Abstract
Background and aims
Rheumatic heart disease is one of the most common cause for heart failure and associated mortalities/morbidities in the young population in developing countries like Nepal imparting huge familial, social and manpower burden.
Materials and methods
This is a hospital based descriptive cross-sectional study during June 2014 to April 2016 over a period of 22 months at College of Medical Sciences-Bharatpur including 235 patients with clinical and/or echocardiographic evidence of definite rheumatic heart disease.
Results
The age of the patients ranged from 7 to 76 years with mean age 39.82 ± 4.2 years with female preponderance (F:M = 2.1:1) (p < 0.01). Majority of the rheumatic heart disease patients belonged to 30–44 years (28.78%) followed by 15–29 years (25.75%) and 45–59 years (25.00%). Majority belonged to the low socioeconomic status (60.60%) (p < 0.05). The predominantly involved isolated valve was mitral in 110 patients (46.80%) followed by isolated aortic valve in 22 patients (9.36%) and 79 (33.62%) had dual valvular involvement. The common rheumatic valvular lesions were pure mitral stenosis in 32 (13.61%), isolated mitral regurgitation in 58 (24.68%), combined mitral stenosis/regurgitation in 36 (15.32%), combined mitral/aortic regurgitation in 23 (9.78%) and combined aortic stenosis/regurgitation in 18 (7.66%) patients with few overlappings. The common complications encountered were heart failure in 90 (38.30%) and arrhythmias in 124 (51.00%) patients.130 patients (55.32%) received injectable benzathine penicillin whereas 45 patients (19.15%) preferred oral penicillin V. Surgical intervention was done in 54 (22.97%) patients. 12 (5.10%) expired in the CCU during the course of treatment.
Conclusion
RHD is a leading cause of heart failure among young populations with requirement of prolonged duration of medical treatment and many of them requiring surgery.
Keywords
Rheumatic heart disease;Sequelae;Complications
1. Background
Rheumatic heart disease (RHD) is one of the most common cardiac disease in developing countries like Nepal. According to the WHO, at least 15.6 million people worldwide have RHD. Of the 500,000 individuals who acquire acute rheumatic fever (ARF) every year, 300,000 go on to develop RHD and 233,000 deaths annually are attributable to ARF or RHD [1] ; [2].
RHD leads to early onset of heart failure in the young population along with multiple complications with lots of mortalities and morbidities each year. It also imparts huge economic and social burden in the Nepalese communities.
We do not have adequate studies on the spectrum and related complications of RHD in central Nepal outside Kathmandu valley. This belt is located in the east–central part of the country covering many patients from nearby zones like Narayani, Lumbini, Gandaki and Janakpur. This study is carried out in our tertiary level hospital in central Nepal to study the magnitude of RHD in the overall cardiac outpatients and inpatients and the spectrum of cardiac involvement along with complications.
2. Aims and objectives
This study is designed to study the overall burden of RHD in the cardiology out and inpatients along with spectrum of cardiac involvement with special focus on pattern of valvular involvement. This study will give a real glimpse of burden of RHD in central Nepal.
3. Materials and methods
This is a hospital based cross-sectional study during June 2014 to April 2016 over a period of 22 months at College of Medical Sciences-Bharatpur including 235 patients with clinical and echocardiographic evidence of definite rheumatic heart disease.
3.1. Inclusion criteria
Consecutive 235 patients with echocardiographic evidence of definite rheumatic heart disease were included in the present study.
2012 World Heart Federation criteria for the echocardiographic diagnosis of rheumatic heart disease was applied to reach the diagnosis [3].
3.2. Definite RHD (A, B, C, or D)
- A. Pathological MR and at least 2 morphological features of RHD of the MV
- B. MS mean gradient ≥ 4 mm Hg
- C. Pathological AR and at least 2 morphological features of RHD of the AV
- D. Borderline disease of both the AV and the MV
3.3. Borderline RHD (A, B, or C)
- A. At least 2 morphological features of RHD of the MV without pathological MR or MS
- B. Pathological MR
- C. Pathological AR
3.4. Criteria for pathological mitral regurgitation
(All 4 Doppler echocardiographic criteria must be met).
Seen in 2 views: In at least 1 view, jet length ≥ 2 cm, velocity ≥ 3 m/s for 1 complete envelope, pan-systolic jet in at least 1 envelope.
Morphological Features of RHD.
Features in Mitral Valve (MV): MV leaflet thickening ≥ 3 mm (age-specific), chordal thickening, restricted leaflet motion, excessive leaflet tip motion during systole.
3.5. Pathological aortic regurgitation
Seen in 2 views: In at least 1 view, jet length ≥ 1 cm, velocity ≥ 3 m/s in early diastole, pan-diastolic jet in at least 1 envelope.
Features in aortic valve (AV): Irregular or focal thickening, coaptation defect, restricted leaflet motion, prolapse.
3.6. Exclusion criteria
Those not fitting into the echocardiographic criteria for diagnosis of definite RHD were excluded from the study.
Verbal consent was taken from each patient during the study period.
Data was regularly entered into the SPSS-16 software.
3.7. Statistical analysis
The datas were analysed by the statistician. Number and sex distribution were expressed in mean and standard deviation. Crosstab analysis was done wherever required. P value of < 0.05 was considered significant.
Data was collected from both cardiology outpatients and inpatients (admitted in cardioward/coronary care unit). Relevant data and information were entered into the pre-structured proforma and then analysed by SPSS-16 software.
Ethical clearance was taken from the Institutional review Board Committee of College of Medical Sciences-Bharatpur, Nepal.
4. Observations and results
Out of total 264 patients who were evaluated with 2-dimensional transthoracic echocardiography, 235 patients were categorized to be having definite RHD and 29 patients had possible or borderline RHD. Those with definite RHD were enrolled in our study for further statistical analysis.
The age of the patients ranged from 7 to 76 years with mean age 39.82 ± 4.2 years with female preponderance (F:M = 2.1:1) (p < 0.01).
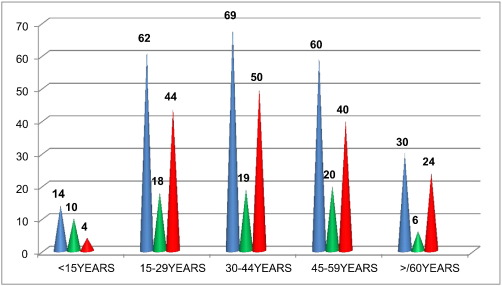
The majority of the RHD patients belonged to 30–44 years (29.36%) followed by 15–29 years (26.38%) and 45–59 years (25.53%) as shown below in the Fig. 1 highlighting the involvement of productive age group.
|
|
|
Fig. 1. RHD distribution with respect to age and sex (n-235). Note: Blue colour represents total number; green represents males and red represents females. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) |
Geographical distribution: The majority of the patients in this study were from Chitwan district (86–36.59%) followed by Nawalparasi (56–23.83%), Makwanpur (26–11.06%), Gorkha (14–5.96%), Tanahun (10–4.25%), Lamjung (8–3.40%) and few from India (10–4.25%).
Majority belonged to low socioeconomic status (63.83%) (p < 0.05). 81 (34.47%) had middle SES and only 4 (1.70%) had good SES.
History of acute rheumatic fever was elicited only in 16 patients (6.80%).
Overcrowding (family members > 6) was noticed in 140 patients (59.57%) (P < 0.01).
22 patients (9.36%) were pregnant all of whom had uneventful antenatal and postnatal history.
Isolated mitral valve was the most commonly affected valve in our study (46.80%) followed by isolated aortic valve. 33.62% had dual involvement of mitral and aortic valve. Only 7.66% had involvement of tricuspid valve and 2.55% had pulmonary valve involvement along with mitral and aortic involvement as shown in Table 1.
| Valve/s involved | Percentage | Number |
|---|---|---|
| Isolated mitral valve | 46.80% | 110 |
| Isolated aortic valve | 9.36% | 22 |
| Mitral and aortic | 33.62% | 79 |
| Mitral, aortic and tricuspid | 7.66% | 18 |
| Mitral, aortic and pulmonary | 2.55% | 6 |
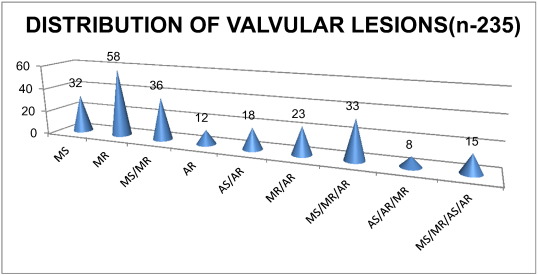
Our patients in this study had both isolated and combined/combination valvular lesions with majority being regurgitant lesions (MR in 173 patients-73.61%). There were many overlappings of the stenotic and regurgitant lesions in a single valve or combined/combination lesions. These are illustrated above in Fig. 2.
|
|
|
Fig. 2. Distribution of Valvular lesions (n-235). |
While assessing the severity of individual valvular lesions, 26 patients (11.06%) had severe MS, 61 patients (25.96%) had severe MR, 9 patients (3.83%) had severe AS and 26 (11.06%) had severe AR. The Table 2 illustrates the severity of valvular lesions in our study.
| Valve lesion | Percentage | Number |
|---|---|---|
| Mitral stenosis | 49.36% | 116 |
| Mild | 15.31% | 36 |
| Moderate | 22.97% | 54 |
| Severe | 11.06% | 26 |
| Mitral regurgitation | 73.62% | 173 |
| Mild | 28.93% | 68 |
| Moderate | 18.72% | 44 |
| Severe | 25.96% | 61 |
| Aortic stenosis | 17.44% | 41 |
| Mild | 11.91% | 28 |
| Moderate | 5.96% | 14 |
| Severe | 3.83% | 9 |
| Aortic regurgitation | 46.38% | 109 |
| Mild | 14.89% | 35 |
| Moderate | 20.42% | 48 |
| Severe | 11.06% | 26 |
Note: Patients has multiple overlappings of rheumatic valvular lesions.
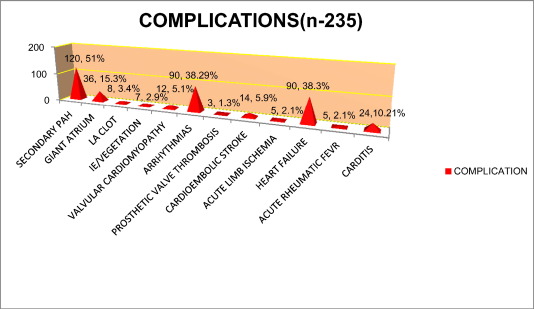
Out of total 235 patients enrolled in this study, secondary pulmonary hypertension (PASP > 30 mm Hg measured by TR jet velocity) was detected in 120 patients (51.06%). Giant atrium (atrial dimension > 7.5 cm) was noted in 36 patients (15.32%) out of whom 2 had upper GI bleeding due to chronic pressure and erosion of esophagus.8 patients (3.40%) had left atrial appendage clot predisposing to risk of thromboembolism like embolic stroke and peripheral lower limbs arterial ischemia. 38.30% of the patients had heart failure and 51.00% had cardiac arrhythmias which is illustrated in the Fig. 3.
|
|
|
Fig. 3. Complications of Rheumatic Heart Disease (n-235). |
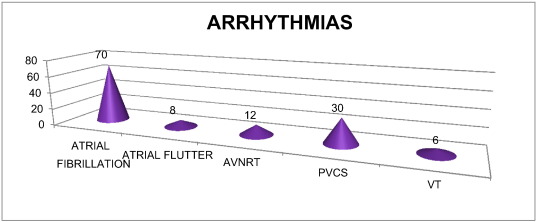
Atrial fibrillation was the most common arrhythmia in our study (70/235–29.78%) as shown in Fig. 4 and was more common with valvular MS (60.24%) (P < 0.05) followed by MR (26.34%).
|
|
|
Fig. 4. Distribution of different arrhythmias (n-126). |
Majority of the patients were managed medically. 54 patients (22.97%) had undergone surgical intervention.
Out of 235 patients, 12 (5.10%) expired in hospital during the study period and the majority of the patients are on regular follow-up.
The causes of death were: refractory heart failure in 7 patients (2.97%), acute pulmonary edema in 4 patients (1.70%) and malignant ventricular arrhythmias in 3 patients (1.27%).
5. Discussion
Rheumatic heart disease is the commonest cardiac disease in children and young adults. It remains a major public health problem in developing countries especially in the rural areas. RHD results from an abnormal autoimmune response to a Group A streptococcal infection in a genetically susceptible host.
There is considerable global variation in acute rheumatic fever incidence and RHD prevalence with the sub-Saharan African and Asian-Pacific regions identified as high disease burden areas [4] ; [5].
Globally there are 15.6–19.6 million patients suffering from RHD out of which majority lie within Asian countries with burden estimated to be 10.8–15.9 million patients [6]. The estimates of RHD in Asian countries indicate that the global burden is significantly higher than the earlier estimates for children as well as all age groups.
The recently published Global Burden of Disease Study reports that the number of years lived with disability due to RHD was estimated in 2010 at 1430 (944 to 2067) worldwide [7]. Lozano et al. [8] reported 345,100 deaths due to RHD in 2010, which represents a 25.40% reduction from 1990, with ages-standardized death rate of 5.2 per 100,000, which was a 53.10% reduction from 1990.
Studies on RHD from different parts of Nepal in the last two decades have shown the prevalence to vary from 1.2 to 1.35 per thousand school aged children [9]; [10]; [11]; [12] ; [13].
The females were predominantly involved due to RHD than males probably because of lack of proper health care and treatment during childhood. The social stigmata and disparity among the genders still prevails in the Nepalese rural community.
Our study highlighted the fact that mitral valve (54.55%) followed by aortic valve and combination/combined valvular lesions are still the most common involvement in RHD.
Mitral regurgiatation isolated along with combined/combination (73.62%) was the most common RHD in our study followed by mitral stenosis, aortic regurgitation and aortic stenosis.
Sanyal S, Berry A, Duggal S, et al. [14] in a prospective study from North India described the chronic MR as the most common form of RHD in children and young adults whereas MS is increasingly common in patients in the fourth to sixth decades of life.
Severe chronic MR may eventually result in ventricular dysfunction with reduced ejection fraction, elevated end-systolic volume and elevated left heart filling pressures.
Rheumatic fever resulting in chronic RHD is the most common cause of mitral stenosis. Symptomatic rheumatic MS may occur as early as second decade of life in children from developing countries of the world. Isolated pure MS was diagnosed in 32 (13.61%) patients but the combined MS lesion with MR contributed 49.36% in our study.
Pure MS occurs in about 25.00% of adults and another 45.00% have combined MS and MR. Women are more likely than men to develop rheumatic mitral stenosis [15].
In mitral stenosis, progressive obstruction to LV inflow develops due to fibrosis and partial fusion of the mitral valve leaflets. Approximately 30.00% of Aboriginal RHD patients in the northern territory aged 10–19 years have mitral stenosis, and the mean age of those with mitral stenosis is 33 years. In the Aboriginal and Torres Strait Islander population, mitral stenosis progresses more rapidly than in the non-Aboriginal and Torres Strait Islander population and patients become symptomatic at a younger age. More rapid progression may be due to undetected recurrences of rheumatic fever [16].
Chronic rheumatic AR occurs because of leaflet thickening, fibrosis and leaflet contracture resulting in abnormal leaflet coaptation and a regurgitant orifice. During compensatory phase, ventricular dilation occurs to maintain forward stroke volume and cardiac output and ejection fraction remains normal. Similar to chronic MR patients, those with chronic severe AR may remain asymptomatic for years. Over time, decompensation may occur resulting in decreased LV function and/or symptoms.
Aortic stenosis occurs 20–40 years after the acute illness as adhesions, leaflet thickening, fibrosis, commissural fusion and calcific nodules may develop over time. Rheumatic AS and AR often occur concurrently usually along with rheumatic mitral valve disease. So, mitral valve should be carefully evaluated in all patients with chronic rheumatic aortic valve disease since coexistent mitral valve involvement is common.
Asymptomatic patients with valvular heart disease can often be followed conservatively as most remain stable for years. Medical management should include serial evaluation to detect interval change and/or the onset of symptoms, prevent complications and to detect change in valvular function, chamber size and ventricular function.
The presence of significant symptoms is a logical and accepted indication but the optimal timing of intervention for asymptomatic children is unclear. Percutaneous balloon valvotomy is effective in many patients with rheumatic mitral stenosis. Mitral valve and aortic valve repair/replacement are performed for adults with severe MR/AR with symptoms and/or left ventricular dysfunction and marked LV enlargement.
The surgical approach to rheumatic tricuspid valve disease is based on the underlying abnormality. A tricuspid annuloplasty may be performed for tricuspid regurgitation owing to annular dilation. Tricuspid commissurotomy is the preferred approach to rheumatic tricuspid stenosis [17].
Many patients in our study had multivalvular involvement. Similar finding is reported from Australia among the aborigines population. Both the mitral and aortic valves may be involved (e.g. aortic regurgitation and mitral stenosis or aortic and mitral regurgitation). Multivalve involvement is seen in 38.00% of patients with MS, with the aortic valve affected in approximately 35.00% and the tricuspid valve in approximately 6.00%. The pulmonic valve is rarely affected. Two thirds of all patients with rheumatic MS are female. The interval between the initial episode of rheumatic fever and clinical evidence of mitral valve obstruction is variable, ranging from a few years to > 20 years [16] ; [18].
The management is usually that of the dominant lesion. However, the proximal valve lesion may modify the effects of the distal lesion e.g. severe mitral stenosis may prevent the development of significant LV dilatation secondary to aortic regurgitation. The progression of the milder valve lesion is variable. However, an Israeli follow-up study (mean 13 ± 7 years) of rheumatic valvular disease patients (mean age 61 ± 10 years) with mild aortic valve disease who had required mitral valve surgery showed that in the vast majority of cases, the aortic valve disease remained stable without disease progression [19] ; [20].
In younger patients, the degree of adherence to antibiotic prophylaxis would be the major determinant of the progression of the non-operated valve disease. The combination of significant mitral and aortic regurgitation is a surgical challenge and carries a high risk of ventricular dysfunction. Surgical intervention is indicated at the onset of symptoms, or if LV dysfunction is identified.
Till date, no primary preventive strategies for acute ARF are available in developing countries like Nepal and India. The only proven cost-effective intervention is secondary prophylaxis i.e., the long-term administration of antibiotics to people with a history of acute ARF or RHD, to prevent ARF recurrences and the development or deterioration of RHD. The best drug for this purpose is intramuscular benzathine penicillin G administered once every 3 weeks. Techniques used to reduce the pain of benzathine penicillin injections include use of small gauze needles, increased injection volumes, addition of 1% lignocaine or procaine penicillin and warming the medication to room temperature. Decisions about duration of secondary prophylaxis relate to the balance between the risk of recurrent ARF and the risk to the patient, should a recurrence occur (higher with increasingly severe heart disease) [21].
5.1. Streptococcus vaccine
Several potential Group A streptococcus vaccines are in development, including a multivalent, M-serotype specific construct [22]. The diversity of M-serotypes limits the efficacy of the vaccines. Various alternative vaccines based on antigens common to all or most strains of Group A streptococcus using either the conserved region of M protein or antigens against non-M protein antigens are in pre-clinical development [23].
Conflict of interest
The authors do not have any conflict of interest including financial in publication of this article.
Acknowledgement
I would like to acknowledge all the staffs of Department of Cardiology for their continuous help and support in taking care of the patients.
References
- [1] J.R. Carapetis; The current evidence of the burden of Group A streptococcal diseases; WHO/FCH/CAH/05–07, World Health Organisation, Geneva (2004), pp. 1–57
- [2] S. Padmavati; Rheumatic fever and rheumatic heart disease in India at the turn of the century; Indian Heart J., 53 (2001), pp. 35–37
- [3] B. Remenyi; World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline; Nat. Rev. Cardiol., 9 (2012), pp. 297–309
- [4] S.F. Rizvi; Status of rheumatic heart disease in rural Pakistan; Heart, 90 (2004), pp. 394–399
- [5] M. Sadiq; Prevalence of rheumatic heart disease in school children of urban Lahore; Heart, 95 (2009), pp. 353–357
- [6] J.R. Carapetis; Rheumatic heart disease in Asia; Circulation, 118 (2008), pp. 2748–2753
- [7] T. Vos, A.D. Flaxman, M. Naghavi, et al.; Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010; Lancet, 380 (2012), pp. 2163–2196
- [8] R. Lozano, M. Naghavi, K. Foreman, et al.; Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010; Lancet, 380 (2012), pp. 2095–2128
- [9] Laudari Shankar, Dhungel Sachin, Sharma K. Sanjib, Gautam Sameer, Dubey Laxman, Subedi Kumudini, et al.; Study of prevalence of rheumatic heart disease and congenital heart disease among school children in central Nepal; World J. Med. Med. Sci. Res., 3 (2015), pp. 14–19
- [10] Dipanker Prajapati, Deewakar Sharma, Prakash Raj Regmi, Harihar Khanal, Sajan Gopal Baidya, Sujeeb Rajbhandari, et al.; Epidemiological survey of rheumatic fever, rheumatic heart disease and congenital heart disease among school children in Kathmandu valley of Nepal; Nepal. Heart J., 10 (2013), pp. 1–5
- [11] K.C. Man Bahadur, D. Sharma, M.P. Shrestha, et al.; Prevalence of rheumatic and congenital heart disease in school children of Kathmandu valley in Nepal; Indian Heart J., 55 (2003), pp. 615–618
- [12] P.R. Regmi, M.R. Pandey; Prevalence of rheumatic fever and rheumatic heart disease in school children of Kathmandu city; Indian Heart J., 49 (1997), pp. 518–520
- [13] Y.R. Limbu, A. Maskey; Current status of rheumatic fever and rheumatic heart disease in Nepal; J. Nepal Med. Assoc., 41 (2002), pp. 514–517
- [14] S. Sanyal, A. Berry, S. Duggal, et al.; Sequelae of the initial attack of acute rheumatic fever in children from North India. A prospective 5-year follow up study; Circulation, 65 (1982), pp. 375–379
- [15] M.R. Essop, V.T. Nkomo; Rheumatic and nonrheumatic valvular heart disease: epidemiology, management, and prevention in Africa; Circulation, 112 (2005), p. 3584
- [16] J.R. Carapetis; Ending the Heartache: The Epidemiology and Control of Acute Rheumatic Fever and Rheumatic Heart Disease in the Top End of the Northern Territory; University of Sydney, Sydney (1998) Unpublished Doctor of Philosophy thesis
- [17] Lloyd Y. Tani; Rheumatic fever and rheumatic heart disease; ,in: Hugh D. Allen, Robert E. Shaddy, David J. Driscoll, Timothy F. Feltes (Eds.), Infants, Children and Adolescents: Moss and Adams' Heart disease (seventh ed.)Vol. 62, , Lippincott Williams and Wilkins, Philadelphia (2008), pp. 1256–1275
- [18] A. Roguin, D. Rinkevich, S. Milo; Long term follow-up of patients with severe rheumatic tricuspid stenosis; Am. Heart J., 136 (1998), pp. 103–108
- [19] C.M. Otto; Echocardiographic Evaluation of Valvular Heart Disease; C.M. Otto, R.O. Bonow (Eds.), Valvular Heart Disease: A Companion to Braunwalds Heart Disease (fourth ed.), Saunders, Philadelphia (2013), pp. 62–85
- [20] V. Mordehay, et al.; The natural history of aortic valve disease after mitral valve surgery; J. Am. Coll. Cardiol., 33 (1999), pp. 2003–2008
- [21] J.R. Carapetis, M. McDonald, N. Wilson; Acute rheumatic fever; Lancet, 366 (2005), pp. 155–168
- [22] K.L. Kotloff, M. Corretti, K. Palmar, et al.; Safety and immunogenicity of a recombinant multivalent group A streptococcal vaccine in healthy adults: a phase 1 trial; JAMA, 292 (2004), pp. 709–715
- [23] K.L. Kotloff, J.B. Dale; Progress in group A streptococcal vaccine development; Pediatr. Infect. Dis. J., 23 (2004), pp. 765–766
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?