Summary
Background/Objective
To investigate the influence of serum anti-thyroglobulin antibody (TgAb) on the prognosis in papillary thyroid cancer (PTC) patients.
Methods
In this retrospective study, the participants were enrolled from 1206 PTC patients (927 women, 279 men; mean age, 42.2 years) with T2 and higher, or N1 or M1 classifications in tumor–node–metastasis staging after total thyroidectomy. We recorded the final serum TgAb data (on thyroxin therapy) at the end of follow-up in 2012. Patients were classified as negative TgAb or positive TgAb groups on the basis of their serum TgAb levels (< 70 IU/mL or ≥ 70 IU/mL).
Results
Among the 1206 patients, after mean follow-up for 11.6 ± 6.1 years (range, 2.0–29.2 years), there were 75 with positive TgAb and 1131 with negative TgAb. Patient categorization depending on the follow-up time (2–5 years after surgery, 5–10 years after surgery, and 10–30 years after surgery) was performed. In comparison to traditional risk factors, such as age, tumor size, and sex, which were important prognostic factors for cancer recurrence and mortality in PTC patients, there was no significant difference in the prognosis between positive TgAb patients and negative TgAb patients by the multivariate analyses (cancer recurrence, p = 0.164, p = 0.112, p = 0.202, respectively; cancer mortality, p = 0.181, p = 0.646, p = 0.656, respectively) based on the different follow-up times.
Conclusion
Positive serum TgAb was not a risk factor, and was not associated with the prognosis of PTC patients.
Keywords
anti-thyroglobulin antibody;mortality;recurrence;total thyroidectomy
1. Introduction
Serum thyroglobulin (Tg) level is an important marker in the follow-up of differentiated thyroid cancer (DTC) patients after total thyroidectomy, and it serves as an indicator for cancer recurrence.1 ; 2 However, it was estimated 10–29% DTC patients may have anti-thyroglobulin antibody (TgAb).3; 4 ; 5 The presence of TgAb is higher in DTC patients, compared with the general population.3 ; 6 TgAb is produced as the result of the presence of thyroid tissue; in addition, the presence of TgAb may influence the measurement of serum Tg levels,7 making the serum Tg levels unreliable. Therefore, the current guideline suggests that all specimens sent for Tg measurement require adjunctive TgAb testing.8
Previous studies show that, after total thyroidectomy, serum TgAb gradually declines over years.5 ; 9 A markedly rising trend in TgAb concentrations during follow-up or persistently high TgAb levels of DTC patients might indicate cancer recurrence or persistence.10; 11 ; 12 Otherwise, conflicting data exist concerning the association of serum TgAb levels after surgery and disease activity in DTC patients after long-term follow-up period.5; 6 ; 13 The aim of this study is to realize whether the presence of high serum TgAb during follow-up is a prognostic factor in patients with papillary thyroid cancers (PTCs).
2. Methods
2.1. Study participants
During the period 1983–2012, a total of 2118 PTC patients with TgAb data underwent treatment and follow-up in Chang Gung Medical Center in Linkou and Keelung, Taiwan. We recruited patients of tumor–node–metastasis (TNM) staging with T2 and higher, or N1 or M1 after total or complete thyroidectomy. Tumor stage had been classified according to the International Union against Cancer TNM criteria (6th edition).14 The enrolled patients were high risk group (any T3 and T4, or any T with N1 or M1 in TNM staging) based on European consensus for the management of DTC patients. In addition, we also included patients with T2N0M0 as most of them had gone total thyroidectomy in our hospital. Patients with T1N0M0 (tumor size, ≤ 2 cm) were excluded from the study because the majority of the patients categorized in T1N0M0 did not undergo total thyroidectomy. Permission was obtained from the Institutional Review Board (IRB) and Ethics Committees of Chang Gung Memorial Hospital for a retrospective review of the medical records of study participants. The IRB waived the requirement for obtaining informed consent. Confidentiality of the research participants was maintained in accordance with the requirements of the IRB of Chang Gung Memorial Hospital. All work was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Follow-up
The patients were treated and followed-up at Chang Gung Medical Center with a minimum postoperative follow-up duration of 2 years. All the patients underwent total or complete thyroidectomy followed by immediate I-131 remnant ablation. We recorded the patients' last serum TgAb levels (on thyroxin therapy) after the mean follow-up of 11.6 ± 6.1 years. The term total thyroidectomy refers to total or near total thyroidectomy with or without central compartment and selective bilateral neck lymph node dissection.
Serum stimulated Tg was measured 1 month postoperatively without thyroxine treatment, and serum Tg, TgAb, and TSH were measured every 3–6 months during thyroxine supplementation. The patients underwent neck ultrasound examination every 6–12 months. Once recurrent lesions were suspected, subsequent larger dose of I-131 were used for treatment. Surgical treatment was performed whenever localized lesions were confirmed. Some patients underwent surgery or I-131 ablation therapy more than once after active thyroid cancer was diagnosed. External beam radiation therapy was used in a few cases of advanced local recurrence or distant bony metastasis.
2.3. Assessment
Patients were classified into negative or positive TgAb groups according to serum TgAb levels (< 70 IU/mL or ≥ 70 IU/mL) at last follow-up data in 2012, and influence of serum TgAb level on prognostic outcome was evaluated. In our institution, TgAb was measured by a competitive radioimmunoassay (Biocode, Liège, Belgium). The analytical sensitivity is 6 IU/mL. The functional sensitivity is measured with a precision of maximum 20% interassay variance and the value is < 15 IU/mL. Interassay precision was mean 90.3 IU/mL: coefficient of variation (CV) 9.3%; 390.0 IU/mL: CV 12.8%; and 985.4 IU/mL: 10.6%. Intra-assay precision was mean 72.6 IU/mL: CV 8.3%; 345.7 IU/mL: CV 7.1%; and 889.9 IU/mL: CV 5.7%. In this study, the manufacturer-recommended cutoff was used in our laboratory. Positivity for TgAb was > 70 IU/mL as suggested according to the instruction for use. Tg was detected by an immunoradiometric kit (CisBio International, CIS Bio International, France, France). The detection limit of the Tg kit is 0.5 ng/mL, and its functional sensitivity has been assessed in our laboratory to be 1.2 ng/mL. The interassay CV was 8.0% at a Tg level of 4.9 ng/mL, 6.9% at a Tg level of 223.2 ng/mL, and 5.1% at a Tg level of 312.9 ng/mL. Normal levels of serum Tg in our laboratory were estimated to be < 25 ng/mL. The Tg level was considered accurate only if the recovery test (performed on all serum samples) was < 80%. Cancer recurrence in this study included recurrence and persistent disease. Recurrence was defined as the reappearance of disease 1 year after total thyroidectomy and was confirmed by neck ultrasound with positive cytology or histopathological findings or extrathyroid I-131 uptake on whole body scanning, fluorine-18 fluorodeoxyglucose positron emission tomography, magnetic resonance imaging, or computed tomography. Persistent disease was defined as the reappearance of disease within 1 year after total thyroidectomy and was confirmed by neck ultrasound with positive cytological or surgical histopathological findings or extrathyroid I-131 uptake on whole body scanning, fluorine-18 fluorodeoxyglucose positron emission tomography, magnetic resonance imaging, or computed tomography.
2.4. Statistical analysis
Discrete data are reported as absolute frequency and percentages, and compared using Chi-squared or Fishers exact tests, where appropriate. Continuous data are reported as means and standard deviation and compared using a Student t test or Mann–Whitney U test, where appropriate. Multivariate analyses were performed using a logistic regression model to identify the association of TgAb with cancer recurrence or cancer mortality after adjusting for other clinicopathological predictors, including age, sex, I-131 treatment dose, and tumor size. All tests were two-tailed, and p < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Study populations
In total, 1206 PTC patients after total thyroidectomy with TgAb data were recruited in the study. There were 927 women and 279 men with a mean age of 42.2 ± 15.3 years. All the patients were followed until the end of 2012 or the date of death.
3.2. Clinical outcomes
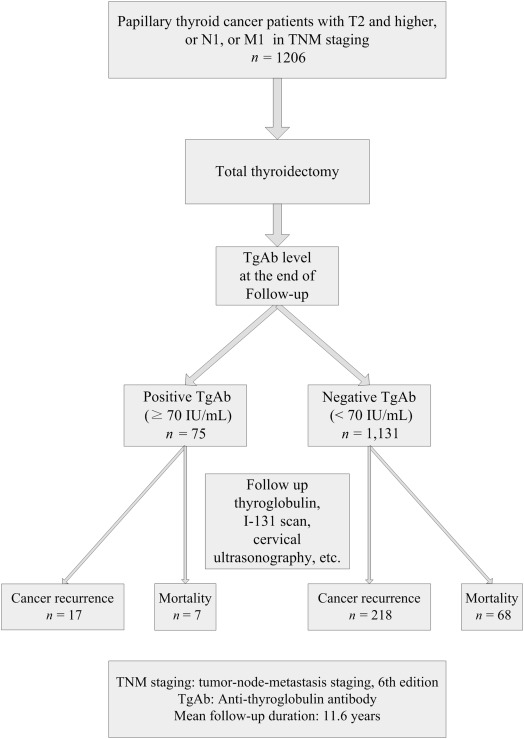
Figure 1 shows the follow-up of the 1206 patients and their clinical outcomes. A total of 75 patients (6.2%) presented with positive TgAb (≥ 70 IU/mL) and 1131 patients (93.8%) presented with negative TgAb (< 70 IU/mL) at the end of follow-up. There were 235 (19.5%) with cancer recurrence, 75 (6.2%) with cancer mortality after the mean follow-up of 11.6 ± 6.1 years (range, 2–27.2 years).
|
|
|
Figure 1. Clinical follow-up of the 1206 patients and therapeutic outcomes of the patients based on their serum anti-thyroglobulin antibody (TgAb) level at the end of follow-up in 2012. |
Because the follow-up time for the patients varied, patient categorization was further performed depending on the follow-up time: 2–5 years after surgery, 5–10 years after surgery, and 10–30 years after surgery. Table 1, Table 2 ; Table 3 compare the clinical characteristics between positive and negative TgAb patients based on the different follow-up time. The tumor size, sex, total I-131 treatment dose during follow-up, and TNM staging were not statistically different between positive and negative TgAb patients based on the different follow-up time, but the patients with positive TgAb during the follow-up period 2–5 years after surgery were younger. The rate of cancer recurrence and cancer mortality between positive and negative TgAb patients were not statistically different based on the different follow-up time.
| TgAb | p | |||
|---|---|---|---|---|
| Total | Negative | Positive | ||
| Number | n = 276 | n = 258 | n = 18 | |
| TgAb (positive) | 18 (6.5%) | |||
| Age (y) | 48.8 ± 15.7 (48; 14–84) | 49.4 ± 15.8 (48; 14–84) | 40.2 ± 10.9 (39.5; 20–58) | 0.016 |
| Sex, Female | 193 (69.9) | 177 (68.6) | 16 (88.9) | 0.700 |
| Male | 83 (30.1) | 81 (31.4) | 2 (11.1) | |
| Tumor size (cm) | 3.4 ± 1.6 (3.0; 0.5–11.1) | 3.4 ± 1.6 (3.0; 0.5–11.1) | 3.3 ± 1.5 (2.75; 1.2–6.0) | 0.897 |
| Postoperative stimulated Tg level (ng/mL) | 633 ± 4678 (4.2; 0–65,320) | 677 ± 4836 (4.3; 0–65,320) | 3.5 ± 4.9 (0; 0–14.8) | 0.556 |
| TNM staging, stages I/II/III/IV (%) | 119/38/90/29 (43.1/13.8/32.6/10.5) | 109/36/87/26 (42.2/14.0/33.7/10.1) | 10/2/3/3 (44.6/11.1/16.7/16.7) | 0.394 |
| Total I-131 therapeutic dose (mCi) | 64 ± 87 (30; 0–600) | 64 ± 88 (30; 0–600) | 63 ± 69 (45; 0–300) | 0.950 |
| Follow-up (y) | 3.1 ± 1.0 (3.0; 1.1–5.0) | 3.1 ± 1.0 (3.0; 1.1–5.0) | 3.2 ± 1.1 (2.9; 1.3–4.8) | 0.622 |
| Cancer recurrence | 68 (24.6) | 63 (24.4) | 5 (27.8) | 0.779 |
| Cancer mortality | 42 (15.2) | 39 (15.1) | 3 (16.7) | 0.743 |
Data are presented as means ± standard deviation (median; range) or n (%), unless otherwise indicated.
Tg = thyroglobulin; TNM = tumor–node–metastasis.
| TgAb | p | |||
|---|---|---|---|---|
| Total | Negative | Positive | ||
| Number | n = 330 | n = 309 | n = 21 | |
| TgAb (positive) | 21 (6.4%) | |||
| Age (y) | 43.7 ± 15.1 (42; 11–83) | 43.7 ± 15.3 (41; 11–83) | 44.1 ± 12.9 (44; 23–66) | 0.912 |
| Sex, Female | 256 (77.6) | 238 (77) | 18 (85.7) | 0.431 |
| Male | 74 (22.4) | 71 (23) | 3 (14.3) | |
| Tumor size (cm) | 3.1 ± 1.3 (2.8; 0.2–10.0) | 3.0 ± 1.3 (2.8; 0.2–10.0) | 3.2 ± 1.3 (2.7; 1.5–7.0) | 0.704 |
| Postoperative stimulated Tg level (ng/mL) | 190 ± 1085 (4.15; 0–12,094) | 202 ± 1120 (4.54; 0–12,094) | 19 ± 83 (0; 0–381) | 0.456 |
| TNM staging, stages I/II/III/IV (%) | 141/41/133/15 (42.7/12.4/40.3/4.5) | 134/38/124/13 (43.4/12.3/40.1/4.2) | 7/3/9/2 (33.3/14.3/42.9/9.5) | 0.766 |
| Total I-131 therapeutic dose (mCi) | 129 ± 205 (60; 0–1800) | 130 ± 205 (60; 0–1800) | 116 ± 216 (60; 29.7–1032.3) | 0.766 |
| Follow-up (y) | 7.8 ± 1.5 (8.0; 5.1–10.0) | 7.8 ± 1.5 (8.0; 5.1–10.0) | 8.1 ± 1.5 (8.25; 5.1–10.0) | 0.395 |
| Cancer recurrence | 56 (17.0) | 52 (16.8) | 4 (19.0) | 0.766 |
| Cancer mortality | 14 (4.2) | 13 (4.2) | 1 (4.8) | 0.609 |
Data are presented as means ± standard deviation (median; range) or n (%), unless otherwise indicated.
Tg = thyroglobulin; TNM = tumor–node–metastasis.
| TgAb | p | |||
|---|---|---|---|---|
| Total | Negative | Positive | ||
| Number | n = 600 | n = 564 | n = 36 | |
| TgAb (positive) | 36 (6.0%) | |||
| Age (y) | 38.3 ± 14.0 (36; 2–75) | 38.3 ± 14.2 (36; 2–75) | 38.2 ± 11.7 (37; 19–65) | 0.967 |
| Sex, Female | 478 (79.7) | 450 (79.8) | 28 (77.8) | 0.771 |
| Male | 122 (20.3) | 114 (20.2) | 8 (22.2) | |
| Tumor size (cm) | 3.3 ± 1.5 (3.0; 0.5–20.0) | 3.3 ± 1.5 [3.0; 0.5–20.0] | 3.1 ± 1.7 (2.8; 1.0–10.0) | 0.589 |
| Postoperative stimulated Tg level (ng/mL) | 44 ± 263 (6; 0–5832) | 46 ± 271 (6; 0–5832) | 13 ± 31 (0; 0–165) | 0.460 |
| TNM staging, stages I/II/III/IV (%) | 301/92/192/15 (50.2/15.3/32.0/2.5) | 287/82/182/13 (50.9/14.5/32.3/2.3) | 14/10/10/2 (38.9/27.8/27.8/5.6) | 0.090 |
| Total I-131 therapeutic dose (mCi) | 168 ± 217 (100; 0–2060) | 168 ± 218 (100; 0–2060) | 171 ± 201 (100; 0–885) | 0.929 |
| Follow-up (y) | 15.6 ± 4.2 (14.7; 10.1–30.2) | 15.7 ± 4.2 (14.8; 10.1–30.2) | 14.4 ± 3.3 (14.1; 10.1–24.7) | 0.039 |
| Cancer recurrence | 111 (18.5) | 103 (18.3) | 8 (22.2) | 0.553 |
| Cancer mortality | 19 (3.2) | 16 (2.8) | 3 (8.31) | 0.099 |
Data are presented as means ± standard deviation (median; range) or n (%), unless otherwise indicated.
Tg = thyroglobulin; TNM = tumor–node–metastasis.
By the multivariate analyses (Tables S1–S3), sex, tumor size at initial diagnosis, and total I-131 treatment dose during follow-up were not related to positive TgAb at the end of follow-up, but being younger patients was associated with positive TgAb during the follow-up period 2–5 years after surgery.
3.3. Multivariate analysis of association between TgAb, cancer recurrence, and cancer mortality
Based on the different follow-up times (2–5 years, 5–10 years, and 10–30 years after surgery), Table 4, Table 5 ; Table 6 summarize the results of multivariate analyses for association of TgAb with cancer recurrence and cancer mortality after adjusting for other clinicopathological predictors, including age, sex, total I-131 treatment dose, and tumor size. The results indicate that traditional risk factors, such as older age and larger tumor size are good predictors for cancer recurrence and cancer mortality. Male sex was also associated with poor prognosis in PTC patients with statistical significance. By contrast, positive serum TgAb levels did not increase the risk of cancer recurrence (p = 0.164, p = 0.112, p = 0.202) or cancer mortality (p = 0.181, p = 0.646, p = 0.656) in PTC patients during the different follow-up time.
| Cancer recurrence | Cancer mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | |||
| lower | upper | lower | upper | |||||
| TgAb | 0.399 | 0.109 | 1.457 | 0.164 | 2.825 | 0.616 | 12.954 | 0.181 |
| Age | 1.041 | 1.017 | 1.066 | 0.001 | 0.938 | 0.911 | 0.967 | < 0.001 |
| Sex | 0.504 | 0.243 | 1.045 | 0.065 | 1.438 | 0.621 | 3.329 | 0.397 |
| I-131 dose | 1.016 | 1.011 | 1.022 | < 0.001 | 0.995 | 0.991 | 0.999 | 0.008 |
| Tumor size | 1.259 | 1.028 | 1.534 | 0.026 | 0.680 | 0.539 | 0.589 | 0.001 |
CI = confidence interval.
| Cancer recurrence | Cancer mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | |||
| lower | upper | lower | upper | |||||
| TgAb | 0.330 | 0.084 | 1.297 | 0.112 | 1.854 | 0.123 | 25.860 | 0.646 |
| Age | 1.030 | 1.003 | 1.057 | 0.027 | 0.899 | 0.848 | 0.954 | < 0.001 |
| Sex | 0.507 | 0.210 | 1.223 | 0.131 | 13.467 | 3.032 | 59.810 | 0.001 |
| I-131 dose | 1.009 | 1.006 | 1.012 | < 0.001 | 0.998 | 0.996 | 1.000 | 0.040 |
| Tumor size | 1.119 | 0.835 | 1.498 | 0.451 | 1.252 | 0.803 | 1.952 | 0.321 |
CI = confidence interval.
| Cancer recurrence | Cancer mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | |||
| lower | upper | lower | upper | |||||
| TgAb | 0.492 | 0.166 | 1.462 | 0.202 | 3.653 | 0.853 | 15.642 | 0.081 |
| Age | 0.982 | 0.962 | 1.002 | 0.080 | 0.954 | 0.920 | 0.991 | 0.014 |
| Sex | 0.671 | 0.351 | 1.284 | 0.228 | 3.384 | 1.209 | 9.470 | 0.020 |
| I-131 dose | 1.008 | 1.006 | 1.010 | < 0.001 | 0.998 | 0.996 | 0.999 | < 0.001 |
| Tumor size | 1.283 | 1.076 | 1.532 | 0.006 | 0.901 | 0.705 | 1.152 | 0.406 |
CI = confidence interval.
4. Discussion
In our laboratory, serum Tg was measured using an immunometric assay method that was prone to interference by TgAb, which might cause an underestimation of serum Tg levels.3; 4; 5 ; 7 The manufacturer-recommended cutoff for TgAb was used in this study and the results of the study showed that the presence of positive TgAb during the different follow-up times (2–5 years, 5–10 years, and 10–30 years after surgery) was not related to sex, tumor size, or total I-131 treatment dose.
Previous study by Görges et al5 reported that 15% of patients had TgAb at 1 year and < 10% at 3 years of follow-up. There was no report of TgAb level after > 10 years follow-up as our finding. Our study found a frequency of 6.2% for positive TgAb with mean follow-up of 11.6 ± 6.1 years (range, 2.0–29.2 years) in PTC patients. Theoretically, TgAb is produced because of the presence of thyroglobulin; therefore, the presence of TgAb may indicate recurrent or persistent disease in DTC patients after total thyroidectomy.15; 16 ; 17 Progressively elevated serum TgAb levels may specifically serve as a marker of the presence of cancer recurrence in DTC patients with low or undetectable serum Tg levels.10; 11 ; 12 However, the clinical significance of postoperative serum TgAb levels as a prognostic marker is controversial.5; 6 ; 13 After total or complete thyroidectomy, close follow-up and aggressive I-131 therapy for high-risk DTC patients without underestimating falsely low Tg values in TgAb-positive patients are important work. In this long-term study, we found that serum TgAb levels during follow-up were unable to predict cancer recurrence or cancer mortality. Although there were no other outcome studies in PTC patients focusing on serum TgAb levels during long-term follow-up, the finding was comparable to the result of Görges et al5 that neither the initial TgAb levels nor the presence of circulating TgAb at the 15–18 month evaluation was associated with the progression of the disease in DTC patients. On the contrary, there was an another study in which they found PTC patients with positive serum TgAb titer during the 1st year after primary treatment were more likely to have persistent or recurrent disease than those who were consistently TgAb-negative.18 However, there is growing recognition that it is the trend in TgAb concentrations that is more prognostic than the presence or absence of TgAb. Sequential TgAb change after total thyroidectomy, changes of serum TgAb levels before and 1–2 years after thyroidectomy, and serum TgAb levels measured at 6–12 months after remnant ablation are all useful for prediction of clinical outcomes in patients with DTC in several recent reports.19; 20 ; 21
Both positive and negative TgAb patients in our study received equally close follow-up and received comparable I-131 dose for ablation therapy, and had comparable clinical outcome. In contrast to serum TgAb levels, large tumor size, and older age, which have long been regarded as good prognostic factors for patients with differentiated thyroid cancer,14; 22; 23 ; 24 were closely associated with cancer recurrence and cancer mortality as well in our study.
There were limitations in this study. TgAb assay was not performed in euthyroid and non-DTC patients in the study. There were only 75 TgAb-positive patients, which might be insufficient to detect differences in prognostic outcome between TgAb-positive and TgAb-negative patients. In addition, we have no data regarding the trend of serum TgAb level, which would be more informative than presence or absence of TgAb.
In summary, unlike traditional risk factors, such as age, tumor size, and serum Tg levels, which are important prognostic factors for cancer recurrence and mortality in PTC patients, positive serum TgAb during follow-up was not related to the prognosis of PTC patients.
Acknowledgments
This work was supported by grants to Sheng-Fong Kuo from the National Science Council in Taiwan (NMRPG2B0012).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1 E.L. Mazzaferri, R.J. Robbins, C.A. Spencer, et al.; A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma; J Clin Endocrinol Metab, 88 (2003), pp. 1433–1441
- 2 M. Schlumberger, A. Hitzel, M.E. Toubert, et al.; Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients; J Clin Endocrinol Metab, 92 (2007), pp. 2487–2495
- 3 A. Kumar, D.H. Shah, U. Shrihari, S.R. Dandekar, U. Vijayan, S.M. Sharma; Significance of antithyroglobulin autoantibodies in differentiated thyroid carcinoma; Thyroid, 4 (1994), pp. 199–202
- 4 F. Pacini, S. Mariotti, N. Formica, et al.; Thyroid autoantibodies in thyroid cancer: incidence and relationship with tumour outcome; Acta Endocrinol (Copenh), 119 (1988), pp. 373–380
- 5 R. Görges, M. Maniecki, W. Jentzen, et al.; Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy; Eur J Endocrinol, 153 (2005), pp. 49–55
- 6 S.L. Souza, L.V. Montalli Da Assumpção, L.S. Ward; Impact of previous thyroid autoimmune diseases on prognosis of patients with well-differentiated thyroid cancer; Thyroid, 13 (2003), pp. 491–495
- 7 C.A. Spencer, M. Takeuchi, M. Kazarosyan, et al.; Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma; J Clin Endocrinol Metab, 83 (1998), pp. 1121–1127
- 8 D.S. Cooper, G.M. Doherty, B.R. Haugen, et al.; Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer; Thyroid, 19 (2009), pp. 1167–1214
- 9 L. Chiovato, F. Latrofa, L.E. Braverman, et al.; Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens; Ann Intern Med, 139 (2003), pp. 346–351
- 10 J.K. Chung, Y.J. Park, T.Y. Kim, et al.; Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation; Clin Endocrinol (Oxf), 57 (2002), pp. 215–221
- 11 O.N. Kucuk, G. Aras, H.A. Kulak, E. Ibis; Clinical importance of anti-thyroglobulin auto-antibodies in patients with differentiated thyroid carcinoma: comparison with 99mTc-MIBI scans; Nucl Med Commun, 27 (2006), pp. 873–876
- 12 C.A. Spencer; Clinical review. Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC); J Clin Endocrinol Metab, 96 (2011), pp. 3615–3627
- 13 O. Soyluk, H. Boztepe, F. Aral, F. Alagol, N.C. Ozbey; Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment; Thyroid, 21 (2011), pp. 1301–1308
- 14 L.H. Sobin; Head and neck tumours; L.H. Sobin, C. Wittekind (Eds.), UICC: TNM Classification of Malignant Tumors (6th ed.), Wiley-Liss, New York, NY (2002), pp. 52–56
- 15 D. Rubello, D. Casara, M.E. Girelli, M. Piccolo, B. Busnardo; Clinical meaning of circulating antithyroglobulin antibodies in differentiated thyroid cancer: a prospective study; J Nucl Med, 33 (1992), pp. 1478–1480
- 16 D. Rubello, M.E. Girelli, D. Casara, M. Piccolo, A. Perin, B. Busnardo; Usefulness of the combined antithyroglobulin antibodies and thyroglobulin assay in the follow-up of patients with differentiated thyroid cancer; J Endocrinol Invest, 13 (1990), pp. 737–742
- 17 P. Hjiyiannakis, J. Mundy, C. Harmer; Thyroglobulin antibodies in differentiated thyroid cancer; Clin Oncol (R Coll Radiol), 11 (1999), pp. 240–244
- 18 C. Durante, S. Tognini, T. Montesano, et al.; Clinical aggressiveness and long-term outcome in patients with papillary thyroid cancer and circulating anti-thyroglobulin autoantibodies; Thyroid, 24 (2014), pp. 1139–1145
- 19 C.J. Hsieh, P.W. Wang; Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma; Thyroid, 24 (2014), pp. 488–493
- 20 Y. Tsushima, A. Miyauchi, Y. Ito, et al.; Prognostic significance of changes in serum thyroglobulin antibody levels of pre- and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients; Endocr J, 60 (2013), pp. 871–876
- 21 W.G. Kim, J.H. Yoon, W.B. Kim, et al.; Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma; J Clin Endocrinol Metab, 93 (2008), pp. 4683–4689
- 22 B. Cady, R. Rossi; An expanded view of risk-group definition in differentiated thyroid carcinoma; Surgery, 104 (1988), pp. 947–953
- 23 E. Yildirim; A model for predicting outcomes in patients with differentiated thyroid cancer and model performance in comparison with other classification systems; J Am Coll Surg, 200 (2005), pp. 378–392
- 24 L.J. DeGroot, E.L. Kaplan, M. McCormick, F.H. Straus; Natural history, treatment, and course of papillary thyroid carcinoma; J Clin Endocrinol Metab, 71 (1990), pp. 414–424
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?