Summary
Intramedullary schwannomas of the spinal cord are rare tumors. They are most commonly observed in the cervical region; however, few have been described in the conus medullaris. The association of intramedullary schwannomas with syringomyelia is also rare. In this report, we present a case of intramedullary schwannoma of the conus medullaris with syringomyelia, which was treated surgically.
Keywords
conus medullaris;intramedullary schwannoma;spinal cord;syringomyelia
1. Introduction
Schwannomas are tumors of Schwann cells and therefore may arise anywhere that a nerve has a Schwann cell sheath.1 Spinal cord schwannomas are one of the most common primary spinal tumors. Most of these lesions are seen intradurally extramedullary. Although they can be seen as pure extradural (25%) and both intradural and extradural (15%) tumors, they are rarely observed at intramedullary locations.2 ; 3 Intramedullary schwannomas correspond to approximately 0.3% of all intraspinal tumors and approximately 1.1% of spinal cord schwannomas.4 Most of them are located in the cervical region and few of them are reported at the level of the conus medullaris.5 They are also rarely associated with syringomyelia.2 ; 4 We report a case of intramedullary schwannoma of the conus medullaris, which was associated with syringomyelia.
2. Case Report
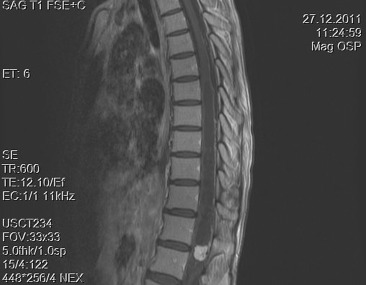
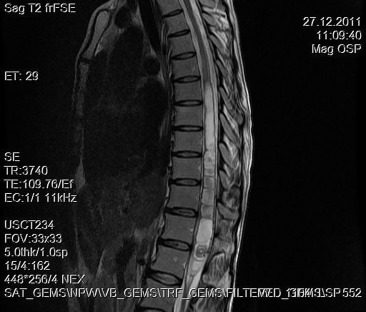
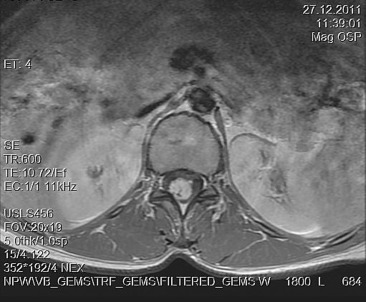
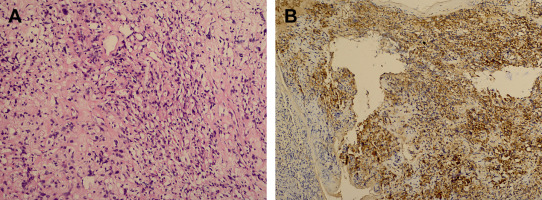
A 30-year-old woman was admitted to our clinic with complaints of back pain, walking disturbance, and progressive numbness in both legs for 2 months. Neurological examination was normal except for mild paraparesis in both legs. She had no bladder or bowel involvement. Magnetic resonance imaging (MRI) disclosed an 18 mm × 13 mm intramedullary tumor at the level of T12–L1 vertebrae. The tumor was hypointense on T1-weighted images (Fig. 1) and hyperintense on T2-weighted images (Fig. 2). Marked homogeneous contrast enhancement was noted (Fig. 3). There was associated syringomyelia extending from T7 down to the level of T12. The patient underwent T12 total laminectomy, and after exposition of the tumor, a biopsy was taken for frozen section analysis. Frozen section results were consistent with schwannoma. The tumor was heavily bleeding and highly adherent to the neural tissue of the spinal cord. Intramedullary tumor was removed subtotally using microsurgical techniques. Neurological examination improved postoperatively. Pathological examination revealed schwannoma (Fig. 4A and B).
|
|
|
Figure 1. Sagittal T1-weighted magnetic resonance imaging with gadolinium contrast demonstrates homogeneous enhancement of the tumor. Syrinx formation is seen. |
|
|
|
Figure 2. Sagittal T2-weighted magnetic resonance imaging between T12 and L1 demonstrates a hyperintense tumor. Syrinx formation is seen. |
|
|
|
Figure 3. Axial T1-weighted magnetic resonance imaging with gadolinium contrast demonstrates homogeneous enhancement of the tumor. |
|
|
|
Figure 4. (A) Photomicrograph illustrating elongated bipolar spindle cells with arrangement of nuclei in a palisade pattern (H&E stain). (B) Most cells are immunoreactive for S-100 protein (diaminobenzidine and H&E). H&E = hematoxylin and eosin. |
3. Discussion
Intramedullary schwannomas are rare tumors. The fibers in the central nervous system do not have Schwann cells, therefore, the pathogenesis of intramedullary schwannomas remains unclear. Hypotheses regarding the origin of intramedullary schwannomas include: (1) ectopic Schwann cells originating from the embryonic neural ridge; (2) Schwann cells ensheathing aberrant intramedullary nerve fibers; (3) Schwann cells extending along the branches of the anterior spinal artery; (4) neoplastic growth of Schwann cells into the cord from areas where the posterior nerve roots enter the pia mater; and (5) transformation of pial cells of neuroectodermal origin into Schwann cells.4; 6; 7 ; 8
Intramedullary schwannomas are more common in men (male/female: 1.4/1). The age at presentation ranges from 9 years to 75 years (mean: 49.2 years).1 ; 6 The cervical cord (58%) is the most common site of occurrence, followed by the thoracic cord (32%) and lumbar cord (10%).2; 3; 4 ; 6 It is rarely seen at the level of the conus medullaris as in our case.
MRI is the most useful method to diagnose spinal intramedullary tumors. Intramedullary schwannomas are usually seen as hypointense on T1-weigted images, with or without isointense areas within. On T2-weighted images, they are usually observed as hyperintense masses.2; 3 ; 4 Schwannomas show intense contrast enhancement, probably due to open-gap junctions, which are short, straight, patent, and freely communicating with a relatively large extracellular space. Although syringomyelia is frequently associated with intramedullary tumors, it is not a common MRI finding of intramedullary schwannomas. Ho et al4 reviewed preoperative MRI features of intramedullary schwannomas that were previously reported in the literature. They concluded that the absence of syringomyelia was a diagnostic MRI finding of intramedullary schwannomas. However, our patient had marked syringomyelia at the level of T7 down to T12 on MRI. The preoperative MRI study report was misleading as intramedullary astrocytoma.
The goal of surgical treatment of spinal schwannomas is total removal. Conti et al8 reported a series of 179 patients who were treated operatively. They achieved total tumor removal in 174 patients and observed recurrence in all cases when the excision was subtotal. There were three cases (1 case with Von-Recklinghausen disease) with intramedullary schwannomas and they highlighted that, unlike more malignant lesions, complete removal of intramedullary schwannomas is achievable.8 However, total resection may be difficult due to adherence of the tumor to the surrounding neural tissue.1; 5 ; 7 In these cases subtotal removal is a viable option to reduce postoperative complications. In our case we performed subtotal resection because frozen section evaluation was consistent with schwannoma and the tumor was tightly adherent to the surrounding neural tissue.
The postoperative treatment plan for intradural extramedullary schwannomas is well understood and based on the amount of tumor removal.9 ; 10 As a result of the benign nature of the lesions, they grow slowly and radiotherapy is rarely used, even after subtotal resection. Reoperation is the primary treatment of choice for recurrence.9 However, because of the rarity of the lesions, the postoperative treatment plan of the intramedullary schwannomas is not clear. Lee et al10 reported the largest series in the literature with surgical treatment of 10 patients. Gross total resection was achieved in eight cases, whereas subtotal resection was achieved in two cases. The mean follow-up period was 75.7 months. They did not observe any recurrence or clinical deterioration in their patients. However, they recommended reoperation rather than radiotherapy in case of recurrence. In the present case there was no recurrence or clinical deterioration after 11 months follow-up.
In conclusion, although intramedullary schwannomas are rare tumors, they should be kept in mind in the differential diagnosis of intramedullary tumors. Due to the high risk of postoperative complications, surgical management of these tumors should be planned according to the intraoperative findings such as adhesion of the tumor to the neural tissue and frozen section examination.
References
- 1 O. Binatli, Y. Ersahin, O. Korkmaz, U. Bayol; Intramedullary schwannoma of the spinal cord. A case report and review of the literature; J Neurosurg Sci, 43 (1999), pp. 163–167
- 2 L. Riffaud, X. Morandi, S. Massengo, B. Carsin-Nicol, N. Heresbach, Y. Guegan; MRI of intramedullary spinal schwannomas: case report and review of the literature; Neuroradiology, 42 (2000), pp. 275–279
- 3 C. Colosimo, A. Cerase, L. Denaro, G. Maira, R. Greco; Magnetic resonance imaging of intramedullary spinal cord schwannomas; J Neurosurg, 99 (Suppl 1) (2003), pp. 114–117
- 4 T. Ho, K.S. Tai, Y.W. Fan, L.L. Leong; Intramedullary spinal schwannoma: case report and review of preoperative magnetic resonance imaging features; Asian J Surg, 29 (2006), pp. 306–308
- 5 T. Ohtonari, N. Nishihara, T. Ota, S. Ota, T. Koyama; Intramedullary schwannoma of the conus medullaris complicated by dense adhesion to neural tissue; Neurol Med Chir, 49 (2009), pp. 536–538
- 6 S.N. Shenoy, A. Raja; Cystic cervical intramedullary schwannoma with syringomyelia; Neurol India, 53 (2005), pp. 224–225
- 7 K.S. Ryu, K.Y. Lee, H.J. Lee, J.K. Park; Thoracic intramedullary schwannoma by extramedullary beads-like daughter schwannomas; J Korean Neurosurg Soc, 49 (2011), pp. 302–304
- 8 P. Conti, G. Pansini, H. Mouchaty, C. Capuano, R. Conti; Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature; Surg Neurol, 61 (2004), pp. 35–44
- 9 P.C. McCormick; Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine; Neurosurgery, 38 (1996), pp. 67–74
- 10 S.E. Lee, C.K. Chung, H.J. Kim; Intramedullary schwannomas: long term outcomes of ten operated cases; J Neurooncol, 113 (2013), pp. 75–81
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?