Summary
The colon can be used for esophageal reconstruction after an esophagectomy. The development of a malignancy in the colonic interposition graft is rare, with less than 15 cases reported in the literature. We present a case of a 60-year-old male with high-grade dysplasia of the colonic interposition graft used for reconstruction of the esophagus, which developed 41 years after the esophageal reconstruction. The long-term complication of the development of a malignancy must be considered when using the colon for reconstruction especially when the esophageal reconstruction is performed for a benign cause.
Keywords
colonic interposition graft;esophageal reconstruction;malignancy
1. Introduction
Various conduits such as the stomach, jejunum, or colon are available for the reconstruction of the esophagus after an esophagectomy. The first colonic conduit used was reported in 1911.1 In the past 50 years,2 the use of the stomach as a conduit has become increasingly popular and is now the conduit of choice after a total esophagectomy. Both conduits carry significant complications such as conduit ischemia, anastomotic leak, and the development of strictures.3 A rare long-term complication is the development of neoplasias in the colonic graft. This complication has been reported by less than 15 cases in the literature. We present a case of a man with tubular adenoma with high-grade dysplasia arising in the interposed colon, 41 years after the reconstruction.
2. Case Report
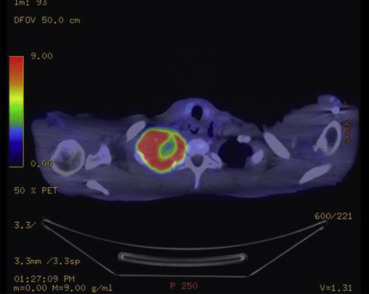
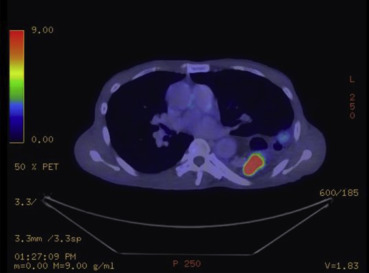
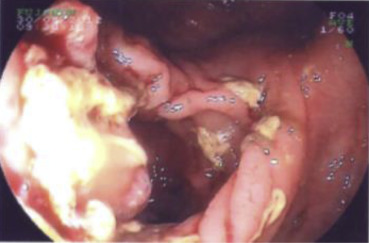
A 60-year-old male patient with a history of extrahepatic portal hypertension secondary to umbilical sepsis underwent splenectomy, total esophagectomy, and colonic interposition in 1971. This was performed in another institution and hence the details are not known. However, we postulate that this was performed owing to the presence of esophageal varices secondary to portal hypertension, and hence a gastric pull-up was not used. He was a nonsmoker and a nonalcoholic. He first presented with right upper limb pain and numbness associated with right upper chest pain for 2 months. There was no dysphagia, regurgitation, or loss of weight and appetite. A chest X-ray scan was performed, and it showed a fracture of the first rib with an associated soft tissue density in the upper zone of the right lung. Computed tomography (CT) of the thorax was then performed, which showed a soft tissue mass in the apical segment of the right upper lobe, abutting the pleura, eroding the first and second ribs, with truncation of the right upper lobe apical segment bronchus. Nodularity of colonic mucosa close to the proximal anastomosis of colonic interposition was also seen. A bronchoscopy and a transbronchial lung biopsy of the right upper lobe mass were performed, and results confirmed it to be a squamous cell carcinoma. A positron emission tomography–CT scan was then performed, which showed a centrally necrotic tumor in the right lung apex measuring 6.1 × 7.4 cm (Fig. 1), eroding through the first and second ribs, showing intense fluorodeoxyglucose (FDG) activity [maximum standardized uptake value (SUV) max = 15.7]. The colonic interposition graft in the left hemithorax showed an FDG avid nodule and a 3.0-cm FDG avid mass (SUV max = 38.1) (Fig. 2), corresponding with the nodularity seen on the CT thorax scan. An endoscopy was performed to investigate the nature of the colonic mass and to exclude a second primary. This showed a polyp and a mass in the colonic interposition graft (Fig. 3). A biopsy of the colonic mass was performed, and results showed mild acute inflammatory infiltrates with hyperplastic features. A polypectomy was also performed, and the histology showed tubular adenoma with low-grade dysplasia. The patient subsequently underwent chemotherapy and radiotherapy for his pancoast tumor. A CT scan of the brain, thorax, abdomen, and pelvis was repeated to evaluate his response after treatment. This showed a reduction in the size of the pancoast tumor; however, there was a stable filling defect within the opacified interposition colon. A repeat endoscopy showed a friable mass in the colonic interposition graft. A biopsy was repeated and showed at least a high-grade dysplasia in the superficial colonic tissue fragments. The case was discussed at a multidisciplinary tumor board, and in view of the rate-limiting disease of the pancoast tumor, conservative treatment of the colonic mass was advised. If symptoms such as bleeding or obstruction were to develop, radiation to the mass or stenting would be performed, respectively.
|
|
|
Figure 1. Positron emission tomography–computed tomography scan showing pancoast tumor. |
|
|
|
Figure 2. Positron emission tomography–computed tomography scan showing FDG avid colonic mass in the colonic interposition graft. |
|
|
|
Figure 3. Endoscopic image of tumor arising from the colonic interposition graft. |
3. Discussion
The stomach, jejunum, and colon can be used as esophageal substitutions after a total eosphagectomy is performed.
Colonic interposition is performed when the stomach cannot be used owing to a previous gastric resection or a lesion in the stomach. A rare, long-term complication of the development of neoplasias in the colonic graft has been described by less than 15 studies in the literature.4 A Japanese group5 has suggested that such changes occur in the interposed colon because of exposure to chemical substances and flora different from its native milieu. In these studies, the time to which the neoplasia arose in the colonic graft ranged from 1 to 40 years.6 Surgery or polypectomy was performed in all but one case, where radiation and chemotherapy was given. In this case, surgery was not performed as the tumor was unresectable because it had invaded the posterior sternum and extended along the mesentery of the transplanted colonic segment into the mediastinum.7 In cases where curative surgery was performed, patients were followed up with good functional status. This suggests that when there is no evidence of metastatic disease, resection of the mass should be considered.
Although other studies8 ; 9 have shown that such alterations in the mucosa of the interposed colon are rare and would more commonly occur in the native colon, in view of the possibility of alterations in the mucosa, a careful preoperative evaluation of the patients history should be undertaken to ensure the absence of any intrinsic diseases or predisposition to alterations in the colonic mucosa. Patients with a high risk of developing colorectal cancer such as those with a family history of colorectal cancer or previous colonic polyps should have alternative options for reconstruction of the esophagus explored.
The current case emphasizes the possibility of the development of a neoplasia when a colonic conduit is used. For patients with a colonic interposition graft, screening of the colonic graft 2 years after the esophageal surgery has been suggested.10 Subsequent endoscopic surveillance is recommended on a 2- to 3-yearly basis owing to the possibility of a development of a polyp or a colonic malignancy. In the event that a lesion is seen, the management should be as for that in the native colon. For example, polypectomies should be performed for polyps and a biopsy for lesions that cannot be removed on endoscopy. Surgical resection should then be considered for these lesions in the absence of metastatic disease as cure can be achieved.
In this case, curative surgery was not performed in view of the rate-limiting disease in the pancoast tumor. If removal of the colonic interposition graft is performed, follow-up of the in situ native colon should be as for colorectal cancer surveillance guidelines. If segmental removal of the graft is performed, surveillance endoscopy of the graft should be performed annually along with the in situ native colon.
References
- 1 G. Kelling; Oesophagoplastic mitt Hilfe des Querkolon; Zentralbl Chir, 38 (1911), pp. 1209–1212
- 2 I.A. May, P.C. Sampson; Esophageal reconstruction and replacements; Ann Thorac Surg, 7 (1969), pp. 249–277
- 3 T.R. DeMeester, K.E. Johansson, I. Franze, et al.; Indications, surgical technique, and long-term function results of colon interposition or bypass; Ann Surg, 208 (1988), pp. 460–474
- 4 C.T. Liau, S. Hsueh, K.M. Yeow; Primary adenocarcinoma arising in esophageal colon interposition: report of a case; Hepatogastro-enterology, 51 (2004), pp. 748–749
- 5 Y. Kuwabara, M. Kimura, A. Mitsui, et al.; Adenocarcinoma arising in a colonic interposition following a total gastrectomy: report of a case; Surg Today, 39 (2009), pp. 800–802
- 6 H. Bando, H. Ikematsu, K. Fu, et al.; A laterally-spreading tumor in a colonic interposition treated by endoscopic submucosal dissection; World J Gastroenterol, 16 (2010), pp. 392–394
- 7 R.W. Haerr, E.M. Higgins, C.H. Seymore, A.M. El-Mahdi; Adenocarcinoma arising in a colonic interposition following resection of squamous cell esophageal cancer; Cancer, 60 (1987), pp. 2304–2307
- 8 J. Isolauri, H. Helin, H. Markkula; Colon interposition for esophageal disease: histologic finding of colonic mucosa after a follow-up of 5 months to 15 years; Am J Gastroenterol, 86 (1991), pp. 277–280
- 9 L. Burgos, S. Barrena, A.M. Andrés, et al.; Colonic interposition for esophageal replacement in children remains a good choice: 33-year median follow-up of 65 patients; J Pediatr Surg, 45 (2010), pp. 341–345
- 10 D.D. Shersher, E. Hong, W. Warren, L. Penfield Faber, M.J. Liptay; Adenocarcinoma in a 40-year-old colonic interposition treated with Ivor Lewis esophagectomy and esophagogastric anastomosis; Ann Thorac Surg, 92 (2011), pp. 113–114
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?