Abstract
Background
Right ventricular dysfunction (RVdysf) is a predictor of poor outcome in patients with heart failure and valvular disease. The aim of this study was to evaluate the evolution and the impact of RVdysf in patients with moderate–severe functional mitral regurgitation (FMR) successfully treated with MitraClip.
Methods and results
From October 2008 to July 2014, 60 consecutive high surgical risk FMR patients were evaluated and stratified into two groups: RVdysf group (TAPSE < 16 mm and/or S′TDI < 10 cm/s, 21 patients) and No-RVdysf group (38 patients). The overall mean age of patients was 73 ± 8 (83% male). Ischemic FMR etiology was present in 67%. Mean LVEF was 30 ± 10%. Overall mean time follow-up was 565 ± 310 days. The only significant difference between the two groups was a greater prevalence of stroke, ICD and use of aldosterone antagonist in RVdysf group. Acute procedural success was achieved in 90% of patients. At 6-month echo-matched analysis significant RV function improvement was observed in patients with baseline RVdysf (TAPSE 15 ± 3.0 vs. 19 ± 4.5, p = 0.007; S′TDI 7 ± 1.2 vs. 11 ± 2.8, p < 0.0001; baseline vs. 6-month, respectively). The mean improvement in the 6-min walking test was significant in both groups (120 and 143 m, RVdysf and No-RVdysf groups, respectively). At Kaplan–Meier analysis, the presence of RVdysf did not affect the outcome in terms of freedom from composite efficacy endpoint.
Conclusions
This study shows that successful MitraClip implantation in patients with FMR and concomitant right ventricular dysfunction yields significant improvement of RV function at mid-term follow-up. Further data on larger population will be required to confirm our observations.
Keywords
MitraClip;Right ventricular dysfunction;Functional mitral regurgitation;Heart failure
1. Introduction
Mitral regurgitation (MR) represents the second most common valvular disease in Europe [1]. This pathology evolves over many years, allowing the heart to adapt to a chronic volume overload, leading to significant and complex hemodynamic and structural changes. In chronic severe MR, left ventricular remodeling leads to the development of significant pulmonary hypertension in almost half of the patients [2] ; [3]. The increase in right ventricular (RV) afterload at first induces right atrial and RV remodeling, and afterwards leads to RV dysfunction. RV dysfunction represents a strong and independent predictor of mortality in left ventricular ischemic and non-ischemic heart failure (HF) [4]; [5]; [6]; [7]; [8]; [9] ; [10]. Alternate effects of surgical mitral valve repair or replacement on preexisting RV dysfunction have been reported in different published series of patients [11]; [12]; [13]; [14]; [15]; [16]; [17] ; [18] Percutaneous mitral valve repair with MitraClip has been shown to be associated with a favorable clinical outcome and left and right ventricular reverse remodeling in high-risk patients with severe MR [19]; [20]; [21] ; [22]. In the present study we evaluated the impact and the evolution of pre-existing RV dysfunction in high-risk functional MR (FMR) patients after successful MitraClip implantation.
2. Methods
2.1. Study population and clinical endpoints
From October 2008 to July 2014, 60 consecutive MitraClip treated patients were evaluated at San Raffaele Hospital and at EMO-GVM Centro Cuore Columbus, Milan, Italy. The MitraClip procedure was considered in patients with symptomatic severe FMR who fulfilled the echocardiographic criteria of eligibility and judged inoperable or at high surgical risk by a ‘heart team’, and had a life expectancy greater than 1 year (according to the ESC/EACTS 2012 recommendation class IIb, level of evidence C). Each patient was informed regarding the possible risk and procedural benefit of MitraClip procedure and written consent was obtained for the procedure, data collection, and subsequent analysis and publication. The study was approved by the Hospital Ethics Committee and no external source of funding supported this study. All patients had symptomatic moderate-to-severe or severe FMR despite optimal medical therapy. Prior to implantation, all patients underwent coronary angiography to exclude relevant coronary artery disease necessitating revascularization. Furthermore, patients underwent transthoracic and transoesophageal echocardiography to quantify MR and to judge morphological suitability for MitraClip implantation. According to the EVEREST II protocol [23], acute procedural success (APS) was defined as implantation of at least one clip and post-procedural MR ≤ 2 +. Advanced congestive heart failure (HF) defined patients with NYHA class III-IV in spite of maximal medical treatment and concomitant presence of LVEDD > 70 mm and/or LVEF < 20% and/or NT-proBNP > 10,000 pg/ml. The transthoracic and transoesophageal echocardiograms were obtained using commercially available ultrasound diagnostic systems (Philips IE 33, Royal Philips Electronics, Amsterdam, The Netherlands, or Vivid 7 and Vivid E9, GE Medical Systems, Milwaukee, WI, USA) by two experienced investigators (MO and EA), according to current ESC/ACC guidelines [21]. Assessment of RV function was performed with the apical four-chamber and parasternal RV inflow and outflow tract views. The following quantitative conventional indices were measured: tricuspid annular plane systolic excursion (TAPSE), peak systolic velocity at the junction of the RV free wall and the tricuspid annulus, assessed with pulsed tissue Doppler imaging (S′TDI). Systolic pulmonary artery pressure (sPAP) was calculated from tricuspid regurgitation maximum pressure gradient by use of the modified Bernoulli equation, with the addition of an estimate of central venous pressure. Tricuspid regurgitation grade was evaluated according to a visual scale ranging from 1 to 4.
All FMR patients were stratified for RVdysf at baseline as with No/Mild RV dysfunction, (No-RVdysf group: TAPSE ≥ 16 mm and/or S′TDI ≥ 10 cm/s) or with moderate/severe RV dysfunction, (RVdysf group: TAPSE < 16 mm and/or S′TDI < 10 cm/s), according to the European Society of Echocardiography guidelines [24]. In case of discrepancy, the S′TDI was utilized as the discriminant value (90% sensitivity and 85% specificity to identify the presence of RVdysf) [25]. Patients were excluded if the morphology of the mitral valve made MitraClip implantation technically impossible or unlikely beyond the classical EVEREST criteria (i.e. short or calcified posterior leaflet without the possibility of leaflet grasping or moderate to severe mitral stenosis). All patients underwent blood sampling for routine complete blood count, basic metabolic panel, liver function test, NT-proBNP (Roche NT-proBNP assay) prior to MitraClip implantation. A 6 min walk test (6-MWT) was performed in eligible patient.
2.2. Study endpoints
The primary endpoint of this study was the echocardiographic evaluation of RV function at 6-month follow-up after successful MitraClip implantation (echo-matched analysis). Additional pre-specified secondary endpoints were: A) composite efficacy endpoint [cardiac death, hospitalizations for acute decompensated heart failure, LVAD (left ventricular assist device) implantation and need for conventional mitral valve surgery after MitraClip (up to 24-month follow-up)]; B) New York Heart Association (NYHA) heart failure class and C) 6 min walking test (6-MWT) variation (at 6-month follow-up).
2.3. Mitraclip implantation procedure
The endovascular edge-to-edge mitral valve repair procedure has been previously described [23]. All procedures were performed using a 24 Fr MitraClip device using CDS01 or CDS02 (Abbott Vascular, Santa Clara, CA, USA). All clips were implanted under general anesthesia and fluoroscopic and transoesophageal echocardiographic control. Hemostasis was achieved by compression of the femoral vein for 5–10 min soon after figure-of-eight suture. Patients were transferred to our intermediate care unit or, if necessary, intensive care unit.
2.4. Clinical and echocardiographic follow-up
Before discharge, all patients were evaluated with NYHA class assessment, 6-MWT, NT-pro-BNP measurement and echocardiography. After MitraClip implantation, patients were prospectively followed up at 1, 6 and up to 24 months. Clinical evaluation of NYHA class, blood sampling for NT-pro-BNP, and echocardiography were performed at every follow-up visit. When feasible, a 6-MWT was performed. Three and nine patients declined to attend, respectively, 6 and 24 months clinical follow-up examination after implantation. Nevertheless, in these cases, follow-up data was obtained by telephone interviews. Echocardiography was performed in 53 eligible patients at 6-months (98%) and in 49 patients after 24-month (91%).
2.5. Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD) or median (inter-quartile range, IQR) and compared with Students t test or Mann–Whitney or Wilcoxon tests, based on the normality (Kolmogorov–Smirnov goodness-of-fit test) of the data. Categorical variables (as frequencies or percentage) were compared with χ2 test with Yates correction for continuity or the Fisher exact test as appropriate for the available data [26]. Differences between matched baseline and 6-month follow-up echocardiographic data (primary endpoint) and NYHA class/6-MWT data (secondary endpoints) were analyzed using the matched-pair t-test or Wilcoxon signed-rank test, according to normality of distribution. Event-free survival during follow-up was evaluated according to the unadjusted Kaplan–Meier method and survival among groups was compared using log-rank test (Cox–Mantel test). Clinical follow-up was censored at the date of last follow-up or up to 24 months follow-up (2-year), whichever came first. Data on patients who were lost to follow-up were censored at the time of the last contact. Cox proportional hazards methods were used to estimate the independent effect of multiple independent variables on the risk of composite efficacy endpoint (secondary endpoint). To avoid multicollinearity, a “low-noise model” has been researched in which each predictor variable correlate at most only minimally with the other. Selection of the variables included in the multivariate model was done with backward elimination (Wald statistic, confirmed using forward and stepwise selection) based on covariates listed in Table 1 ; Table 2. Only the covariates that were significantly associated with the risk of composite efficacy endpoint at univariate analysis (p < 0.05 for model inclusion and p > 0.10 for exclusion) and that considered clinically relevant were included, and the convention of limiting the number of independent variables to 1 for every 10 events was followed [27] ; [28]. The proportional hazards assumption was checked both graphically and by hypothesis testing. Graphical examination was done using a log-cumulative hazard plot. Linearity was checked graphically using the smoothed martingale residuals from the null model plotted against the covariate variables. The results are reported as adjusted hazard ratios (HRs) with associated 95% Confidence Intervals (CI). Two-side p-values < 0.05 were considered statistically significant. The statistical analyses were performed using SPSS 16.0.2 (SPSS Inc., Chicago, IL, USA) and NCSS 2007 (NCSS, Kaysville, UT, USA). Kaplan–Meier survival curves were generated with the use of GraphPad Prism software (version 4; GraphPad, Inc., San Diego, CA).
| FMR patients (n = 60) | No-RVdysf group (n = 38) | RVdysf group (n = 22) | p value | |
|---|---|---|---|---|
| Clinical characteristics (%) | ||||
| Age, years | 73 ± 8 | 73.6 ± 7 | 72 ± 8 | 0.478 |
| Proportion aged > 80 years | 9 (15) | 7 (18) | 2 (9) | 0.329 |
| Male gender | 50 (83) | 31 (82) | 19 (86) | 0.632 |
| BMI, kg/m2 | 25 ± 3.6 | 25 ± 2.8 | 24 ± 4 | 0.651 |
| BSA, m2 | 1.8 ± 0.19 | 1.8 ± 0.18 | 1.8 ± 0.21 | 0.655 |
| Hypertension | 43 (72) | 29 (76) | 14 (64) | 0.294 |
| Hypercholesterolemia | 32 (53) | 21 (55) | 11 (50) | 0.662 |
| Previous/Current smoker | 27 (45) | 16 (42) | 11 (50) | 0.554 |
| Diabetes mellitus (DM) | 18 (30) | 10 (26) | 8 (36) | 0.413 |
| Insulin-dependent DM | 10 (17) | 5 (13) | 5 (23) | 0.338 |
| Moderate/Severe COPD | 19 (32) | 11 (29) | 8 (36) | 0.647 |
| CRF⁎ | 36 (60) | 21 (55) | 15 (68) | 0.325 |
| Atrial fibrillation | 21 (35) | 11 (29) | 11 (50) | 0.103 |
| Logistic EuroSCORE | 27.2 ± 20.5 | 23.8 ± 18 | 33 ± 23 | 0.101 |
| Logistic EuroSCORE > 25 | 30 (50) | 16 (42) | 14 (64) | 0.089 |
| STS score mortality | 7.9 ± 8.5 | 8.0 ± 8.7 | 7.7 ± 8.4 | 0.883 |
| Advanced congestive HF | 21 (35) | 10 (26) | 11 (50) | 0.093 |
| §6-MWT, m | 293 ± 97 | 290 ± 92 | 298 ± 112 | 0.815 |
| Ischemic FMR etiology | 40 (67) | 24 (63) | 16 (73) | 0.449 |
| NYHA functional class (III-IV) | 42 (70) | 27 (71) | 15 (68) | 0.815 |
| Pulmonary hypertension⁎⁎⁎ | 11 (18) | 7 (18) | 4 (18) | 0.916 |
| Coronary artery disease | 42 (70) | 24 (63) | 16 (73) | 0.115 |
| Three-vessel disease | 22 (37) | 14 (37) | 8 (36) | 0.970 |
| Chronic stable angina | 9 (15) | 5 (13) | 4 (18) | 0.606 |
| Previous pulmonary edema | 28 (47) | 16 (42) | 12 (54) | 0.355 |
| Previous AMI | 38 (63) | 21 (55) | 17 (77) | 0.119 |
| Previous PCI | 31 (52) | 18 (47) | 13 (59) | 0.384 |
| Previous CABG | 17 (28) | 10 (26) | 7 (32) | 0.658 |
| Peripheral vascular disease | 13 (22) | 6 (16) | 7 (32) | 0.146 |
| Previous stroke | 3 (5) | 0 (0) | 3 (14) | 0.020 |
| Laboratory analysis | ||||
| NT-pro-BNP, pg/ml | 9004 ± 15958 | 8323 ± 16258 | 10206 ± 15833 | 0.702 |
| NT-pro-BNP ≥ 10.000 pg/ml | 10 (17) | 4 (10) | 6 (27) | 0.077 |
| Sodium, mEq/l | 138 ± 4 | 138 ± 4 | 138 ± 4 | 0.967 |
| Hemoglobin, gr/dl | 11.8 ± 1.8 | 11.9 ± 1.7 | 11.7 ± 1.8 | 0.752 |
| RDW > 15% | 37 (62) | 24 (63) | 13 (59) | 0.755 |
| RDW, % | 16.1 ± 2.1 | 15.9 ± 2.0 | 16.3 ± 2.1 | 0.551 |
| Creatinine, mg/dl | 1.06 ± 0.7 | 1.7 ± 0.7 | 1.6 ± 0.6 | 0.600 |
| Bilirubin, mg/dl | 0.86 ± 0.5 | 0.78 ± 0.55 | 0.99 ± 0.4 | 0.172 |
| AST, units/L | 59 ± 224 | 80 ± 283 | 24 ± 8 | 0.364 |
| ALT, units/L | 49 ± 165 | 67 ± 208 | 20 ± 9 | 0.309 |
| Treatment history (%) | ||||
| Cardiovascular medication | ||||
| Loop diuretic | 56 (93) | 34 (89) | 22 (100) | 0.170 |
| Loop diuretic, mg | 138 ± 111 | 144 ± 113 | 128 ± 111 | 0.603 |
| Aldosterone antagonist | 29 (48) | 14 (37) | 15 (68) | 0.024 |
| Aldosterone antagonist, mg | 19.5 ± 16.6 | 14.9 ± 15.3 | 23.4 ± 17.1 | 0.180 |
| Beta-blocker | 47 (78) | 31 (82) | 16 (73) | 0.308 |
| CCB | 3 (5) | 3 (8) | 0 (0) | 0.170 |
| ACE-I/ARB | 31 (52) | 21 (55) | 10 (45) | 0.213 |
| Ivabradine | 6 (10) | 3 (8) | 3 (14) | 0.497 |
| Digoxin | 9 (15) | 6 (16) | 3 (14) | 0.778 |
| Cardioaspirin | 39 (60) | 25 (66) | 14 (64) | 0.708 |
| Dual antiplatelet therapy | 17 (28) | 11 (29) | 6 (27) | 1.000 |
| Oral anticoagulant therapy | 22 (37) | 12 (32) | 10 (45) | 0.317 |
| Electrical therapy | ||||
| ICD | 36 (60) | 19 (50) | 17 (77) | 0.038 |
| CRT-D | 11 (18) | 5 (13) | 6 (27) | 0.173 |
| CRT-PM | 10 (17) | 5 (13) | 5 (23) | 0.234 |
Data are presented as absolute numbers and percentages (for categorical variables) or mean value ± SD (for continuous variables) unless otherwise specified.
Students unpaired t-test for continuous data; Chi-square test for categorical data.
AMI = acute myocardial infarction; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blocker; AST = aspartate aminotransferase; ALT = alanine transaminase; BMI = body mass index; BSA = body surface area; CABG = coronary artery bypass grafting; CCB = Calcium channel blockers; CRT D = cardiac resynchronization therapy with defibrillator; CRT-PM = cardiac resynchronization therapy without defibrillator; COPD = chronic obstructive pulmonary disease; ICD = implantable cardioverter defibrillator; NYHA = New York Heart Association. NT-pro-BNP = N-terminal protype-brain natriuretic peptide; PCI = percutaneous coronary intervention; RDW = red cell distribution with.
Bold numbers indicate significance at p value < 0.05.
⁎Chronic renal failure (CRF) was defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2.
⁎⁎Severe pulmonary hypertension was defined as a pulmonary systolic pressure ≥ 60 mm Hg as estimated by doppler echocardiography.
§6-MWT = six minutes walking test, available in 34 patients (60% of patients).
| FMR patients (n = 60) | No-RVdysf group (n = 38) | RVdysf group (n = 22) | p value | |
|---|---|---|---|---|
| Left ventricle and atrium | ||||
| LVEDD, mm | 66.8 ± 9.2 | 66.1 ± 9.4 | 67.8 ± 9.1 | 0.507 |
| LVEDD > 55 mm | 51 (85) | 32 (84) | 19 (86) | 0.851 |
| LVESD, mm | 56.1 ± 10.2 | 55.6 ± 10.1 | 57.0 ± 11.0 | 0.772 |
| LVEDV, ml | 210 ± 78 | 210 ± 85 | 211 ± 66 | 0.954 |
| LVESV, ml | 147 ± 69 | 146 ± 77 | 148 ± 55 | 0.914 |
| LVEF, % | 30 ± 10 | 31 ± 11 | 27 ± 7 | 0.097 |
| LVEF ≤ 35% | 44 (73) | 25 (66) | 19 (86) | 0.082 |
| MR 4 + (severe) | 50 (83) | 31 (82) | 19 (86) | 0.628 |
| Eccentric mitral regurgitation jet | 13 (22) | 8 (21) | 5 (23) | 0.950 |
| Tenting area, cm2 | 2.93 ± 1.38 | 3.12 ± 1.86 | 2.79 ± 1.07 | 0.700 |
| Coaptation depth, mm | 12.63 ± 4.87 | 11.98 ± 3.29 | 13.51 ± 6.44 | 0.331 |
| Coaptation length, mm | 2.25 ± 1.98 | 2.55 ± 2.07 | 1.87 ± 1.65 | 0.293 |
| Restrictive transmitral filling patterns | 22 (37) | 13 (34) | 9 (41) | 0.861 |
| Atrium volume, ml | 134 ± 46 | 135 ± 53 | 131 ± 34 | 0.754 |
| Right ventricle and atrium | ||||
| RV basal diameter, mm | 40 ± 8 | 38 ± 7 | 44 ± 8 | 0.048 |
| RV basal diameter > 42 mm | 12 (20) | 6 (16) | 6 (27) | 0.052 |
| TR grade > 2 + | 15 (25) | 8 (21) | 7 (32) | 0.428 |
| sPAP, mmHg | 50 ± 16 | 49 ± 17 | 51 ± 14 | 0.626 |
| sPAP > 60 mmHg | 12 (20) | 7 (18) | 5 (23) | 0.737 |
| TAPSE, mm | 19.3 ± 4.2 | 21.3 ± 3.4 | 15.5 ± 3.0 | 0.000 |
| TAPSE < 16 mm | 12 (20) | 0 (0) | 12 (54) | 0.000 |
| SʹTDI, cm/s | 10 ± 2.5 | 11.7 ± 1.4 | 7.3 ± 1.2 | 0.000 |
| SʹTDI < 10 cm/s | 21 (35) | 0 (0) | 21 (95) | 0.000 |
LVEDD = left ventricular end diastolic diameter, LVESD = left ventricular end systolic diameter, LVEDV = left ventricular end diastolic volume, LVESV = left ventricular end systolic volume, LVEF = left ventricular ejection fraction, LA vol = left atrial volume; MR = mitral regurgitation, RV = right ventricle, TR = tricuspid regurgitation, PAP = pulmonary arterial pressure, TAPSE = tricuspid annular plane systolic excursion, SʹTDI = systolic wave with tissue Doppler imaging.
Bold numbers indicate significance at p value < 0.05.
3. Results
The principal baseline characteristics of the overall population of patients with FMR (60 patients) are reported in Table 1. Briefly, the mean age of patients was 73 ± 8 (15% were > 80 years old) and the majority of them were male (83%). Most patients had an increased surgical mortality risk as assessed by the logistic EuroSCORE (27.2 ± 20.5) and STS score (7.9 ± 8.5). 30 patients (50%) had a logistic EuroSCORE > 20 and were thus considered very high risk patients for surgery. Ischemic FMR etiology was present in 67% of cases. 42 patients (70%) were in NYHA class III-IV. Preoperative echocardiographic features are reported in Table 2. Mean LVEF was 30 ± 10% and 73% of cases had LVEF < 35% at presentation. Of these, 10 patients (17%) had an NT-proBNP > 10.000 pg/mL. In most of these cases, MitraClip procedure was the last treatment option before considering left ventricular assist devices implantation as destination therapy. According to echocardiographic diagnostic criteria, we identified 38 patients (63%) with no/mild RV dysfunction (No-RVdysf group) and 21 patients (37%) with moderate/severe RV dysfunction (RVdysf group). Between the two patients' groups there were no significant differences in terms of clinical and echocardiographic characteristics except for history of previous stroke, implantable cardioverter-defibrillator (ICD) and use of aldosterone antagonist which were significantly more often present in RVdysf group, Table 1 ; Table 2.
3.1. Acute MitraClip results
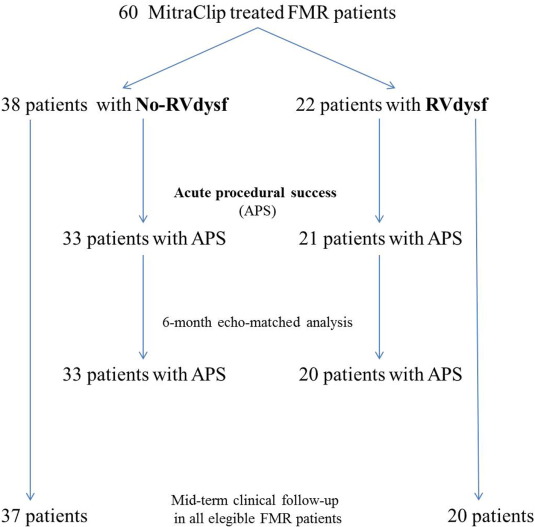
Acute procedural success (APS) was achieved in 54 patients (90%), Fig. 1. Four patients had post-procedural MR > 2 +, in one case the procedure was interrupted because of pericardial tamponade soon after transeptal puncture requiring percutaneous drainage. One patient had retroperitoneal bleeding from femoral access site, which was managed with manual compression and urgent embolization with ethylcellulose microspheres. APS was obtained in all patients who implanted one MitraClip (23 patients), in 94% of patients who implanted two MitraClips (31 patients), in none of patients treated with ≥ 3 MitraClips (5 patients). Peri-procedural complications are listed in Table 3. Thirty-day mortality rate was 5% (three deaths, all in-hospital): two cardiac-deaths (in patients with end-stage heart failure) and one non cardiac-death (acute respiratory distress syndrome in obstructive and restrictive lung disease).
|
|
|
Fig. 1. Study flow-chart. |
| FMR patients (n = 60) | No-RVdysf group (n = 38) | RVdysf group (n = 22) | p value | |
|---|---|---|---|---|
| Acute procedural success (APS) | 54 (90) | 34 (89) | 20 (91) | 0.858 |
| Procedure time, min | 97 ± 90 | 108 ± 87 | 70 ± 35 | 0.326 |
| Fluoroscopy duration, min | 50 ± 45 | 56 ± 53 | 38 ± 22 | 0.202 |
| Number of clip implanted | 1.63 ± 0.62 | 1.64 ± 0.63 | 1.62 ± 0.59 | 0.908 |
| Clip embolization | 0 | 0 | 0 | NA |
| Inotropic support | 20 (33) | 11 (29) | 9 (41) | 0.348 |
| IABP support | 8 (13) | 5 (13) | 3 (14) | 0.967 |
| In-hospital outcome | ||||
| Overall mortality | 3 (5) | 2 (5) | 1 (4) | 0.897 |
| Cardiac mortality | 2 (3) | 1 (3) | 1 (4) | 0.695 |
| Acute kidney injury | 1 (2) | 0 (0) | 1 (4) | 0.187 |
| Need for CVVH | 1 (2) | 0 (0) | 1 (4) | 0.176 |
| Major infection/sepsis | 2 (3) | 1 (3) | 1 (4) | 0.695 |
| Stroke | 0 | 0 | 0 | NA |
| AMI | 0 | 0 | 0 | NA |
| Partial clip detachment | 1 (2) | 1 (3) | 0 | 0.441 |
| Need for blood transfusion | 2 (3) | 0 (0) | 2 (9) | 0.132 |
| LOS, days | 7.6 ± 4.8 | 7.9 ± 5.4 | 7.0 ± 3.3 | 0.465 |
CVVH = continuous venous-venous hemofiltration; AMI = acute myocardial infarction; LOS = length of stay.
3.2. Echocardiographic outcome
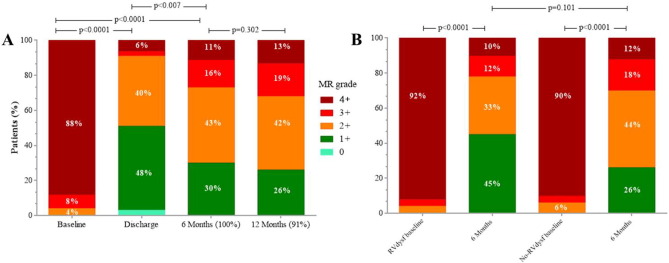
Echocardiographic data at baseline and at 6-month follow-up in 53 eligible patients are reported in Table 4, (one patient was missing for 6-month echo-matched analysis). Between baseline and 6-month follow-up, a persistent and significant reduction of MR grade was observed in all FMR patients and in the 2-subgroups, (Table 4 and Fig. 2, panel A). No significant differences were found in terms of LV end-diastolic and end-systolic volumes and diameter, as well as LVEF, in both groups (No-RVdysf and RVdysf). On the contrary, a significant improvement of RV function was observed in the overall population. RV function improvement was mainly driven by the cohort of patients with baseline RVdysf (TAPSE 15 ± 3.0 vs. 19 ± 4.5, p = 0.007; S′TDI 7 ± 1.2 vs. 11 ± 2.8, p < 0.0001; baseline vs. 6-month follow-up, respectively), and was observed in 79% of patients with baseline RVdysf (15/19 patients). In all patients, mean systolic pulmonary arterial pressure (sPAP) was significantly reduced at follow-up (49 ± 14 vs. 45 ± 13, baseline vs follow-up, p = 0.039), while tricuspid regurgitation grade did not change significantly (2.1 ± 1.2 vs. 1.9 ± 1.0, p = 0.302); however, a sub-analysis including only 16 patients with moderate to severe tricuspid regurgitation (TR ≥ 2), showed a trend in reduction of TR grade after MitraClip (2.9 ± 0.9 vs. 2.4 ± 1.0, p = 0.08). We did not find significant differences in RV function improvement according to MR etiology (ischemic vs. non-ischemic cardiomyopathy).
| FMR patients (n = 53) | No-RVdysf group (n = 33) | RVdysf group (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Month | p value | Baseline | 6-Month | p value | Baseline | 6-Month | p value | |
| LVEDV, mm | 217 ± 73 | 220 ± 66 | 0.697 | 216 ± 69 | 217 ± 56 | 0.880 | 218 ± 81 | 223 ± 82 | 0.707 |
| LVESV, mm | 155 ± 64 | 154 ± 64 | 0.880 | 152 ± 61 | 150 ± 55 | 0.739 | 159 ± 71 | 159 ± 77 | 0.980 |
| LVEDD, mm | 66 ± 7 | 64 ± 10 | 0.153 | 66 ± 6.5 | 63 ± 9.5 | 0.132 | 65 ± 10 | 64 ± 13 | 0.815 |
| LVESD, mm | 56 ± 15 | 54 ± 19 | 0.580 | 63 ± 14 | 64 ± 12 | 0.500 | 64 ± 12 | 65 ± 13 | 0.632 |
| LVEF, % | 30 ± 9 | 32 ± 10 | 0.066 | 32 ± 10 | 34 ± 10 | 0.083 | 28 ± 7 | 29 ± 10 | 0.446 |
| LA volume, ml | 139 ± 36 | 125 ± 54 | 0.139 | 138 ± 42 | 122 ± 31 | 0.094 | 141 ± 23 | 131 ± 81 | 0.623 |
| MR grade | 3.8 ± 0.6 | 2.0 ± 1.0 | 0.000 | 3.8 ± 0.6 | 2.1 ± 0.9 | 0.000 | 3.8 ± 0.5 | 1.6 ± 1.0 | 0.000 |
| sPAP, mmHg | 49 ± 14 | 45 ± 13 | 0.039 | 46 ± 15 | 42 ± 13 | 0.045 | 53 ± 12 | 50 ± 13 | 0.035 |
| TAPSE, mm | 19 ± 4.5 | 21 ± 4 | 0.018 | 21 ± 3 | 22 ± 3 | 0.669 | 15 ± 3 | 19 ± 4.5 | 0.007 |
| SʹTDI, cm/s | 10 ± 2.5 | 11.5 ± 2.3 | 0.002 | 12 ± 1.2 | 12 ± 1.9 | 0.863 | 7 ± 1.2 | 11 ± 2.8 | 0.000 |
| TR grade | 2.1 ± 1.2 | 1.9 ± 1.0 | 0.302 | 1.9 ± 1.1 | 1.9 ± 0.9 | 0.840 | 2.4 ± 1.2 | 2 ± 1.1 | 0.088 |
| RV diameter, mm | 39 ± 9 | 41 ± 7 | 0.067 | 36 ± 8 | 39 ± 5.5 | 0.125 | 43 ± 10 | 45 ± 7 | 0.366 |
Bold numbers indicate significance at p value < 0.05.
|
|
|
Fig. 2. Mitral regurgitation grade at baseline, discharge, 6 months and 12 months after MitraClip implantation in all FMR patients (panel A) and at baseline and 6 months in patients stratified for RV dysfunction (RVdysf, panel B). |
3.3. Clinical follow-up
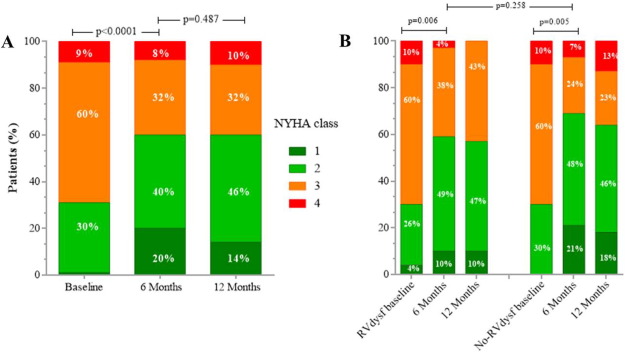
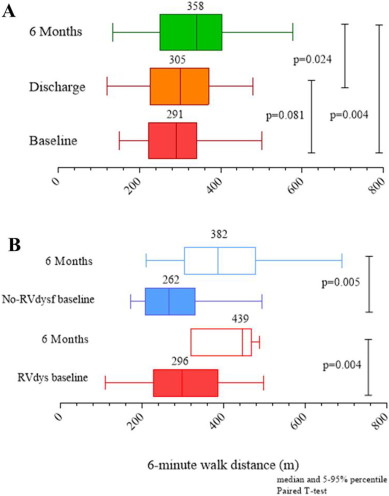
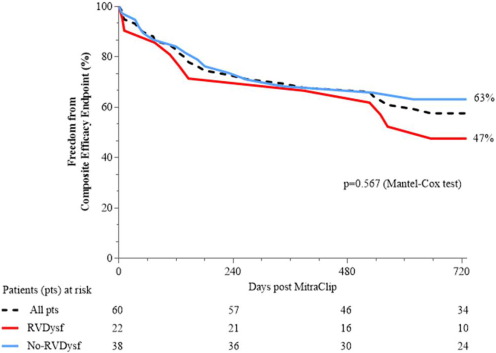
Mid-term follow-up events are listed in Table 5. The overall mean follow-up time was 565 ± 310 days (median 395 days). The duration of clinical follow-up was not statistically different between RVdysf and No-RVdysf groups. Significant NYHA improvement of 1 or 2 functional classes was observed in all patients and was persistent at 12-month follow-up (Fig. 3, panel A), without difference within the 2 groups (Fig. 3, panel B). The mean improvement in the 6-min walking test was significant in both groups (120 and 143 m, RVdysf and No-RVdysf groups, respectively; Fig. 4). Six patients (10%) died between the first month and the end of the sixth month after discharge: 5 cardiac deaths (4 due to refractory heart failure and 1 sudden death), 1 non-cardiac death (bowel occlusion). During follow-up, 2 cases of partial clip detachment were observed. Two patients received LVAD due to progressive heart failure (median time period after MitraClip 185 days, IQR 45–240). One case of redo-procedure was performed successfully for the patient with pericardial tamponade during the index procedure. At Kaplan–Meier and Cox-regression analysis, the presence of RV dysfunction did not affect the outcome in patients with APS (final MR ≤ 2) in terms of freedom from composite efficacy endpoint (47% vs. 63%, RVdysf vs. No-RVdysf, respectively, p = 0.567), Fig. 5. Multivariable Cox regression analysis was used to identify clinical independent predictors of composite efficacy endpoint in all patients. The adjusted Cox proportional-hazard analysis identified advanced congestive HF and Logistic EuroSCORE > 25 as significant independent predictors of composite efficacy endpoint (Table 6).
| FMR patients (n = 57) | No-RVdysf (n = 37) | RVdysf (n = 20) | p value | |
|---|---|---|---|---|
| Days follow-up | 605 ± 310 | 671 ± 280 | 605 ± 322 | 0.553 |
| All death | 18 (32) | 10 (27) | 8 (40) | 0.315 |
| Cardiac death | 15 (26) | 7 (19) | 8 (40) | 0.085 |
| Sudden cardiac death | 5 (9) | 1 (3) | 4 (20) | 0.031 |
| AMI | 0 | 0 | 0 | 1.000 |
| Stroke | 1 (2) | 1 (3) | 0 (0) | 0.458 |
| Bleeding | 4 (7) | 3 (8) | 1 (5) | 0.664 |
| Re-hospitalization for HF | 20 (35) | 12 (32) | 8 (40) | 0.470 |
| Need for LVAD implantation | 2 (3) | 2 (5) | 0 (0) | 0.294 |
| Composite efficacy endpoint | 26 (46) | 15 (40) | 11 (55) | 0.339 |
| NYHA class improvement | ||||
| 1 or 2 class improvement | 24 (42) | 16 (43) | 8 (40) | 0.764 |
| 3 class improvement | 6 (10) | 4 (11) | 2 (10) | 0.910 |
|
|
|
Fig. 3. Distributions of New York Heart Association functional (NYHA) class at baseline, 6 months and 12 months after MitraClip implantation in all FMR patients (panel A) and in patients stratified for RV dysfunction (panel B). Matched analysis available for 57 patients at 6-month and 43 patients at 12-month. |
|
|
|
Fig. 4. Six-minute walk test distance at baseline, discharge, 6 months in all FMR patients (panel A) and at baseline and 6 months in patients stratified for RV dysfunction (RVdysf, panel B). Matched analysis available for 45 patients at 6-month. |
|
|
|
Fig. 5. Kaplan–Meyer curves of Freedom from combined primary endpoint according to presence or not of RV dysfunction (RVdysf). |
| Univariate analysis (p value) | Multivariate analysis (p value) | HR (95% CI) | |
|---|---|---|---|
| RVdysf | 0.416 | 0.602 | 0.76 (0.2–2.1) |
| Chronic renal failure (CRF) | 0.795 | 0.287 | 1.79 (0.6–5.2) |
| Logistic EuroSCORE > 25 | 0.009 | 0.030 | 3.82 (1.1–12.8) |
| Advanced congestive HF | 0.000 | 0.001 | 6.67 (2.1–20) |
| Acute procedural success (APS) | 0.159 | 0.585 | 0.69 (0.1–2.5) |
Bold numbers indicate significance at p value < 0.05.
4. Discussion
This retrospective, observational, unblinded study involving high risk patients with FMR shows that: 1) successful MitraClip correction induces RV function improvement at 6-month follow-up in a sizeable proportion of patients; 2) RV dysfunction is not a predictor of unfavorable medium term clinical outcome; and 3) MitraClip implantation reduces significantly the grade of tricuspid regurgitation in those patients with baseline moderate to severe tricuspid regurgitation. Additionally, our results confirm a significant and persistent reduction of FMR grade during follow-up, which is associated to concomitant improvements in NYHA functional class and six-minute walking test, as previously reported [29]; [30]; [31] ; [32].
This study confirms previous observations reporting that reduction in LV filling pressure obtained after MitraClip implantation yields a positive effect on the hemodynamic of the right sections. At 6-month echo-matched analysis, significant reduction in sPAP and significant increase in longitudinal RV systolic function (increase in TAPSE and RV S′TDI) were observed in 79% of patients with baseline RV dysfunction. These findings could be related to the fact that RV performance is influenced by elevated sPAP, which is a common finding in severe chronic MR. Reduced or normalized sPAP after surgical mitral valve repair could at least in part be the mechanism of the observed improvement of RV impairment in such patients [33] ; [34]. In the present study, we did not observe an overall positive left ventricular remodeling effect, as reported in other experience [31]; [32] ; [35]. However, an improvement of mean LVEF (of at least > 5%) at echo-follow-up was observed in 34% of patients (17/53 echo-matched); these patients were different from patient with no LVEF improvement only for significantly lower baseline LVEDV (183 ml vs. 234 ml, p = 0.039). It is plausible that a better left ventricular functional outcome could be observed in a larger study population. Right ventricle could be more sensible to volume overload than left ventricle is, because of their anatomical structures. It is conceivable that a reduction in volume overload could be initially beneficial for the right ventricle and then for LV. A longer follow up could show changes in left ventricle function. As a matter of fact, in chronic MR and LV dysfunction, subclinical LV damage could be present well before the appearance of LV function impairment [36] ; [37]. Similarly, irreversible myocardial damage was likely already present in our study population, in the context of severe MR and LV dysfunction. The lack of further deterioration of LV function could therefore be considered as a therapeutic goal, even in absence of significant improvement.
4.1. RV dysfunction and outcome
RV outcome after MitraClip procedure is not yet clearly defined [21] ; [22]. RV dysfunction (mainly due to impairment of the longitudinal shortening of the ventricular fibers) is the result of a complex interaction between the remodeled and enlarged left ventricle and atrial, septal performance, and sPAP in presence of significant MR [4]. Different studies in this context have reported conflicting results. RV dysfunction has been reported to be a strong predictor of poor cardiovascular and overall survival in patients with heart failure [10]; [17]; [39]; [38] ; [40]. Conversely, isolated tricuspid regurgitation has been associated with a poor prognosis, independent of age, biventricular systolic function, RV size, and dilation of the inferior vena cava [41]. Ghio et al. observed that RV function may be preserved despite elevated sPAP and that RV dysfunction may be observed even in patients with normal sPAP [8]. On the other hand, other studies have established an inverse relation between RV systolic function and pulmonary arterial pressure [42]. A recent observation indicates that RV dysfunction, but not significant TR, is independently associated with late survival after left heart valve surgical procedure [43]. In fact, recurrence or new development of significant tricuspid regurgitation late after left heart valve surgical procedure is frequently found and varies between 9% and 49% [44]; [45] ; [46]. It can be speculated that in patients with severe right heart dysfunction, the higher mean trans-mitral pressure gradient determined by MitraClip acting as a “iatrogenic” mild mitral stenosis, may prevent progressive dilation of LV, but it could further overload an already malfunctioning right ventricle [47]. In our series of patients, the presence of RV dysfunction did not affect clinical outcome in terms of composite efficacy endpoint rate, which was not significantly higher compared to patient without RV dysfunction (63% vs. 47%, p = 0.567, Fig. 4). In fact, RV dysfunction improved while TR grade remained unchanged. On the other hand, the Cox proportional-hazard analysis identified advanced congestive HF and Logistic EuroSCORE > 25 as the only significant independent predictors of composite efficacy endpoint. Pre-existing RV dysfunction before mitral valve repair has been associated to further deterioration during follow up. On this basis, surgical repair before the occurrence of biventricular impairment has been advocated. The most plausible explanations of our results may be related, first, to the follow-up analysis focused on patients with successful MitraClip procedure, second, to the mild baseline RV dysfunction (TAPSE 15.5 mm, S′TDI 7.3 cm/s) of the RVdysf group. Finally, we should also take into account that mitral surgery may generate more myocardial damage than a MitraClip procedure. However, the effects of open heart surgery and extracorporeal circulation by themselves could well play a crucial role on an already diseased RV, which is known to be more vulnerable as compared to the left heart [48]. Recently, Orban et al. [49], reported a significantly higher rate of mortality in a subgroup of patient with severe biventricular heart failure and high-risk profile (mean LVEF 19.8%, TAPSE 13 mm, Logistic EuroSCORE 31.1) and found RV dysfunction and pulmonary hypertension as predictors of poor outcome (1-year mortality of 77%). Additional explanations for this difference compared to our results could be related to a different patient selection (higher LVEF and smaller LVEDD in our patients) and definition of procedural success.
The major limits of the present study are related to its observational nature, relatively small sample size and lack of a reference standard for RV functional evaluation (such as magnetic resonance imaging or three-dimensional echocardiography). Moreover, it reflects the initial experience of our center and thus the results must be regarded as hypothesis generating and exploratory and require validation in further larger studies.
4.2. Conclusions
This observational study shows that successful MitraClip implantation in patients with FMR and RV dysfunction yields significant improvement of RV function at mid-term follow-up. The presence of RV dysfunction does not appear to be a predictor of unfavorable clinical outcome in these patients. The minimally invasive approach of MitraClip could have played a significant role in avoiding the late deterioration of RV function and occurrence of tricuspid regurgitation, frequently observed after surgical mitral valve repair.
Disclosures
None.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] A. Vahanian, O. Alfieri, F. Andreotti, et al.; Guidelines on the management of valvular heart disease (version 2012); Eur. Heart J., 33 (2012), pp. 2451–2496
- [2] J.S. Borer, R.O. Bonow; Contemporary approach to aortic and mitral regurgitation; Circulation, 108 (2003), pp. 2432–2438
- [3] V.V. McLaughlin, S. Rich; Pulmonary hypertension; Curr. Probl. Cardiol., 29 (2004), pp. 575–634
- [4] Y. Juilliere, G. Barbier, L. Feldmann, et al.; Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy; Eur. Heart J., 18 (1997), pp. 276–280
- [5] P. de Groote, A. Millaire, C. Foucher-Hossein, et al.; Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure; J. Am. Coll. Cardiol., 32 (1998), pp. 948–954
- [6] T.G. Di Salvo, M. Mathier, M.J. Semigran, G.W. Dec; Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure; J. Am. Coll. Cardiol., 25 (1995), pp. 1143–1153
- [7] A. Gavazzi, C. Berzuini, C. Campana, et al.; Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure; J. Heart Lung Transplant., 16 (1997), pp. 774–785
- [8] S. Ghio, A. Gavazzi, C. Campana, et al.; Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure; J. Am. Coll. Cardiol., 37 (2001), pp. 183–188
- [9] J.F. Polak, B.L. Holman, J. Wynne, W.S. Colucci; Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease; J. Am. Coll. Cardiol., 2 (1983), pp. 217–224
- [10] D. Wencker, J.S. Borer, C. Hochreiter, et al.; Preoperative predictors of late postoperative outcome among patients with nonischemic mitral regurgitation with'high risk'descriptors and comparison with unoperated patients; Cardiology, 93 (1999), pp. 37–42
- [11] C. Ward, B. Hancock; Extreme pulmonary hypertension caused by mitral valve disease. Natural history and results of surgery; Br. Heart J., 37 (1975), pp. 74–78
- [12] S. Hyllén, S. Nozohoor, A. Ingvarsson, et al.; Right ventricular performance after valve repair for chronic degenerative mitral regurgitation; Ann. Thorac. Surg., 98 (2014), pp. 2023–2030
- [13] J. Grapsa, D. Dawson, D. Pandis, et al.; Mitral valve repair results in better right ventricular remodelling than valve replacement for degenerative mitral regurgitation: a three-dimensional echocardiographic study; Hell. J. Cardiol., 53 (2012), pp. 279–286
- [14] F. Onorati, G. Santarpino, D. Marturano, et al.; Successful surgical treatment of chronic ischemic mitral regurgitation achieves left ventricular reverse remodeling but does not affect right ventricular function; J. Thorac. Cardiovasc. Surg., 138 (2009), pp. 341–351
- [15] R.R. Desai, L.M.V. Abello, A.L. Klein, et al.; Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure; J. Thorac. Cardiovasc. Surg., 146 (2013) (1126-32.e10)
- [16] B.G. Rajbanshi, R.M. Suri, V.T. Nkomo, et al.; Influence of mitral valve repair versus replacement on the development of late functional tricuspid regurgitation; J. Thorac. Cardiovasc. Surg., 148 (2014), pp. 1957–1962
- [17] T. Le Tourneau, G. Deswarte, N. Lamblin, et al.; Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment; Circulation (2013) (CIRCULATIONAHA. 112.000999)
- [18] X. Sun, J. Ellis, L. Kanda, P.J. Corso; The role of right ventricular function in mitral valve surgery; The heart surgery forum, Carden Jennings (2013), p. E170-E6
- [19] S. Scandura, G.P. Ussia, P. Capranzano, et al.; Left cardiac chambers reverse remodeling after percutaneous mitral valve repair with the MitraClip system; J. Am. Soc. Echocardiogr., 25 (2012), pp. 1099–1105
- [20] S.T. Pleger, M. Schulz-Schönhagen, N. Geis, et al.; One year clinical efficacy and reverse cardiac remodelling in patients with severe mitral regurgitation and reduced ejection fraction after MitraClip© implantation; Eur. J. Heart Fail., 15 (2013), pp. 919–927
- [21] C. Giannini, A.S. Petronio, M. De Carlo, et al.; Integrated reverse left and right ventricular remodelling after MitraClip implantation in functional mitral regurgitation: an echocardiographic study; Eur. Heart J. Cardiovasc. Imaging (2013), p. jet141
- [22] A.C. van Riel, K. Boerlage-van Dijk, R.H. de Bruin-Bon, et al.; Percutaneous mitral valve repair preserves right ventricular function; J. Am. Soc. Echocardiogr., 27 (2014), pp. 1098–1106
- [23] T. Feldman, E. Foster, D.D. Glower, et al.; Percutaneous repair or surgery for mitral regurgitation; N. Engl. J. Med., 364 (2011), pp. 1395–1406
- [24] L.G. Rudski, W.W. Lai, J. Afilalo, et al.; Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography; J. Am. Soc. Echocardiogr., 23 (2010), pp. 685–713
- [25] J. Meluzin, L. Spinarova, J. Bakala, et al.; Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function; Eur. Heart J., 22 (2001), pp. 340–348
- [26] W. Conover; Practical Nonparametric Statistics; John Wiley&Sons Inc., New York (1999)
- [27] J. Concato, P. Peduzzi, T.R. Holford, A.R. Feinstein; Importance of events per independent variable in proportional hazards analysis I. Background, goals, and general strategy; J. Clin. Epidemiol., 48 (1995), pp. 1495–1501
- [28] P. Peduzzi, J. Concato, A.R. Feinstein, T.R. Holford; Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates; J. Clin. Epidemiol., 48 (1995), pp. 1503–1510
- [29] F. Maisano, O. Franzen, S. Baldus, et al.; Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe; J. Am. Coll. Cardiol., 62 (2013), pp. 1052–1061
- [30] A. Auricchio, W. Schillinger, S. Meyer, et al.; Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling; J. Am. Coll. Cardiol., 58 (2011), pp. 2183–2189
- [31] O. Franzen, J. van der Heyden, S. Baldus, et al.; MitraClip® therapy in patients with end-stage systolic heart failure; Eur. J. Heart Fail., 13 (2011), pp. 569–576
- [32] Y. Ohno, G.F. Attizzani, D. Capodanno, et al.; Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip system: 30-day and 12-month follow-up from the GRASP registry; Eur. Heart J. Cardiovasc. Imaging, 15 (2014), pp. 1246–1255
- [33] R. Grose, J. Strain, T. Yipintosoi; Right ventricular function in valvular heart disease: relation to pulmonary artery pressure; J. Am. Coll. Cardiol., 2 (1983), pp. 225–232
- [34] A.B. Goldstone, J. Chikwe, S.P. Pinney, et al.; Incidence, epidemiology, and prognosis of residual pulmonary hypertension after mitral valve repair for degenerative mitral regurgitation; Am. J. Cardiol., 107 (2011), pp. 755–760
- [35] P.L. Whitlow, T. Feldman, W.R. Pedersen, et al.; Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (endovascular valve edge-to-edge repair) high risk study; J. Am. Coll. Cardiol., 59 (2012), pp. 130–139
- [36] F.A. Tibayan, F. Rodriguez, F. Langer, et al.; Alterations in left ventricular torsion and diastolic recoil after myocardial infarction with and without chronic ischemic mitral regurgitation; Circulation, 110 (2004), pp. II-109–II-114
- [37] J. Zheng, D.M. Yancey, M.I. Ahmed, et al.; Increased sarcolipin expression and adrenergic drive in humans with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation; Circ. Heart Fail., 7 (2014), pp. 194–202
- [38] F.L. Dini, U. Conti, P. Fontanive, et al.; Right ventricular dysfunction is a major predictor of outcome in patients with moderate to severe mitral regurgitation and left ventricular dysfunction; Am. Heart J., 154 (2007), pp. 172–179
- [39] J. Meluzin, L. Spinarová, P. Hude, et al.; Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure; J. Am. Soc. Echocardiogr., 18 (2005), pp. 435–444
- [40] M. Neuss, T. Schau, M. Schoepp, et al.; Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure; Eur. J. Heart Fail., 15 (2013), pp. 786–795
- [41] J. Nath, E. Foster, P.A. Heidenreich; Impact of tricuspid regurgitation on long-term survival; J. Am. Coll. Cardiol., 43 (2004), pp. 405–409
- [42] B.N. Brent, H.J. Berger, R.A. Matthay, et al.; Physiologic correlates of right ventricular ejection fraction in chronic obstructive pulmonary disease: a combined radionuclide and hemodynamic study; Am. J. Cardiol., 50 (1982), pp. 255–262
- [43] A.A. Kammerlander, B.A. Marzluf, A. Graf, et al.; Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure; J. Am. Coll. Cardiol., 64 (2014), pp. 2633–2642
- [44] R.G. Fuster, A. Vázquez, A.G. Peláez, et al.; Factors for development of late significant tricuspid regurgitation after mitral valve replacement: the impact of subvalvular preservation; Eur. J. Cardiothorac. Surg., 39 (2011), pp. 866–874
- [45] J.-J. Kwak, Y.-J. Kim, M.-K. Kim, et al.; Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations; Am. Heart J., 155 (2008), pp. 732–737
- [46] A. Matsunaga, C.M. Duran; Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation; Circulation, 112 (2005), pp. I-453–I-457
- [47] J. Magne, M. Sénéchal, P. Mathieu, et al.; Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis; J. Am. Coll. Cardiol., 51 (2008), pp. 1692–1701
- [48] G. Christakis, R. Weisel, D. Mickle, et al.; Right ventricular function and metabolism; Circulation, 82 (1990), pp. IV332–IV340
- [49] M. Orban, D. Braun, M. Orban, et al.; Long-term outcome of patients with severe biventricular heart failure and severe mitral regurgitation after percutaneous edge-to-edge mitral valve repair; J. Interv. Cardiol., 28 (2015), pp. 164–171
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?