Abstract
Background
Sex specific temporal trends in the incidence and prevalence of hospitalization for heart failure (HF), particularly in conjunction with reduced and preserved left ventricular ejection fraction (EF) remain unclear, especially in Asian general populations.
Methods
We conducted a community based HF registration study over a 10 year period in an aging cohort of the Japanese general population.
Results
A total of 2598 cases of hospitalized HF were registered during the survey period. Of these 1413 cases (55%) were initial admissions for HF (incident case). The study period was divided into five 2-year terms (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012), and data were compared among the terms. Age adjusted incidence of HF (per 105 person-year) remained stable in men, but decreased significantly by 25% in women (from 104 at T1 to 79 at T5; p for trend < 0.05). Among incident cases who underwent echocardiography (≈ 90%), the proportion of HF with preserved EF increased in men (from 32% at T1 to 43% at T5; p for trend < 0.05), and was relatively high and remained stable throughout the study period in women (from 52% at T1 to 58% at T5; p for trend; NS).
Conclusion
Although the incidence of HF has declined especially in women between 2003 and 2012 in the study population, the proportion of HF with preserved EF has increased over time. These trends suggest a future prevalence of HF with preserved EF rather than HF with reduced EF in aging Asian populations.
Keywords
Cohort;Community;Population
1. Introduction
It has been recognized that heart failure (HF) is a growing public health problem in industrialized nations with aging populations [1]; [2] ; [3]. Although many reports have described the incidence and hospitalization rates for HF in various districts and countries mainly in North America and Europe [4]; [5]; [6]; [7]; [8] ; [9], population based studies about recent temporal trends in HF incidence in terms of subtypes with reduced ejection fraction (HF reduced-EF) or preserved ejection fraction (HF preserved-EF) are very limited, especially in Asian countries.
Among elderly population, the proportion of HF preserved-EF within overall HF is higher in women than in men [10]; [11]; [12]; [13] ; [14], whereas HF reduced-EF is more common in men than in women. There may be some differences between the genders in terms of the etiology and prevalence of structural heart disease underlying HF. In fact, the Framingham heart study has reported a significantly lower prevalence of coronary artery disease in women compared to men among patients with HF [12]. Moreover, other underlying risk factors for HF such as valvular heart disease, hypertension and diabetes are more common in women than in men [12]. It may therefore be important to elucidate sex specific differences in the incidence and prevalence of HF according to ejection fraction and how these differences may have changed over time.
However, few sex specific population based studies have examined temporal changes in the incidence (initial admission for HF) and prevalence (initial or recurrent admission for HF) of hospitalized HF in relation to subtypes of left ventricular dysfunction. Owan et al. have studied the number of admissions for HF in Mayo clinic hospitals from 1986 to 2002, and described an increase in the prevalence of HF preserved-EF relative to HF reduced-EF over this period [11]. However, this previous study did not indicate whether temporal trends were similar in both sexes or whether temporal trends in incidence parallel those for prevalence. Steinberg et al. have reported that the percentage of HF preserved-EF among voluntary based patients with HF increased gradually by approximately 5% during a 5 year study period [15]. However, this study may have included an inherent selection bias for HF patients, and also did not attempt any sex specific analysis of temporal trends of HF according to left ventricular function subtypes.
We have therefore performed a prospective population based registration study for hospitalized HF with the specific aim of defining temporal trends in the incidence of HF in the northern part of Iwate, Japan, over a 10 year period. We also investigated whether changes in the incidence and prevalence of HF preserved-EF and HF reduced-EF differed between men and women.
2. Methods
2.1. Study population
The study area comprised the districts of Ninohe and Kuji, a rural area situated in the northern part of Iwate prefecture, Honshu, Japan (Fig. 1). The population of this region decreased gradually from 135,395 in 2003 to 119,597 in 2012. However, the proportion of elderly (aged ≥ 65 years) increased from 26% in 2003 to 31% in 2012. The number of elderly women was 1.5 times higher than that of men. This area has a low migration rate with a relatively stable population (i.e. in 2010, 93 elderly persons moved into the area and 151 moved out).
|
|
|
Fig. 1. Study area. Black zone indicates the northern part of Iwate prefecture. |
2.2. Case registration
The study area had only six general hospitals (Ninohe, Ichinohe, Karumai, Ibonai, Hirono, and Kuji hospitals) and no other medical providers in the area had cardiology admission facilities. Patients with decompensated HF were mostly admitted to the two hospitals (Ninohe and Kuji) which had fulltime attending cardiologists. The other 4 branch hospitals were usually not equipped to perform specialized tests and provide intensive care for overt HF. For these hospitals, part-time cardiologists from the major two hospitals visited regularly to provide cardiac examination such as 2-dimensional transthoracic echocardiographic (TTE) and medical advice to attending internists. Other medical facilities including local general practitioners in the study area did not provide care for symptomatic patients with HF on a regular basis. Thus, the above 6 general hospitals were selected for prospective registration. In addition, the registration study was extended to include 5 teaching hospitals located in remote urban areas such as Morioka city and Hachinohe city to capture patients who may have directly visited medical centers located outside of the study area (Fig. 1). During the 10 year study period, the study team of cardiologists and trained research nurses regularly retrieved and reviewed medical charts and/or discharge summaries for nearly all patients admitted to the cardiology and internal medicine wards at each of these 11 hospitals. Patients who had been transferred to another hospital were counted at the index admission only.
TTE evaluation such as left ventricular ejection fraction assessment (Simpson or Teichholz method) was performed for nearly all patients with HF by full time attending cardiologists. However, in the branch hospitals without attending cardiologists, left ventricular ejection fraction was occasionally missing (≈ 10% in the incidence cohort; Table 1). Patients with a left ventricular ejection fraction of 50% or higher were classified as having HF preserved-EF, whereas those with an ejection fraction of less than 50% were classified as HF reduced-EF [16] ; [17].
| All terms 2003-2012 | T1 2003-2004 | T2 2005-2006 | T3 2007-2008 | T4 2009-2010 | T5 2011-2012 | p values⁎ | |
|---|---|---|---|---|---|---|---|
| Incident cohort (n = 1413) | |||||||
| Men | |||||||
| Number | 632 | 144 | 96 | 131 | 124 | 137 | |
| Mean age ± SD (year) | 75 ± 13 | 73 ± 12 | 74 ± 13 | 76 ± 13 | 76 ± 13 | 77 ± 12 | < 0.05 |
| Atrial fibrillation | 58% | 62% | 62% | 55% | 55% | 59% | NS |
| Transthoracic echocardiography | 92% | 92% | 82% | 94% | 94% | 94% | NS |
| Age-adjusted mortality (95%CI) per 105 person-year | 13 (6 -20) | 12 (5 -19) | 22 (13 - 31) | 15 (7 - 22) | 7 (2 -13) | 11 (4 -17) | NS |
| Women | |||||||
| Number | 781 | 152 | 170 | 162 | 145 | 152 | |
| Mean age ± SD (year) | 83 ± 11 | 81 ± 10 | 81 ± 12 | 83 ± 12 | 82 ± 11 | 85 ± 8 | < 0.01 |
| Atrial fibrillation | 50% | 44% | 52% | 48% | 52% | 52% | NS |
| Transthoracic echocardiography | 88% | 89% | 81% | 90% | 90% | 91% | NS |
| Age-adjusted mortality (95%CI) per 105 person-year | 11 (4 -17) | 9 (3 -15) | 18 (10 - 26) | 12 (5 -19) | 6 (1 - 11) | 10 (4-16) | NS |
| Prevalent cohort (n = 2598) | |||||||
| Men | |||||||
| Number | 1156 | 235 | 189 | 244 | 234 | 254 | |
| Mean age ± SD (year) | 76 ± 12 | 74 ± 13 | 76 ± 12 | 76 ± 13 | 77 ± 11 | 78 ± 11 | < 0.05 |
| Atrial fibrillation | 62% | 60% | 62% | 60% | 63% | 64% | NS |
| Transthoracic echocardiography | 89% | 87% | 80% | 91% | 94% | 92% | < 0.01 |
| Age-adjusted mortality (95%CI) per 105 person-year | 16 (8 - 24) | 11 (4 -17) | 15 (7 - 22) | 21 (12 - 30) | 18 (10 - 23) | 16 (8 - 24) | NS |
| Women | |||||||
| Number | 1442 | 256 | 299 | 311 | 273 | 303 | |
| Mean age ± SD (year) | 83 ± 10 | 81 ± 11 | 83 ± 12 | 83 ± 11 | 84 ± 10 | 86 ± 8 | < 0.01 |
| Atrial fibrillation | 53% | 45% | 53% | 48% | 58% | 57% | < 0.01 |
| Transthoracic echocardiography | 84% | 80% | 75% | 86% | 88% | 92% | < 0.01 |
| Age-adjusted mortality (95%CI) per 105 person-year | 26 (16 - 36) | 20 (11 - 29) | 38 (26 - 50) | 29 (18 - 39) | 21 (12 - 30) | 23 (14 - 32) | NS |
⁎. p = among T1-T5
2.3. Inclusion criteria and enrolment
Decompensated HF cases were registered from January 1, 2003 to December 31, 2012 within the study area. Inclusion criteria were based on the Framingham HF criteria [18]. Patients were enrolled if they had been hospitalized and fulfilled the following conditions: 1) were established residents of the study district, 2) were admitted during the study period. Patients were excluded if they had been hospitalized: 1) to undergo planned cardiac examination and therapies, 2) with an advanced stage malignant tumor and/or preceding apparent pneumonia, 3) within 4 weeks after onset of acute myocardial infarction, 4) with end stage renal failure without apparent cardiac dysfunction.
2.4. Ethics
Approval was obtained from the ethics review board of each participating hospital and our University Ethics Committee prior to commencement of the study.
2.5. Data analysis
The 10 year study period was divided into five 2-year terms (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012), and data were compared accordingly among the five time periods. Crude incidence rates were calculated as the observed number of HF cases divided by the area population (person-year). In addition, the incidence rate was adjusted by the 2010 Japanese standard population. Continuous variables are expressed as mean ± SD. Group comparisons were based on Students T test, one-way ANOVA or Kruskal–Wallis test, as appropriate. The significance of trends in several parameters among T1 to T5 were tested by Poisson trend analysis. A significant difference was defined as p < 0.05.
3. Results
3.1. Clinical characteristics
During the 10 year survey period, a total of 2598 cases of hospitalized decompensated HF were registered (men 1156, women 1442, M:F = 1:1.2; prevalent cohort in Table 1). More than half (n = 1413) were initial hospitalizations for HF (incident cohort in Table 1). In both cohorts, the mean age of patients with HF increased significantly over time, and clinical class of HF was relatively severe with approximately 60% of cases being NYHA 3 or 4. Atrial fibrillation was present in approximately 60% of men and 50% of women. Echocardiographic parameters were obtained in nearly 90% of the incident cohort (men 92%; women 88%). The performance rate of TTE was stable across the 5 terms in the incident cohort (Table 1). In terms of temporal trends of age adjusted in-hospital mortality for both incident and prevalent cohorts, no significant changes were found during the study period (Table 1).
3.2. Incidence rate
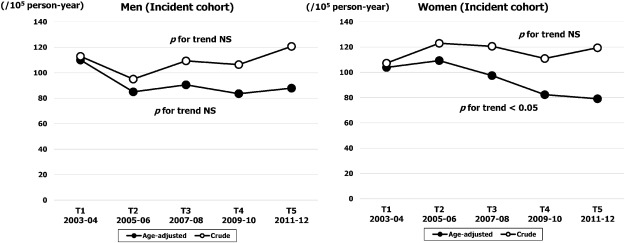
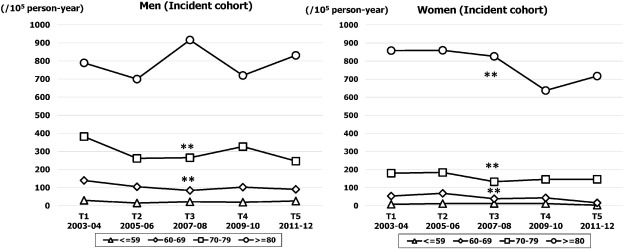
The crude and age adjusted incidence rates (per 100,000 person-year) are shown in Fig. 2. The temporal trend in crude incidence rate did not change throughout the study period in either sex. Although the age adjusted incidence rate in men was stable over time (from 110 at T1 to 88 at T5; NS), the rate in women decreased gradually from 104 (95% CI, 84–124) in T1 to 79 (95% CI, 61–96) in T5 (p for trend < 0.05). The age specific temporal trend in incidence rates are shown in Fig. 3. The highest rate was observed in the ≥ 80 years age band in both sexes. The rate in men was stable at around 800 throughout the study period (NS), whereas the rate in women was decreased significantly from 858 (95% CI, 801–915) in T1 to 717 (95% CI, 665–769) in T5 (p for trend < 0.01). For the younger age bands of 60–69 and 70–79 years, the incidence decreased gradually over time in both sexes (p for trend < 0.01: Fig. 3).
|
|
|
Fig. 2. Sex specific temporal trends in crude and age adjusted incidence rates of HF over five time periods (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012). |
|
|
|
Fig. 3. Sex and age specific incidence rates of HF over five time periods (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012). **p for trend < 0.01. |
3.3. Prevalence rate
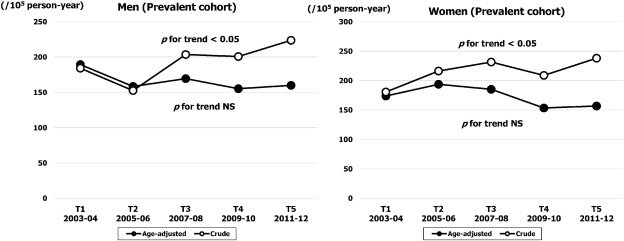
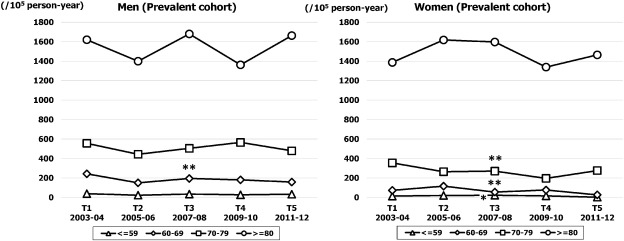
As shown in Fig. 4, the crude prevalence rate (per 100,000 person-year) increased significantly from 184 (95% CI, 157–210) in T1 to 224 (95% CI, 195–256) in T5 in men (p for trend < 0.05), and from 181 (95% CI, 155–207) in T1 to 238 (95% CI, 208–268) in T5 in women (p for trend < 0.05). However, the temporal trend for age adjusted prevalence rate did not change in either sex. The age specific trend for prevalence rate is demonstrated in Fig. 5. In both sexes, the rate for the highest age band (≥ 80 years) was stable throughout the study period, while in the younger age bands the rate decreased gradually over time in both sexes.
|
|
|
Fig. 4. Sex specific temporal trends in crude and age adjusted prevalence rates of HF over five time periods (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012). |
|
|
|
Fig. 5. Sex and age specific prevalence rate of HF over five time periods (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012). *p for trend < 0.05, **p for trend < 0.01. |
3.4. Left ventricular function subtype
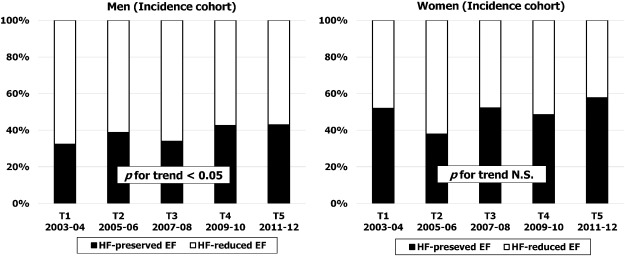
In cases who underwent 2-dimensional TTE (≅ 90% of the incident cohort), actual case number per term for HF preserved-EF in men tended to increase over time (from 42 in Term 1 to 55 in Term 5), while in women the case number increased gradually and overtook the rate for HF reduced-EF in Term 5 (HF preserved-EF, n = 79 versus HF reduced-EF, n = 58). The percentage of HF preserved-EF in men increased significantly from 32% in T1 to 43% in T5 (p for trend < 0.05; Fig. 6), while in women, the proportion of HF preserved-EF was relatively high at more than 50% and this remained stable throughout the study period (from 52% at T1 to 58%. at T5; p for trend NS): Fig. 6.
|
|
|
Fig. 6. Sex specific temporal trends in proportion of patients with HF reduced-EF versus HF preserved-EF within incident cohort over five time periods (T1, 2003–2004; T2, 2005–2006; T3, 2007–2008; T4, 2009–2010; T5, 2011–2012). |
4. Discussion
The proportion of elderly in the study area population is growing rapidly, providing a basis for projection of future population structures in developed countries. We found the following: 1) the age of hospitalized patients with HF has increased significantly over the last decade in both sexes; 2) age adjusted incidence over the 10 year study period did not change in men, but decreased significantly by approximately 25% in women; 3) in-hospital mortality did not change across the time periods; 4) the number and proportion of HF preserved-EF cases tended to increase during the study period.
In the research region, only six general hospitals have admission facilities, while other private clinics in the area have outpatient facilities only and rarely provide clinical care for overt HF patients. Thus patients with HF who have clinical symptoms are usually transferred for admission to one of these six hospitals. In addition, to capture patients who were hospitalized for HF in remote areas outside of the study district, our registration study was extended to include several teaching hospitals located out of the study area. We are therefore confident that the registration process will have captured almost all hospitalized patients with HF within the area concerned. These findings of the present study may therefore be able to clarify the community based incidence and prevalence rates of acute decompensated HF in our population.
The present study found that the age adjusted incidence of HF in men did not change significantly over the 10 year study period. In contrast, the rate in women decreased gradually by 25% over this time. Few previous reports have described sex specific temporal trends in the population based incidence of HF. This makes it difficult to compare our research findings with the results of previous studies from other populations, as the definition of HF differs between reports (i.e., the Framingham definition [4]; [5]; [6] ; [7], the Boston criteria [7], the European Society of Cardiology definition [8], and the International Classification of Disease [9]). In addition, categories of HF (i.e., hospitalized patients only or including outpatients) also differ. However, when the present data are compared to previous studies for hospitalized HF, the rates found in this study are lower than those found in other locations such as North America [19] ; [20] and Scotland [9] ; [21]. Definitive reasons for this lower incidence of HF in our population remain unknown, but the prevalence of coronary heart disease, which is the main burden of HF, is clearly lower among the Japanese than among North American and European populations [22]. In accord with the lower incidence of HF in our population, Ng et al. have reported using national public hospital and mortality data in Singapore to show that the hospitalization rate for HF in Chinese was 35% lower than in Malays or Indians [23]. Bahrami et al. demonstrated in a US multiethnic cohort study that the incidence of HF in Chinese Americans was the lowest in a comparison among African Americans, Hispanic, and Whites [24]. In view of these reports, the lower incidence and prevalence of HF observed in our population is likely to have been due to ethnic differences specifically lower incidence of atherosclerotic cardiac disorders. Another possible factor underlying the low incidence of HF may be Japans relatively affordable and accessible health care system [25] which allows easy access to medical facilities before overt HF and hospitalization.
The present study has shown a sex difference in temporal trends in HF over a recent 10 year period. There are a few population based reports describing sex specific chronological changes in the incidence of HF. In the Framingham study, during a recent 50 year period, the rate of HF has not changed significantly in men but has fallen by 30% in women [4]. Conversely, in the Olmsted study, the rate of HF in the Mayo clinic group did not change in either sex over a recent 30 year period [11]. The present study appears to agree with the Framingham study by finding that HF rates have remained stable in men while decreasing significantly by about 25% in women. The reasons for these sex differences remain unclear on the basis of this research, although an official report has shown that the prevalence of several cardiovascular risk factors such as diabetes, obesity, hypertension and smoking are lower in women than in men [26]. In addition, the prevalence of hypertension in Japanese population has been reported to decrease during the recent years in all 10-year age groups of women (30–79 years) and younger men (30 s and 40 s). However, this trend did not occur in men aged ≥ 50 years [27]. These sex differences in epidemiological trends for CV risk factors will reduce the risk of onset of various types of heart disease and thus contribute to the observed decrease in the incidence and prevalence of HF in women.
The present study also suggests that the number and proportion of HF preserved-EF increased over the study period. Although the reasons for this were not clarified, the mean age of HF increased significantly in both sexes over time. The aging progress leads to an increase in the prevalence of the comorbidities of hypertensive left ventricular hypertrophy and diabetes mellitus, both of which are commonly associated with HF preserved-EF. In addition, several studies have demonstrated that aging increases diastolic left ventricular elastance and vascular stiffness [28]. Cardiovascular stiffening is most prominent in the population at risk for HF preserved-EF and is therefore considered a major risk factor for HF preserved-EF. The observed sustained or increased number and percentage of HF preserved-EF may therefore be due to the increasing age of HF patients along with general population aging.
The rate of in-hospital mortality did not improve significantly across the study period in either the incident or the prevalent HF cohort. During the past decade, there have been important advances in the management of cardiovascular disease, including greater use of vasodilators, renin–angiotensin–aldosterone blockers, and beta-blockers for improving prognosis of HF. However, limited data exist to show whether survival rates for real-world HF have changed over time. MacIntyre et al. reported that the 30 day case fatality rate in hospitalized patients with HF in Scotland improved 26% in men and 17% in women [29]. Ni et al. also demonstrated that in-hospital deaths with a primary diagnosis of HF in Oregon hospitals decreased [30]. Although these results appear to vary from our findings, previous reports concerning temporal trends for in-hospital prognosis may be inconsistent. In fact, crude and age adjusted 30 day mortality for hospitalized patients with HF has been reported to remain steadily high between 1997 and 2007 [31]. Roger et al. have reported that age adjusted 30 day mortality did not improve significantly in women or elderly persons [6]. These incongruent estimates could in part come from regional variations in patient population, availability of health services, and the selection and definition of HF applied. Indeed, the present HF cohort was relatively advanced in age with the possible presence of comorbidities and preserved-EF, thus HF in this group may have been refractory to recent standard medical therapy.
Despite the advantages afforded by our prospective population based study design using standard HF criteria over a 10 year period, several limitations must be considered. First, as this was an epidemiological study, detailed echocardiographic parameters for left ventricular diastolic function could not be obtained and ejection fraction could not be measured in approximately 10% of patients registered. As a result, although the proportion of patients who underwent TTE was approximately 90% and remained stable during the study period, the analysis of left ventricular function subtype may have some inherent bias. As the ejection fraction data tended to be lacking more among female cases (Table 1), numbers of patients with HF preserved-EF may have been underestimated. Second, the east Japan earthquake and tsunami which occurred in the Iwate prefecture in 2011, thus during the study period, may have had an impact on the present results. However, official statistics for the disaster showed that there were only two victims and no refugees in the present study area (northern Iwate sea coast and inlands areas) [32]. This suggests that damage caused by the disaster in the study areas was much less serious. In addition, our previous study demonstrated that the incidence of HF in the same area was stable for several months after the disaster [33]. On the basis of these data we believe that any bias induced by the disaster in the incidence of HF regardless of the presence or absence of preserved EF in the present study area may be small. Third, the present study did not attempt to determine the etiology of HF as a large proportion of cases were very elderly and may thus have had overlapping etiologies (ie, coronary artery and hypertensive heart disease), and been unsuitable for detailed cardiac examination such as invasive angiography or stress myocardial perfusion imaging.
In conclusion, although the incidence of HF declined over the recent decade in our population, especially in women, the number and proportion of HF preserved-EF is increasing. These trends suggest a future prevalence of HF preserved-EF rather than HF reduced-EF in the aging general population.
Sources of funding
This study was supported in part by grants-in-aid from the scientific research fund of the Ministry of Education, Science, and Culture of Japan (26461082), Tokyo, Japan; the Japan Arteriosclerosis Prevention Fund (JAPF), Tokyo, Japan; and the Takeda Science Foundation, Osaka, Japan (2012).
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We gratefully acknowledge and appreciate the support of RNs Miwako Ozawa, Yukiko Ito, Yurie Kojima, Yumiko Okuyama, Etsuko Shinozaki, Junko Sakuraba, Yasuko Ube, Akiko Fujimori, Seiko Yonai, Reiko Ochiai, Masako Iwasawa, Nobuko Kumagai, Kazuko Terasawa, Teruko Sasaki, Ayako Uemura, Sadako Ogasawara, Yumiko Sano.
References
- [1] P.A. Heidenreich, N.M. Albert, L.A. Allen, et al.; Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association; Circ. Heart Fail., 6 (2013), pp. 606–619
- [2] P.A. McCullough, E.F. Philbin, J.A. Spertus, S. Kaatz, K.R. Sandberg, W.D. Weaver; Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive 3. Heart Failure (REACH) study; J. Am. Coll. Cardiol., 39 (2002), pp. 60–69
- [3] A.W. Hoes, A. Mosterd, D.E. Grobbee; An epidemic of heart failure? Recent evidence from Europe; Eur. Heart J., 19 (Suppl. L) (1998), pp. L2–L9
- [4] D. Levy, S. Kenchaiah, M.G. Larson, et al.; Long-term trends in the incidence of and survival with heart failure; N. Engl. J. Med., 347 (2002), pp. 1397–1402
- [5] F.M. Gomez-Soto, J.L. Andrey, A.A. Garcia-Egido, et al.; Incidence and mortality of heart failure: a community-based study; Int. J. Cardiol., 151 (2011), pp. 40–45
- [6] V.L. Roger, S.A. Weston, M.M. Redfield, et al.; Trends in heart failure incidence and survival in a community-based population; J. Am. Med. Assoc., 292 (2004), pp. 344–350
- [7] J. Remes, A. Reunanen, A. Aromaa, K. Pyörälä; Incidence of heart failure in eastern Finland: a population-based surveillance study; Eur. Heart J., 13 (1992), pp. 588–593
- [8] M.R. Cowie, D.A. Wood, A.J. Coats, et al.; Incidence and aetiology of heart failure; a population-based study; Eur. Heart J., 20 (1999), pp. 421–428
- [9] J. McMurray, T. McDonagh, C.E. Morrison, H.J. Dargie; Trends in hospitalization for heart failure in Scotland 1980–1990; Eur. Heart J., 14 (1993), pp. 1158–1162
- [10] R.B. Devereux, M.J. Roman, J.E. Liu, et al.; Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study; Am. J. Cardiol., 86 (2000), pp. 1090–1096
- [11] T.E. Owan, D.O. Hodge, R.M. Herges, S.J. Jacobsen, V.L. Roger, M.M. Redfield; Trends in prevalence and outcome of heart failure with preserved ejection fraction; N. Engl. J. Med., 355 (2006), pp. 251–259
- [12] K.K. Ho, J.L. Pinsky, W.B. Kannel, D. Levy; The epidemiology of heart failure: the Framingham Study; J. Am. Coll. Cardiol., 22 (4 Suppl A) (1993), pp. 6A–13A
- [13] B.A. Borlaug, M.M. Redfield; Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum; Circulation, 123 (2011), pp. 2006–2013
- [14] D.C. Scantlebury, B.A. Borlaug; Why are women more likely than men to develop heart failure with preserved ejection fraction?; Curr. Opin. Cardiol., 26 (2011), pp. 562–568
- [15] B.A. Steinberg, X. Zhao, P.A. Heidenreich, et al.; Get with the guidelines scientific advisory committee and investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes; Circulation, 126 (2012), pp. 65–75
- [16] R.S. Vasan, E.J. Benjamin, D. Levy; Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective; J. Am. Coll. Cardiol., 26 (1995), pp. 1565–1574
- [17] T.E. Owan, M.M. Redfield; Epidemiology of diastolic heart failure; Prog. Cardiovasc. Dis., 47 (2005), pp. 320–332
- [18] P.A. McKee, W.P. Castelli, P.M. McNamara, W.B. Kannel; The natural history of congestive heart failure: the Framingham study; N. Engl. J. Med., 285 (1971), pp. 1441–1446
- [19] J.B. Croft, W.H. Giles, R.A. Pollard, M.L. Casper, R.F. Anda, J.R. Livengood; National trends in the initial hospitalization for heart failure; J. Am. Geriatr. Soc., 45 (1997), pp. 270–275
- [20] R.J. Goldberg, F.A. Spencer, C. Farmer, T.E. Meyer, S. Pezzella; Incidence and hospital death rates associated with heart failure: a community-wide perspective; Am. J. Med., 118 (2005), pp. 728–734
- [21] S. Stewart, K. MacIntyre, M.M. MacLeod, A.E. Bailey, S. Capewell, J.J. McMurray; Trends in hospitalization for heart failure in Scotland, 1990–1996. An epidemic that has reached its peak?; Eur. Heart J, 22 (2001), pp. 209–217
- [22] A. Sekikawa, T. Satoh, T. Hayakawa, H. Ueshima, L.H. Kuller; Coronary heart disease mortality among men aged 35–44 years by prefecture in Japan in 1995–1999 compared with that among white men aged 35–44 by state in the United States in 1995-1998: vital statistics data in recent birth cohort; Jpn. Circ. J., 65 (2001), pp. 887–892
- [23] T.P. Ng, M. Niti; Trends and ethnic differences in hospital admissions and mortality for congestive heart failure in the elderly in Singapore, 1991 to 1998; Heart, 89 (2003), pp. 865–870
- [24] H. Bahrami, R. Kronmal, D.A. Bluemke, et al.; Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis; Arch. Intern. Med., 168 (2008), pp. 2138–2145
- [25] K. Kojima, S. Ito, M. Sayama, L. Kim, G. Greer; Outline of the medical insurance system in Japan—the change on 1 January 2001; Jpn. Hosp., 20 (2001), pp. 27–33
- [26] http://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/0000032813.pdf ([In Japanese] accessed 28 May 2015)
- [27] K. Miura, M. Nagai, T. Ohkubo; Epidemiology of hypertension in Japan: where are we now?; Circ. J., 77 (2013), pp. 2226–2231
- [28] M.M. Redfield, S.J. Jacobsen, B.A. Borlaug, R.J. Rodeheffer, D.A. Kass; Age- and gender-related ventricular–vascular stiffening: a community-based study; Circulation, 112 (2005), pp. 2254–2262
- [29] K. MacIntyre, S. Capewell, S. Stewart, et al.; Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995; Circulation, 102 (2000), pp. 1126–1131
- [30] H. Ni, D.J. Nauman, R.E. Hershberger; Analysis of trends in hospitalizations for heart failure; J. Card. Fail., 5 (1999), pp. 79–84
- [31] D.F. Yeung, N.K. Boom, H. Guo, D.S. Lee, S.E. Schultz, J.V. Tu; Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007; CMAJ, 184 (2012), pp. E765–E773
- [32] http://www2.pref.iwate.jp/~bousai/ ([In Japanese] accessed 30 June 2015)
- [33] M. Nakamura, F. Tanaka, S. Nakajima, et al.; Comparison of the incidence of acute decompensated heart failure before and after the major tsunami in Northeast Japan; Am. J. Cardiol., 110 (2012), pp. 1856–1860
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?