., 2014 , Zhu et al., 2014 and Monsel et al., 2015 ). Zhu YG et al. confirmed that MSC-released microvesicles reduced extravascular lung water, neutrophil influx and macrophage inflammatory protein-2 levels in the bronchoalveolar lavage (BAL) fluid in an acute lung injury mouse model, partly through KGF mRNA expression (Zhu et al., 2014 ). There is limited knowledge on the role of hUCMSC-exosomes in severe burn-induced excessive inflammation.
Excessive inflammation is an early systemic inflammatory response that can result in multiple organ failure and even death (Leclerc et al., 2011 ). miRNAs enriched in exosomes play a pivotal role in regulating inflammation (Wang et al., 2015 ). miR-155 and miR-146a are two critical regulators of inflammation, as exosomal miR-146a administration represses inflammatory gene expression and reduces endotoxin-induced inflammation in mice (Alexander et al., 2015 ). miR-181c abrogates TLR4 expression by directly binding to its 3′-UTR to restrict the inflammatory response (Zhang et al., 2015 ).
Our study demonstrated a significant beneficial effect from hUCMSC-exosome administration on severe burn-induced excessive inflammation. We found that hUCMSC-exosomes reduced the number of total white blood cells and downregulated TLR4, NF-κB/P65 and p-P65 protein expression, which prevented the release of inflammatory factors such as IL-1β and TNF-α. The therapeutic effects of hUCMSC-exosome administration in our study were achieved through tail vein injection, suggesting that the homing characteristic of hUCMSC-exosomes may help them reach the site of inflammation.
TLR4 is a key protein that has been reported to play an important role in inflammation (Ping et al., 2012 ). Since TLR4 was identified as the LPS receptor, it has been suggested to trigger all LPS responses, and macrophage is key causative participants in this process (Plociennikowska et al., 2015 ). Previous studies have shown that TLR4 expression in activated microglia mediates neuroinflammation through nuclear factor-κB (NF-κB) in response to brain injury (Mao et al., 2012 ). The NF-κB family consists of five members, including p105/p50, p100/p52, p65/Rel A, Rel B and c-Rel (Yu et al., 2009 ). In silent cells, NF-κB is kept inactive by binding to IκB proteins. Upon LPS stimulation, IκB proteins are targeted for degradation by the 26S proteasome after IκB kinase (IKK) phosphorylation (Yu et al., 2009 ). p65 can then translocate into the nucleus and initiate inflammatory gene expression.
Additionally, we demonstrated that through inflammatory signaling, the TLR4 receptor regulates miR-181 and that reduced miR-181 levels after severe burn-induced excessive inflammation may be a pro-survival mechanism. The results of our study showed that after severe burn or LPS stimulation of macrophages, hUCMSC-exosome or miR-181c-exosome administration successfully restricted TLR4 expression, which may subsequently reduce p65 phosphorylation to regulate inflammatory factor expression.
Hutchison ER et al. previously showed that miR-181 knockdown enhanced LPS-induced production of TNF-a, IL-6, IL-1β, IL-8 and HMGB1, while miR-181 overexpression resulted in a significant increase in anti-inflammatory cytokine IL-10 expression in the neuroinflammatory responses of astrocytes (Hutchison et al., 2013 ). In the serum of severe burn rats, hUCMSC-exosomes suppressed TNF-α and IL-1β secretion and increased IL-10 secretion. IL-10 is considered an anti-inflammatory cytokine, and a previous study found that macrophage-derived IL-10 is necessary for the effect of BMSCs in mouse sepsis models (Nemeth et al., 2009 ). In mouse macrophage cell line cultures, hUCMSC-exosomes downregulated TNF-α and IL-1β levels and upregulated IL-10 levels after LPS stimulation, suggesting an immunomodulatory effect in animal models similar to cultured cells. However, hUCMSC-exosomes do not affect the expression of TNF-α, IL-1β and IL-10 after 24 h, suggesting that a second dose may be necessary in the burn rat models in future studies.
In summary, our study elucidated the importance of the mechanism by which miR-181c regulates burn-induced inflammation. hUCMSC-exosome-derived miR-181c decreased TLR4 expression and subsequently reduced NF-κB/p65 activation, which was an important mediator in regulating inflammatory factor expression and restricting burn-induced inflammation in rats. Therefore, we have defined a critical role for exosomal miR-181c in regulating burn-induced inflammation and provided a potential target for clinical therapy of burn patients.
Abbreviations
hUCMSCs- human umbilical cord mesenchymal stem cells
hSFC- human skin fibroblast cell
TLR4- toll-like receptor 4
LPS- lipopolysaccharide
TNF-α- tumor necrosis factor α
IL- interleukin
NF-κB- nuclear factor kappa B
siRNA- small interference RNA
TSBA- total surface burn area
MPO- myeloperoxidase
The following is the supplementary data related to this article.
|
|
|
Fig. S1. The markers of MSC identified by flow cytometry. |
Author Contributions
X.L., L.Y.L., YH.Y. and J.K.C. conceived the study; X.L., L.Y.L., Y.J. and Y.H.Y. performed the experiments; X.L., Y.H.Y., L.Y·W., L.M., and H.N.Y. analyzed the data; X.L. and Y.H.Y. wrote the manuscript; J.K.C. approved the manuscript.
Conflict of Interest Statements
The authors have no conflict of interest to declare.
Acknowledgments
The authors would like to thank Dr. Feng Xu for dynamic light scattering analysis and Dr. Sa Zhang for transmission electron microscopy analysis. The current study was supported by the National Science Foundation of China (grant nos. NSFC81372052 , NSFC81571894 , NSFC81501694 , NSFC81471873 ), and the Nursery Fund of PLA General Hospital14KMM22 .
References
- Akyurekli et al., 2015 C. Akyurekli, Y. Le, R.B. Richardson, D. Fergusson, J. Tay, D.S. Allan; A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles; Stem Cell Rev., 11 (2015), pp. 150–160
- Alexander et al., 2015 M. Alexander, R. Hu, M.C. Runtsch, D.A. Kagele, T.L. Mosbruger, T. Tolmachova, M.C. Seabra, J.L. Round, D.M. Ward, R.M. O'Connell; Exosome-delivered microRNAs modulate the inflammatory response to endotoxin; Nat. Commun., 6 (2015), p. 7321
- Cardoso et al., 2015 A.L. Cardoso, J.R. Guedes, M.C. de Lima; Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions; Curr. Opin. Pharmacol., 26 (2015), pp. 1–9
- Chiu and Wu, 2015 C.C. Chiu, W.S. Wu; Investigation of microRNAs in mouse macrophage responses to lipopolysaccharide-stimulation by combining gene expression with microRNA-target information; BMC Genomics, 16 (Suppl. 12) (2015), p. S13
- Choi et al., 2014 M. Choi, T. Ban, T. Rhim; Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell; Mol. Cell, 37 (2014), pp. 133–139
- Colombo et al., 2014 M. Colombo, G. Raposo, C. Thery; Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles; Annu. Rev. Cell Dev. Biol., 30 (2014), pp. 255–289
- E.L. et al., 2013 A.S. E.L., I. Mager, X.O. Breakefield, M.J. Wood; Extracellular vesicles: biology and emerging therapeutic opportunities; Nat. Rev. Drug Discov., 12 (2013), pp. 347–357
- Frausin et al., 2015 S. Frausin, S. Viventi, L. Verga Falzacappa, M.J. Quattromani, G. Leanza, A. Tommasini, E. Valencic; Whartons jelly derived mesenchymal stromal cells: biological properties, induction of neuronal phenotype and current applications in neurodegeneration research; Acta Histochem., 117 (2015), pp. 329–338
- Gangoda et al., 2015 L. Gangoda, S. Boukouris, M. Liem, H. Kalra, S. Mathivanan; Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic?; Proteomics, 15 (2015), pp. 260–271
- Haijun et al., 2015 Z. Haijun, Y. Yonghui, C. Jiake, D. Hongjie; Detection of the MicroRNA expression profile in skeletal muscles of burn trauma at the early stage in rats; Ulus. Travma Acil Cerrahi Derg., 21 (2015), pp. 241–247
- Hoshino et al., 2013 D. Hoshino, K.C. Kirkbride, K. Costello, E.S. Clark, S. Sinha, N. Grega-Larson, M.J. Tyska, A.M. Weaver; Exosome secretion is enhanced by invadopodia and drives invasive behavior; Cell Rep., 5 (2013), pp. 1159–1168
- Hu et al., 2012 G. Hu, K.M. Drescher, X.M. Chen; Exosomal miRNAs: biological properties and therapeutic potential; Front. Genet., 3 (2012), p. 56
- Hutchison et al., 2013 E.R. Hutchison, E.M. Kawamoto, D.D. Taub, A. Lal, K. Abdelmohsen, Y. Zhang, W.H. Wood 3rd, E. Lehrmann, S. Camandola, K.G. Becker, M. Gorospe, M.P. Mattson; Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes; Glia, 61 (2013), pp. 1018–1028
- Karahuseyinoglu et al., 2007 S. Karahuseyinoglu, O. Cinar, E. Kilic, F. Kara, G.G. Akay, D.O. Demiralp, A. Tukun, D. Uckan, A. Can; Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys; Stem Cells, 25 (2007), pp. 319–331
- Katsuda et al., 2013 T. Katsuda, N. Kosaka, F. Takeshita, T. Ochiya; The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles; Proteomics, 13 (2013), pp. 1637–1653
- Kowal et al., 2014 J. Kowal, M. Tkach, C. Thery; Biogenesis and secretion of exosomes; Curr. Opin. Cell Biol., 29 (2014), pp. 116–125
- Leclerc et al., 2011 T. Leclerc, C. Thepenier, P. Jault, E. Bey, J. Peltzer, M. Trouillas, P. Duhamel, L. Bargues, M. Prat, M. Bonderriter, J.J. Lataillade; Cell therapy of burns; Cell Prolif., 44 (Suppl. 1) (2011), pp. 48–54
- Lee et al., 1993 R.C. Lee, R.L. Feinbaum, V. Ambros; The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14 ; Cell, 75 (1993), pp. 843–854
- Li et al., 2012 J. Li, D. Li, X. Liu, S. Tang, F. Wei; Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats; J. Inflamm. (Lond), 9 (2012), p. 33
- Liang et al., 2011 L. Liang, C. Dong, X. Chen, Z. Fang, J. Xu, M. Liu, X. Zhang, D.S. Gu, D. Wang, W. Du, D. Zhu, Z.C. Han; Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis; Cell Transplant., 20 (2011), pp. 1395–1408
- Liang et al., 2014 X. Liang, Y. Ding, Y. Zhang, H.F. Tse, Q. Lian; Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives; Cell Transplant., 23 (2014), pp. 1045–1059
- Lin et al., 2012 C.M. Lin, J. Gu, Y. Zhang, L.J. Shen, L. Ma, J. Ni, Z.Q. Wang, W. Wu; [Effect of UC-MSCs on inflammation and thrombosis of the rats with collagen type II induced arthritis]; Zhonghua Xue Ye Xue Za Zhi, 33 (2012), pp. 215–219
- Liu et al., 2014 L. Liu, Y. Yu, Y. Hou, J. Chai, H. Duan, W. Chu, H. Zhang, Q. Hu, J. Du; Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats; PLoS One, 9 (2014), Article e88348
- Mao et al., 2012 S.S. Mao, R. Hua, X.P. Zhao, X. Qin, Z.Q. Sun, Y. Zhang, Y.Q. Wu, M.X. Jia, J.L. Cao, Y.M. Zhang; Exogenous administration of PACAP alleviates traumatic brain injury in rats through a mechanism involving the TLR4/MyD88/NF-kappaB pathway; J. Neurotrauma, 29 (2012), pp. 1941–1959
- Monsel et al., 2015 A. Monsel, Y.G. Zhu, S. Gennai, Q. Hao, S. Hu, J.J. Rouby, M. Rosenzwajg, M.A. Matthay, J.W. Lee; Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice; Am. J. Respir. Crit. Care Med., 192 (2015), pp. 324–336
- Nemeth et al., 2009 K. Nemeth, A. Leelahavanichkul, P.S. Yuen, B. Mayer, A. Parmelee, K. Doi, P.G. Robey, K. Leelahavanichkul, B.H. Koller, J.M. Brown, X. Hu, I. Jelinek, R.A. Star, E. Mezey; Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production; Nat. Med., 15 (2009), pp. 42–49
- Ping et al., 2012 L. Ping, N. Ogawa, Y. Zhang, S. Sugai, Y. Masaki, X. Weiguo; p38 mitogen-activated protein kinase and nuclear factor-kappaB facilitate CD40-mediated salivary epithelial cell death; J. Rheumatol., 39 (2012), pp. 1256–1264
- Plociennikowska et al., 2015 A. Plociennikowska, A. Hromada-Judycka, K. Borzecka, K. Kwiatkowska; Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling; Cell. Mol. Life Sci., 72 (2015), pp. 557–581
- Rani et al., 2015 S. Rani, A.E. Ryan, M.D. Griffin, T. Ritter; Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications; Mol. Ther., 23 (2015), pp. 812–823
- Rebane and Akdis, 2013 A. Rebane, C.A. Akdis; MicroRNAs: essential players in the regulation of inflammation; J. Allergy Clin. Immunol., 132 (2013), pp. 15–26
- Salama et al., 2014 A. Salama, N. Fichou, M. Allard, L. Dubreil, L. De Beaurepaire, A. Viel, D. Jegou, S. Bosch, J.M. Bach; MicroRNA-29b modulates innate and antigen-specific immune responses in mouse models of autoimmunity; PLoS One, 9 (2014), Article e106153
- Takahashi et al., 2012 N. Takahashi, T. Nakaoka, N. Yamashita; Profiling of immune-related microRNA expression in human cord blood and adult peripheral blood cells upon proinflammatory stimulation; Eur. J. Haematol., 88 (2012), pp. 31–38
- Tetta et al., 2013 C. Tetta, E. Ghigo, L. Silengo, M.C. Deregibus, G. Camussi; Extracellular vesicles as an emerging mechanism of cell-to-cell communication; Endocrine, 44 (2013), pp. 11–19
- Thery et al., 2006 Thery, C., Amigorena, S., Raposo, G., Clayton, A., 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3 22.
- Turturici et al., 2014 G. Turturici, R. Tinnirello, G. Sconzo, F. Geraci; Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages; Am. J. Phys. Cell Physiol., 306 (2014), pp. C621–C633
- Wang et al., 2015 X. Wang, H. Gu, D. Qin, L. Yang, W. Huang, K. Essandoh, Y. Wang, C.C. Caldwell, T. Peng, B. Zingarelli, G.C. Fan; Exosomal miR-223 contributes to mesenchymal stem cell-elicited Cardioprotection in Polymicrobial sepsis; Sci. Rep., 5 (2015), p. 13721
- Yu et al., 2009 Y. Yu, Y. Wan, C. Huang; The biological functions of NF-kappaB1 (p50) and its potential as an anti-cancer target; Curr. Cancer Drug Targets, 9 (2009), pp. 566–571
- Yu et al., 2014 Y. Yu, J. Chai, H. Zhang, W. Chu, L. Liu, L. Ma, H. Duan, B. Li, D. Li; miR-194 promotes burn-induced hyperglycemia via attenuating IGF-IR expression; Shock, 42 (2014), pp. 578–584
- Zhang et al., 2012 L. Zhang, L.Y. Dong, Y.J. Li, Z. Hong, W.S. Wei; The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor; J. Neuroinflammation, 9 (2012), p. 211
- Zhang et al., 2015 L. Zhang, Y.J. Li, X.Y. Wu, Z. Hong, W.S. Wei; MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4; J. Neurochem., 132 (2015), pp. 713–723
- Zhu et al., 2014 Y.G. Zhu, X.M. Feng, J. Abbott, X.H. Fang, Q. Hao, A. Monsel, J.M. Qu, M.A. Matthay, J.W. Lee; Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice ; Stem Cells, 32 (2014), pp. 116–125
Document information
Published on 06/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?