Summary
Aim
The aim of this study was to analyze our experience with rectal cancer patients who underwent surgical excision at our institution.
Methods
Data on 112 rectal cancer patients who underwent surgical resection with total mesorectal excision, from January 2005 to December 2008, were evaluated retrospectively.
Results
We achieved an initial complete remission rate of 74.1%. Overall, 92.8% of patients had a complete total mesorectal excision. The overall survival analysis for all patients showed a 1-year survival rate of 98%, a 3-year survival rate of 82%, and a 5-year survival rate of 70%. We report a 41.9% rate of postoperative complications. The 1-, 3-, and 5-year survival rates for females were 100%, 90%, and 72%, respectively and for males, they were 90%, 80%, and 68%, respectively. Differences in overall survival by sex were not statistically significant (p > 0.05). Those patients who were treated with only surgery had the best outcomes with survival being worse in those treated with surgery and adjuvant therapy. Neoadjuvant treatment followed by surgery led to better results.
Conclusion
We conclude that we have been successful in achieving high rates of curative resection, complete remission, and overall survival. Neoadjuvant and adjuvant chemotherapy significantly impact rates of remission.
Keywords
colorectal cancer;Pakistan;rectal adenocarcinoma;rectal cancer;surgical oncology
1. Introduction
Although rectal cancer is often seen as part of the much broader entity of colorectal cancer, the pathological course and treatment regimens of cancers of the rectum and the colon differ considerably,1 and outcomes of treatment are also different. Colorectal cancer is one of the 10 most common malignancies worldwide, with high rates being reported from North America, Australia, New Zealand, Western Europe, and Japan. Asian and African countries are generally considered areas of low incidence. In 1995, cancers of the rectum and colon taken together were the seventh most common cancer in males and the ninth most common in females in Pakistan.2 Gradually increasing rates are now being reported from South Asian as well as European countries.3; 4 ; 5 Bhurgri et al,3 reporting on results from Pakistan’s only population-based cancer registry, reported the crude incidence rate of rectal cancer increasing from 1.7/100,000 in 1995–1997 to 2.3/100,000 in 1998–2002 in Karachi, Pakistan. As well as increasing incidence, younger age at diagnosis is also now being widely reported.3; 4 ; 6
The cornerstone of management of rectal cancer patients is optimal surgical excision. Total mesorectal excision (TME) guarantees complete excision of the lymphatics around the rectum, contained within the mesorectum, and ensures adequate resection margins and circumferential tumor clearance.7 As well as improved techniques of surgical excision, the past two decades have seen major advances in the application of neoadjuvant and adjuvant treatment, using chemotherapy and radiation therapy. Today, these are integral parts of the treatment regimens for these patients.6 Neoadjuvant chemoradiation has been shown to be beneficial in leading to a reduction in local recurrence in rectal cancer.8 Neoadjuvant therapy has also been shown to result in complete pathological response in stage IV rectal cancer.9 According to Naiken et al,9 10–20% of patients achieve complete pathological response after chemoradiation. Primary surgical excision is currently recommended for stage I patients, whereas neoadjuvant chemotherapy and radiation followed by surgery, with or without adjuvant treatment, is considered appropriate for stages II and III rectal cancer.10
Radical surgery in these patients has led to high rates of morbidity associated with the surgical procedure itself as well as with prolonged hospital stay. Surgery also requires either a defunctioning or permanent colostomy, which many patients have difficulty accepting and becoming accustomed to, leading to a reduced quality of life.7
The Shaukat Khanum Memorial Cancer Hospital and Research Center is a tertiary-level, dedicated cancer hospital located in Lahore, Pakistan, a city with a population of 16 million.2 The aim of this study was to analyze the outcomes of patients with rectal cancer who underwent surgical excision at our institution, and to review their postoperative morbidity, curative resection rates, recurrence rates, and overall survival.
2. Patients and methods
As a follow-up of a previous publication from our institution that focused on colonic as well as rectal cancer patients,2 we retrieved retrospectively data on rectal cancer patients treated at our institution from January 2005 to December 2008. A total of 336 rectal malignancies were registered during this 4-year period. Of this total, 112 patients, all histologically confirmed, who underwent surgery [abdominoperineal resection (APR) and low anterior resection (LAR)], along with total mesorectal resection, at the hospital were selected for complete review in this study. Rectal cancer was defined as a tumor occurring above the anal canal and within 15 cm of the anal verge. Patients were staged according to the American Joint Committee for Cancer (AJCC) staging of cancers for the colon and rectum.11 To facilitate the review, stages I and II were combined into “Early Stage Cancer” and stages III and IV were grouped into “Late Stage Cancer.” All patients with rectal cancer were staged in a uniform manner with contrast-enhanced computerized tomographic scans of the chest and abdomen, and contrast-enhanced magnetic resonance scan of the pelvis. Imaging was usually done at our center, and any studies performed elsewhere were reviewed by radiologists at our institution for adequacy, prior to rereporting. Where imaging studies performed elsewhere were felt to be inadequate, these were repeated at our institution. Only those carcinoembryonic antigen (CEA) levels done at our center were included. Prior to surgery, all rectal cancer patients treated at our institution are usually given neoadjuvant chemoradiation. This consists of induction chemotherapy with CapOx regimen (oral capecitabine 1000 mg/m2, twice a day, D1–14, and intravenous oxaliplatin 130 mg/m2, D1) given three times weekly for four cycles. This is followed by pelvic radiation to a dose of 50.4 Gy in 28 fractions with concurrent oral capecitabine 825 mg/m2, twice a day throughout radiation. Radiation is usually computerized tomography-planned with target volume encompassing the primary tumor and locoregional lymph nodes. Overall survival interval was defined as date of diagnosis to date of death. Disease-free survival (DFS) was taken as the period from the date of surgery/end of treatment to the date of relapse or death. Patients were deemed as lost to follow-up if they had missed their last scheduled appointment and a period of at least 3 months had lapsed since.
Disease response was defined using the World Health Organization or Response Evaluation Criteria in Solid Tumors criteria.12 Complete response (CR) was defined as disappearance of all the target lesions confirmed on imaging 4–6 weeks after treatment. Partial response was defined as at least 30% reduction in the sum of the diameters of the target lesions confirmed at 4–6 weeks after treatment. Progressive disease (PD) was taken as an increase of at least 20% in the diameters of the target lesions or appearance of a new lesion. Stable disease was specified as neither PD nor partial remission. Remission was defined as persistent complete response for at least 1 year. Patients who were lost to follow-up were censored during survival analysis.
File review was completed in April 2012. Attempts were made to contact telephonically all patients who had been lost to follow-up.
The variables that were included in the analysis were age, sex, body mass index, disease stage, family history, histology, CEA levels, treatment modality, disease response after treatment, perioperative blood transfusion, preoperative hemoglobin levels, and postoperative complications. We also looked at follow-up times, DFS, and overall survival. Postoperative complications were cross-tabulated with both body mass index (BMI) and hemoglobin level.
The survival distributions were evaluated by means of the Kaplan–Meier survival analysis. The distributions were compared using the log-rank Chi-square test. All tests were considered statistically significant at an < level of 0.05. A PubMed literature search was carried out to review similar data available for outcomes of surgically treated rectal cancer patients.
3. Results
Of the 112 patients selected for this study, 69 (61.6%) were males and 43 (38.4%) were females, with a male/female ratio of 1.6:1. The mean age at presentation was 43.6 (range 16–79) years.
Rectal bleeding was the only presenting complaint in 13 (11.6%) patients. The most common combination of symptoms was altered bowel habit and rectal bleeding, which was seen in 33 (29.4%) of the patients. Twenty-three (20.5%) patients had a combination of rectal bleeding, weight loss, and pain, and 43 (38.3%) patients had a variety of miscellaneous symptoms including loss of appetite, abdominal pain, nausea and vomiting, and general malaise.
Seven (6.2%) patients had a positive family history of colorectal cancer in a first-degree relative. Eight (7.1%) patients had a history of cancers other than colorectal cancer in a first-degree relative. Ninety-seven (86.6%) patients had no family history of cancer.
The most common stage at presentation was stage III, as shown in Table 1.
| Frequency | % | |
|---|---|---|
| Presenting symptoms | ||
| Rectal bleeding | 13 | 11.6 |
| ABH and rectal bleeding | 33 | 29.4 |
| Rectal bleeding, weight loss and pain | 23 | 20.5 |
| Miscellaneous | 43 | 38.3 |
| Total | 112 | 100 |
| Stage | ||
| Early stage | ||
| Stage I | 3 | 2.7 |
| Stage II | 17 | 15.3 |
| Total | 20 | 18 |
| Late stage | ||
| Stage III | 91 | 81.2 |
| Stage IV | 1 | 0.89 |
| Total | 92 | 82.1 |
| Total | 112 | 100 |
| Histology | ||
| Adenocarcinoma | 107 | 95.5 |
| Squamous cell carcinoma | 1 | 0.89 |
| Carcinoma NOS | 4 | 3.57 |
| Total | 112 | 100 |
ABH = altered bowel habits; NOS = not otherwise specified.
A total of 107 (95.5%) patients had adenocarcinoma, one (0.89%) patient had squamous cell carcinoma, and four (3.57%) patients had carcinoma not otherwise specified.
Sixty-six (58.9%) patients had moderately differentiated carcinoma, whereas in 19 (16.9%) patients the histology grade could not be assessed. The rest had poorly differentiated (14, 12.5%), well-differentiated (12, 10.7%), and undifferentiated (1, 0.89%) tumors.
The majority of our patients (64, 57.1%) had normal (<2.5 ng/ml) CEA levels. Thirty-eight (33.9%) patients had high levels (>2.5 ng/ml), whereas in 10 (8.9%) patients, the initial CEA levels were not available for review. Of the 38 patients with elevated CEA levels, the majority had stage III disease (32, 84.2%), with six (15.7%) presenting with early stage (stages I and II) disease.
Sixty (57.1%) patients underwent APR, whereas 48 (42.8%) had LAR. Of the 64 APR procedures performed, 28 (43.7%) were carried out using a laparoscopic approach.
One hundred and four (92.8%) of the TME procedures were complete resections. Only eight (7.1%) specimens showed an incomplete mesorectum on examination after resection.
R0 resection was achieved in 85 (75.9%) of 112 patients, whereas 27 (24.1%) patients had positive margins on examination of resected specimens. Of these, 13 (48.1%) were R1 resections and 14 (51.8%) were R2 resections. Eighteen (66.6%) of the 27 patients with positive margins eventually relapsed.
A total of 104 patients underwent chemotherapy (neoadjuvant, adjuvant, or both). The most common chemotherapeutic regimen used was capecitabine with oxaliplatin (CapOx) in 53 (50.9%) patients. 5-Flurouracil with folinic acid was used in 21 (20.2%) patients. Chemotherapy had to be delayed, stopped, or the dose reduced in 26 (25%) of these patients, because of either toxicity or patient intolerance. Five of these 26 patients subsequently had positive surgical margins and 10 had relapse of disease.
The most commonly used treatment regimen (50, 44.6%) was neoadjuvant chemotherapy and radiation, followed by surgical resection, then by adjuvant therapy. Eight patients were selected to undergo surgery only. These included five patients who had early-stage disease and three patients who had late stage disease.
Eighty-three (74.1%) patients achieved complete remission. Of these, 38 (45.7%) were treated with neoadjuvant therapy, surgery, and then adjuvant therapy, whereas 28 (33.7%) patients received neoadjuvant therapy followed by surgery (Table 2). Comparing CR rates for the two types of surgical procedures performed revealed that of the patients who underwent LAR, 68% achieved CR, compared to 46.8% in those undergoing APR.
| Treatment group | Number | Disease response after treatment | ||||
|---|---|---|---|---|---|---|
| N/A | PR | PD | CR | SD | ||
| Neoadjuvant–surgery | 40 | 2 | 3 | 6 | 28 | 1 |
| Surgery | 8 | 1 | 6 | 1 | ||
| Surgery–adjuvant | 14 | 1 | 1 | 11 | 1 | |
| Neoadjuvant–surgery–adjuvant | 50 | 3 | 6 | 2 | 38 | 1 |
| Total | 112 | 6 | 9 | 10 | 83 | 4 |
CR = complete response; N/A = not applicable; PD = progressive disease; PR = partial remission; SD = stable disease.
Postoperative complications were classified according to the Clavien–Dindo classification.13 We experienced an overall complication rate of 41.9% (Table 3). The majority of the complications encountered were of grade 3a (19, 40.4%). Of these, obstructive uropathy was the most common (15, 78.9%). Sixteen (34%) patients had grade 2 complications, including wound infections (14, 87.5%). Twenty-four (51%) of all complications were seen in patients with BMIs in the normal range (18.5–25). Only nine (19%) complications occurred in patients with low BMIs (<18.5; Table 4). There was no statistically significant association between BMI values and the presence or absence of postoperative complications (p = 0.65). Nor was there any statistically significant association found between preoperative hemoglobin categories (low and normal) and the presence or absence of postoperative complications (p = 0.46; Table 4).
| Clavien–Dindo grades | Frequency | % |
|---|---|---|
| Grade 1 | 7 | 14.89 |
| Fecal incontinence | 7 | 100 |
| Grade 2 | 16 | 34 |
| Wound infections | 14 | 87.5 |
| Pneumonia | 1 | 6.25 |
| Obstructive uropathy | 1 | 6.25 |
| Grade 3a | 19 | 40.4 |
| Obstructive uropathy | 15 | 78.9 |
| Urethral strictures | 4 | 21 |
| Grade 3b | 5 | 10.6 |
| Anastomotic leak | 3 | 60 |
| Anastomotic stricture | 1 | 20 |
| Obstructive uropathy | 1 | 20 |
| Total | 47 | 100 |
| Overall rate | 47/112 | 41.9 |
| Frequency of complications | % | |
|---|---|---|
| BMI | ||
| Low (<18.5) | 9 | 19.1 |
| Normal (18.5–25) | 24 | 51 |
| High (>25) | 14 | 29.7 |
| Total | 47 | 100 |
| Hemoglobin | ||
| Low (<12) | 24 | 51 |
| Normal (12–17) | 23 | 48.9 |
| High (>17) | 0 | 0 |
| Total | 47 | 100 |
Forty-four (39.2%) patients received perioperative blood transfusions. Perioperative transfusion was defined as a blood transfusion occurring within 7 days prior to, during, or up to 7 days after surgery. Fourteen (31.8%) of the 44 patients who received perioperative blood transfusions also suffered from postoperative complications. We were not able to demonstrate a clinical association between perioperative blood transfusions and postoperative complications.
On the last review (disease status at last visit), 60 (53.5%) patients were in CR, whereas 21 patients had relapsed and two had died. Of the patients who achieved partial remission, only two remained in partial remission and eight patients experienced PD, leading to a total of 17 patients with PD. Four patients maintained stable disease, whereas disease status for six could not be determined.
Overall, 36 of 112 (32.1 %) patients had been lost to follow-up, whereas 72 (64.2%) were under regular review. Efforts were made, for the purpose of this review, to contact all patients who had been lost to follow-up to determine patient status. Of these 112 patients, 64 (57.1%) were still alive, 22 (19.6%) had died, and current status could not be determined for 26 (23.2%).
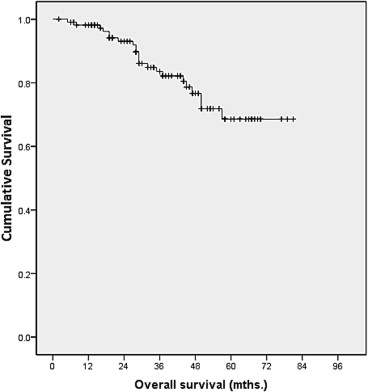
Overall survival was defined as the time interval between the dates of diagnosis and death. Death was taken as the endpoint of interest. Survival analysis was carried out using the Kaplan–Meier method (Table 5). The overall survival analysis for all patients showed a 1-year survival rate of 98%, a 3-year survival rate of 82%, and a 5-year survival rate of 70% (Fig. 1).
| Overall survival | Total | Number of events | Censored | |
|---|---|---|---|---|
| N | % | |||
| Overall | 112 | 22 | 90 | 80 |
| By sex | ||||
| Female | 43 | 6 | 37 | 86 |
| Male | 69 | 16 | 53 | 76 |
| Disease-free survival | ||||
| By sex | ||||
| Female | 35 | 11 | 24 | 69 |
| Male | 48 | 12 Relapse—21 Death—2 | 36 | 75 |
| By stage | ||||
| Early | 17 | 3 | 14 | 82 |
| Late | 66 | 20 | 46 | 69 |
| By treatment modality | ||||
| Surgery | 6 | 0 | 6 | 100 |
| Neoadjuvant + surgery | 28 | 4 | 24 | 86 |
| Neoadjuvant + surgery + adjuvant | 38 | 12 | 26 | 68 |
| Surgery + adjuvant | 11 | 7 | 4 | 36 |
| Disease-free survival by stage and treatment modality | ||||
| Early stage: | ||||
| Surgery | 3 | 0 | 3 | 100 |
| Neoadjuvant + surgery | 8 | 1 | 7 | 88 |
| Neoadjuvant + surgery + adjuvant | 5 | 1 | 4 | 80 |
| Surgery + adjuvant | 1 | 1 Relapse—2 Death—1 | 0 | 0 |
| Late stage: | ||||
| Surgery | 3 | 0 | 3 | 100 |
| Neoadjuvant + surgery | 20 | 3 | 17 | 85 |
| Neoadjuvant + surgery + adjuvant | 33 | 11 | 22 | 67 |
| Surgery + adjuvant | 10 | 6 Relapse—19 Death—1 | 4 | 40 |
|
|
|
Figure 1. Kaplan–Meier analysis on overall survival. |
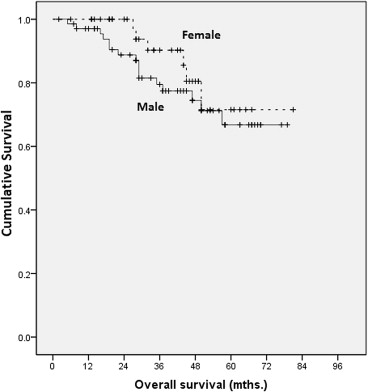
Overall survival was also analyzed by sex (Fig. 2). The 1-, 3-, and 5-year survival rates for females were 100%, 90%, and 72%, respectively. For men, they were 90%, 80%, and 68%, respectively. The difference was not found to be statistically significant (p = 0.356).
|
|
|
Figure 2. Kaplan–Meier analysis on overall survival by sex. |
DFS was computed as the time interval between treatment and relapse, or between treatment and death. The average DFS for the entire group (n = 83) was 40.5 months. The longest mean DFS of 49.3 months was achieved by the “surgery only” group, followed closely by the “neoadjuvant therapy–surgery” group at 49.2 months.
The median survival times could not be calculated for overall survival as survival did not drop to 50%.
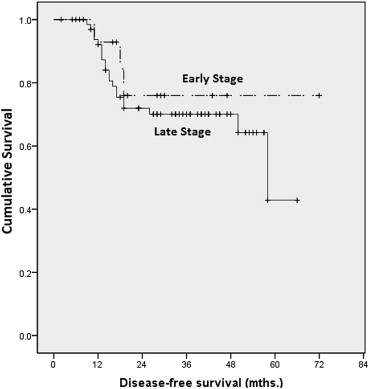
Our 1-, 3-, and 5-year DFS rates for late stage cancer were 92%, 70%, and 42%, respectively. For early stage cancer, 1-, 3-, and 5-year DFS rates were 92%, 78%, and 78%, respectively. There were no statistically significant differences found (p = 0.469; Fig. 3).
|
|
|
Figure 3. Kaplan–Meier analysis on disease-free survival by stage. |
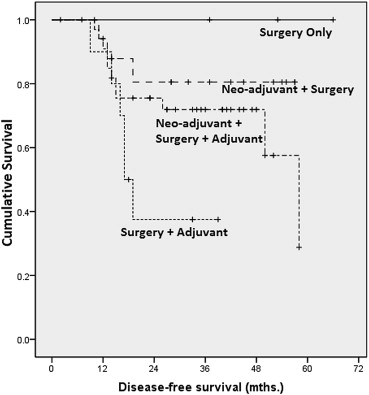
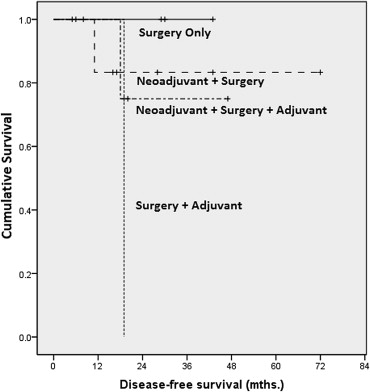
Analyzing data by treatment modality and late stage disease, we found statistically significant differences between outcomes related to surgery versus surgery–adjuvant (p = 0.015), surgery–adjuvant versus neoadjuvant–surgery (p = 0.015), and surgery–adjuvant versus neoadjuvant–surgery–adjuvant (p = 0.039; Fig. 4).
|
|
|
Figure 4. Kaplan–Meier analysis on disease-free survival by treatment (late stage). |
No such differences were found in the early stage group when stratified by treatment modality (Fig. 5).
|
|
|
Figure 5. Kaplan–Meier analysis on disease-free survival by treatment (early stage). |
DFS for the “surgery only” group was 100% at 1-, 3-, and 5-years. The “surgery–adjuvant” group showed 90% survival at 1 year followed by 36% at 3 years. The patients in this group did not survive until 5 years. For the “neoadjuvant–surgery” group, survival was 86% at 1 year and 86% at 3 years and 5 years. The “neoadjuvant–surgery–adjuvant” group showed 88% and 76% for 1 year and 3 years, respectively. None of the patients in this group survived for 5 years.
DFS when computed by sex did not show any statistically significant difference (p = 0.867).
DFS was also analyzed by stage and treatment modality. In late stage disease, there is a statistically significant difference when comparing the “neoadjuvant–surgery” group with the “surgery–adjuvant group” (p = 0.03). There is no other statistically significant difference when comparing various subgroups with one another, either in early or late stage disease.
4. Discussion
Our data demonstrate rates of rectal cancer in men and women that are consistent with previous reports from the region. Our male/female ratio was 1.6:1 compared to the 2:1 reported by Bhurgri et al.3 However, similar sex distribution is not seen with reports from other parts of the world where women had slightly higher rates of rectal cancer, especially locally advanced rectal cancer.14 The higher incidence in men seen in our study may be because we see patients from all over Pakistan as well as neighboring Afghanistan. Women may not always be able to travel as freely as men, and in some rural areas, their health issues may be minimized or ignored.
As with other studies, we also found rectal bleeding and altered bowel habit to be the most common combination of presenting symptoms in patients with rectal cancer.15
Overall, 81.2% of our patients presented with AJCC stage III. Andreoni et al1 in Italy saw UICC (Union for International Cancer Control) stage III, which has the same stage definitions as the AJCC, as the most common stage at presentation in their rectal cancer patients. This indicates a global trend toward stage III cancers being the most common stage at presentation. Innos et al,10 however, found slightly higher rates of stage IV cancer (29.7%) compared to stage III (22%) in Estonia.
Of the two different surgical procedures, LAR was seen to be the more successful one in achieving complete remission despite the fact that stage distribution was similar between the two groups in our cohort. Our results show an initial complete remission rate of 74.1%.
Our rate of R0 resections (75.9%) was slightly lower than that reported by Andreoni et al1 (84.7%). We had a high rate (92.8%) of complete TME. However, a comparative analysis could not be performed, as rates of TME have not been reported in other recently conducted studies. TME has been seen to be associated with good outcomes, and where TME was not performed or was incomplete, lower survival rates have been seen.4; 6 ; 10
Of the 83 patients who had a complete response to treatment, 21 subsequently relapsed. A case note review showed this to be because of positive surgical margins, interrupted or reduced chemotherapy and/or radiation, or advanced cancer stage at diagnosis. Most relapses were seen in stage III, but this may simply be because the majority of our patients presented with stage III tumors.
The probability of survival for those who underwent surgery only was 100% at 1 year, 3 years, and 5 years after treatment. This is likely to be because these patients presented with lower stage tumors and thus experienced longer DFS. The group treated with neoadjuvant therapy followed by surgery had the next longest survival, which is likely because of downstaging of these patients prior to surgery, presumably leading to a higher rate of surgical tumor clearance. Shorter disease-free intervals as well as a higher relapse rate were seen in patients who were treated with neoadjuvant therapy, surgery, and adjuvant treatment, in keeping with the fact that these patients tended to have more advanced stage and/or residual tumor following surgery.
DFS rates were not found to be statistically significant between early stage and late stage cancers. This result may be confounded by our sample size as the majority of our patients fell into the late stage category. The survival curves for late stage and early stage disease begin to diverge starting at about 48 months after treatment, and it may be that, with more prolonged follow-up, this difference may become significant.
In the late-stage group, DFS, when analyzed by treatment modality showed several significant differences. The worst survival rate was seen in the group treated with surgery and adjuvant chemotherapy. Survival was better in the other three treatment groups (Fig. 4), and the differences in survival between each of these three groups and the fourth group (surgery and adjuvant chemotherapy) were statistically significant. This is attributable to the fact that those patients selected for only surgery had less extensive disease and were better surgical candidates, and that for all others neoadjuvant treatment followed by surgery led to better local control of disease.
Evaluation of our survival rates was significantly affected by the fact that many of our patients were lost to follow-up after the immediate postoperative period. This is in part because many of our patients travel long distances to come to our institution for treatment, and find it difficult to subsequently return for follow-up, especially if they feel well. Although attempts were made to contact these patients, these were unsuccessful in 23.2% of patients. Men suffered a steeper decline of survival rates compared to women. This is possibly attributable to the higher incidence and often more advanced stage of rectal cancers in men compared to women in our cohort.
We report a 41.9% rate of postoperative complications. This rate is close to that of 37.2% reported by Andreoni et al1 for rectal cancer patients and the 39% (23% early complications and 16% late complications) reported by a Polish study.13 Our higher rate may be attributable to the fact that we included late complications as well as early ones in our review, whereas it is unclear whether Andreoni et al1 included late complications. Our rate is comparable to that in the Polish study13 as the authors included both early and late complications in their review. According to Campos et al,14 the rates of postoperative complications vary from 20% to 42%. Improving patient follow-up might lead to better postoperative care and minimize the rate of complications.
Overall, 39.2% of our patients received perioperative blood transfusions. Andreoni et al1 have reported an association between these two factors, and postulated that this could be because those requiring preoperative blood transfusions tend to be in poorer general health and are thus more likely to suffer postoperative complications, rather than the transfusions themselves having an adverse effect. However, we were unable to show an association between preoperative hemoglobin level or the need for blood transfusion and the likelihood of postoperative complications. We also examined the association between postoperative complications and body mass index and again found no significant association.
The limitations of our study included the small sample size, which may have affected the survival estimates, similar to other studies.10 The small sample size also limited us in evaluating survival differences between treatment modalities. Also, we did not compare the outcomes of patients who underwent open procedures with those of patients who had laparoscopic procedures. This would have undeniably had an impact on postoperative morbidity and is worth evaluating in a prospective study. The large number of patients who failed to follow-up adversely affected our analysis of 5-year survival rates.
5. Conclusion
Our study proves that surgical techniques currently used at our center are successful in achieving a curative resection rate of 75.9%, a complete remission rate of 74.1%, and an overall survival rates at 1 year of 98%. Neoadjuvant and adjuvant chemotherapy significantly impact rates of remission, with relapse in almost 40% of those who were unable to complete their planned chemotherapy and radiation regimens. Efforts should be made to reduce chemotherapy and radiation toxicities in order to allow these patients to fully benefit from standard-of-care treatment protocols.
In conclusion, appropriate surgery with complete TME and appropriate chemotherapy and radiation, when given as planned, ensure optimal survival in rectal cancer patients.
Acknowledgments
The authors acknowledge the efforts of Dr Noureen Irfan and Dr Fouzia Meerza in the process of data collection.
References
- 1 B. Andreoni, A. Chiappa, E. Bertani, et al.; Surgical outcomes for colon and rectal cancer over a decade: results from a consecutive monocentric experience in 902 unselected patients; World J Surg Oncol, 5 (2007), p. 73
- 2 N. Anwar, F. Badar, M.A. Yusuf; Profile of patients with colorectal cancer at a tertiary care cancer hospital in Pakistan; Ann N Y Acad Sci, 1138 (2008), pp. 199–203
- 3 Y. Bhurgri, T. Khan, N. Kayani, et al.; Incidence and current trends of colorectal malignancies in an unscreened, low risk Pakistan population; Asian Pac J Cancer Prev, 12 (2011), pp. 703–708
- 4 E.B. Ostenfeld, R. Erichsen, L.H. Iversen, et al.; Survival of patients with colon and rectal cancer in central and northern Denmark, 1998–2009; Clin Epidemiol, 31 (2011), pp. 27–34
- 5 H.E. Karim-Kos, L.A. Kiemeney, M.W. Louwman, et al.; Progress against cancer in the Netherlands since the late 1980s: an epidemiological evaluation; Int J Cancer, 130 (2012), pp. 2981–2989
- 6 R. Itah, R. Greenberg, N. Werbin, et al.; Current changes in the management and outcome of patients with curable colorectal cancer; Isr Med Assoc J, 13 (2011), pp. 300–303
- 7 R.P. Kennelly, A. Heeney, A. White, et al.; A prospective analysis of patient outcome following treatment of T3 rectal cancer with neo-adjuvant chemoradiotherapy and transanal excision; Int J Colorectal Dis, 27 (2012), pp. 759–764
- 8 Y.K. Lim, W.L. Law, R. Liu, et al.; Impact of neoadjuvant treatment on total mesorectal excision for ultra-low rectal cancers; World J Surg Oncol, 8 (2010), p. 23
- 9 S.P. Naiken, C. Toso, L. Rubbia-Brandt, et al.; Complete pathological response (ypT0N0M0) after preoperative chemotherapy alone for stage IV rectal cancer; BMC Surg, 14 (2014), p. 4
- 10 K. Innos, J. Soplepmann, T. Suuroja, et al.; Survival for colon and rectal cancer in Estonia: role of staging and treatment; Acta Oncol, 51 (2012), pp. 521–527
- 11 AJCC; American Joint Committee on Cancer Staging manual; (6th ed.)Springer-Verlag, New York (2002)
- 12 E.A. Eisenhauer, P. Therasse, J. Bogaerts, et al.; New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1); Eur J Cancer, 45 (2009), pp. 228–247
- 13 B. Szynglarewicz, R. Matkowski, D. Sydor, et al.; Postoperative complications of curative treatment for rectal cancer in males with sphincter-preserving total mesorectal excision; Pol Merkur Lekarski, 23 (2007), pp. 348–351
- 14 F.G. Campos, M.C. Calijuri-Hamra, A.R. Imperiale, et al.; Locally advanced colorectal cancer: results of surgical treatment and prognostic factors; Arq Gastroenterol, 48 (2011), pp. 270–275
- 15 B.A. Adelstein, P. Macaskill, S.F. Chan, et al.; Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review; BMC Gastroenterol, 11 (2011), p. 65
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?