(Created page with "==Abstract== ====Objective==== We determined the effectiveness of the HATCH score in patients with typical atrial flutter (AFl) undergoing cavotricuspid isthmus (CTI) ablati...") |
m (Scipediacontent moved page Draft Content 655811175 to Garcia-Seara et al 2016a) |
(No difference)
| |
Latest revision as of 11:31, 19 May 2017
Abstract
Objective
We determined the effectiveness of the HATCH score in patients with typical atrial flutter (AFl) undergoing cavotricuspid isthmus (CTI) ablation to predict long-term atrial fibrillation (AF).
Methods
We conducted an observational retrospective single-center cohort study including all patients admitted to our hospital for a CTI ablation between 1998 and 2010. The patients were divided into four categories: 1) new-onset AF (no prior AF and AF during follow-up (FU)); 2) old AF (prior AF and no AF during FU); 3) prior and post AF (AF prior and post CTI ablation); and 4) no AF.
Results
Four hundred and eight patients were included. In patients without prior AF, the hazard ratio (HR) for new-onset AF during FU was 0.98 (CI 95%: 0.65–1.50; p = 0.95) and 1.00 (CI 95%: 0.57–1.77; p = 0.98) for HATCH ≥ 2 and HATCH ≥ 3, respectively. In patients with prior AF, the HR for AF was 1.41 (CI 95%: 0.87–2.28; p = 0.17) and 1.79 (CI 95%: 0.96–3.35; p = 0.06), for HATCH ≥ 2 and HATCH ≥ 3, respectively. Left atrial enlargement was positively correlated with the occurrence of AF during FU, especially in the subgroup without prior AF, which had a HR of 2.44 (CI 95%: 1.35–4.40; p = 0.003), a HR of 2.88 (CI 95%: 1.36–6.10; p = 0.006) and a HR of 3.68 (CI 95%: 1.71–7.94; p = 0.001), for slight, moderate and severely dilated left atrial dimension, respectively, compared with a normal value.
Conclusions
HATCH score did not predict AF in patients with typical AFl who underwent CTI ablation. Basal left atrium dimension could help predict new-onset AF.
Abbreviations
AFl, atrial flutter;AF, atrial fibrillation;CTI, cavotricuspid isthmus;AAD, antiarrhythmic drugs;LAD, left atrial dimension
Keywords
Atrial flutter;Cavotricuspid isthmus ablation;Atrial fibrillation;HATCH;CHA2DS2VASc
1. Introduction
Atrial fibrillation (AF) occurrence in patients with typical atrial flutter (AFl) who undergo cavotricuspid isthmus (CTI) ablation is common during follow-up despite antiarrhythmic drug (AAD) treatment, and the occurrence of AF may be favored by similar electrophysiologic triggers and substrates [1]; [2]; [3]; [4] ; [5]. AF is a progressive arrhythmia and the risk of progression is quantified by the HATCH score, which includes factors of underlying heart disease rather than characteristics of the arrhythmia [6]. The development of AF after successful ablation of typical AFl could be a result of disease progression precipitated by advanced age and comorbidities. Therefore, the HATCH score may be able to predict AF especially in isolated AFl patients. This would be clinically relevant for the anticoagulation therapy strategy after CTI ablation. We have already described that chronic obstructive pulmonary disease (COPD) and prior AF were the main predictors to transition to AF after CTI ablation [7]. This study aimed to investigate the value of the HATCH score for predicting AF development in this population.
2. Material and methods
2.1. Study designs
We conducted an observational retrospective single-centre cohort study including all patients admitted to our hospital for a CTI ablation for typical AFl between 1998 and 2010. We assessed whether the HATCH score (hypertension; age > 75 years; transient ischemic attack or stroke; COPD; and heart failure) would be predictive of new-onset AF after ablation of typical AFL. Each component has a value of 1 point except for stroke and heart failure, which have values of 2 points each.
2.2. Patients
Four hundred and eight consecutive patients with typical AFl who successfully underwent radiofrequency catheter ablation were included. Patients with intra-atrial re-entrant tachycardia following reparative surgery for complex congenital heart disease were excluded, as were patients who underwent concomitant AF ablation during the same procedure. Patients were classified into four groups: 1) new-onset AF (patients with no prior AF who developed AF during follow-up); 2) old AF (patients with prior AF and without AF during follow-up); 3) prior and post AF (patients with AF prior and post CTI ablation); and 4) no AF (patients without AF prior to ablation or during follow-up) (Table 1).
| New-onset AF (n = 97) | Old AF (n = 49) | Prior and post AF (n = 75) | No AF (n = 187) | p | |
|---|---|---|---|---|---|
| Age | 64.8 ± 10 | 65.6 ± 10 | 62.0 ± 12 | 65.2 ± 11 | 0.14 |
| Sex male (n, %) | 83 (85.6) | 42 (85.7) | 62 (82.7) | 157 (84.0) | 0.94 |
| Obesity (n, %) | 24 (24.7) | 10 (20.4) | 14 (18.7) | 55 (29.4) | 0.25 |
| Tobacco (n, %) | 23 (24.7) | 10 (20.4) | 14 (18.7) | 55 (29.4) | 0.29 |
| Alcohol (n, %) | 15 (19.2) | 7 (17.5) | 19 (29.7) | 70 (21.1) | 0.47 |
| Diabetes (n, %) | 27 (27.8) | 8 (16.3) | 9 (12.0) | 41 (21.9) | 0.06 |
| Hypertension (n, %) | 56 (58.3) | 22 (44.9) | 35 (47.3) | 106 (57.0) | 0.22 |
| Dyslipidemia (n, %) | 41 (42.7) | 17 (34.7) | 28 (37.3) | 75 (40.5) | 0.77 |
| Peripheral vascular disease (n, %) | 6 (6.2) | 1 (2.0) | 6 (8.0) | 9 (4.8) | 0.50 |
| CKD (n, %) | 13 (14.3) | 7 (14.6) | 7 (9.5) | 35 (19.9) | 0.20 |
| Pulmonar HT (n, %) | 8 (11.1) | 3 (7.5) | 3 (4.3) | 12 (7.7) | 0.52 |
| No cardiopathy (n, %) | 30 (32.6) | 20 (40.8) | 27 (36.0) | 52 (28.1) | 0.37 |
| IHD (n, %) | 16 (17.4) | 6 (12.2) | 13 (17.6) | 35 (19.4) | 0.71 |
| Heart failure (n, %) | 12 (13.5) | 8 (16.3) | 16 (21.6) | 38 (21.2) | 0.40 |
| COPD (n, %) | 31 (32.0) | 9 (18.4) | 12 (16.0) | 44 (23.5) | 0.07 |
| Dilated cardiomyopathy (n, %) | 11 (12.5) | 4 (8.2) | 10 (13.5) | 14 (7.8) | 0.43 |
| Prior stroke (n, %) | 2 (2.1) | 0 | 0 | 1 (0.5) | 0.31 |
| Stroke post (n, %) | 7 (7.2) | 2 (4.1) | 5 (6.7) | 3 (1.6) | 0.08 |
| HATCH | 1.35 ± 1.23 | 1.18 ± 1.09 | 1.23 ± 1.27 | 1.43 ± 1.25 | 0.31 |
| Persistent AFl, n (%) | 47 (53.4) | 20 (40.8) | 24 (32.4) | 114 (63.7) | 0.43 |
| AAD prior – No – Amiodarone – Flecainide – Propafenone | 50 (63.3) 23 (29.1) 6 (7.6) 0 | 14 (31.1) 21 (46.7) 10 (22.2) 0 | 21 (29.6) 42 (59.2) 7 (9.9) 1 (1.4) | 126 (78.8) 26 (16.2) 7 (4.4) 1 (0.6) | 0.00 |
| AAD post – No – Amiodarone – Flecainide – Propafenone | 84 (86.6) 11 (11.3) 2 (2.1) 0 | 29 (59.2) 15 (30.6) 5 (10.2) 0 | 43 (57.3) 24 (32.0) 7 (9.3) 1 (1.3) | 159 (85) 27 (14.4) 1 (0.3) 0 | 0.00 |
| OAC, n (%) | 60 (61.9) | 36 (73.5) | 52 (71.2) | 93 (49.7) | 0.00 |
| RAAS i, n (%) | 26 (40.7) | 13 (38.2) | 16 (48.1) | 69 (49.0) | 0.86 |
| Statins, n (%) | 23 (34.8) | 13 (37.1) | 11 (25.6) | 48 (33.8) | 0.68 |
| Betablockers, n (%) | 16 (25.0) | 12 (33.3) | 13 (30.2) | 33 (23.6) | 0.62 |
| LVEF, n (%) | 53.7 ± 14.3 | 49.7 ± 14.5 | 58.7 ± 14.2 | 52.7 ± 14.3 | 0.22 |
| Exitus, n (%) | 23 (23.7) | 7 (14.3) | 14 (18.7) | 31 (16.6) | 0.42 |
Continuous variables expressed as mean ± SD. Categorical variables expressed as number, percentage. CKD: chronic kidney disease; HT: hypertension; IHD: ischemic heart diseases; COPD: chronic obstructive pulmonar disease. AAD: antiarrythmic drug; OAC: oral anticoagulation; RAAS i: renin–angiotensin–aldosterone system inhibitors. LVEF: left ventricle ejection fraction.
We performed an initial clinical evaluation that included a medical history, physical examination, 12-lead surface ECG, 24-h ECG monitoring, X-ray examination, blood chemistry tests and two-dimensional echocardiography with color-flow Doppler measurements for all patients. The use of medications, including beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and AAD was at the discretion of the responsible clinician. The use of antithrombotic drugs was determined based on the CHADS2/CHA2DS2VASc risk score. All the patients were classified based on the HATCH score. Left atrial dimension (LAD) was measured using the M-mode anteroposterior linear dimension obtained from the parasternal long-axis view. The reference limits for LAD were according to recommendations for echocardiographic chamber quantification by the American Society of Echocardiography and the European Association of Echocardiography: women: mildly abnormal (3.9–4.2 cm), moderately abnormal: 4.3–4.6 cm, and severely abnormal: ≥ 4.7 cm; and men: mildly abnormal: 4.1–4.6 cm, moderately abnormal: 4.7–5.2 cm, and severely abnormal: ≥ 5.2 cm [8].
2.3. Electrophysiological study
The electrophysiological procedure was performed as previously described [5]. Briefly, a standard quadripolar catheter (Usci-Bard Inc., MA, USA) was used to map the bundle of His, a 10-pole catheter (Usci-Bard Inc.) was used to locate the coronary sinus and a 12-pole Halo XP catheter (Cordis-Webster Inc., CA, USA) was used to record activation in the anterolateral aspect of the right atrium. An 8-mm-tip catheter was used for radiofrequency (RF) ablation in 97.9% of patients and an irrigated-tip catheter was used in the remaining 2.1%. RF was delivered for 60 s at each location. The maximum power output was 90 W, and the maximum temperature was 55 °C. CTI-dependence was confirmed by concealed entrainment if the AFl rhythm was either present at the beginning of the electrophysiological analysis or induced in the laboratory. If the patient was in sinus rhythm, bidirectional CTI permeability was determined before ablation.
The objective of the ablation procedure was to achieve a bidirectional conduction block across the CTI. Bidirectional block was determined by the sequence of electrical activity between the right atrium, bundle of His and coronary sinus following a 600-ms stimulation in the coronary sinus and in the inferolateral wall of the right atrium. Persistence of bidirectional CTI conduction block was tested 20 min after the procedure.
2.4. Post-ablation management and follow-up
All patients underwent continuous ECG monitoring for at least 24 h before hospital discharge. The strategy for anticoagulation therapy was to maintain anticoagulation treatment for four weeks after the procedure in all patients. Thereafter, anticoagulation therapy was suspended in isolated AFl and maintained in prior-AF patients, according to the CHADS2 or CHA2DS2VASc score (as appropriate because patients were included from 1998 to 2010). The patients returned for a follow-up visit every 6 months, and 24-h Holter monitoring for assessing asymptomatic arrhythmia episodes was performed every year. Each time a patient went to the emergency room or saw his general practitioner, a report was filed into the patients electronic history and an ECG recording was performed. Long-term maintenance of oral anticoagulant (OAC) treatment was determined at the discretion of the responsible clinician depending on the occurrence of AF during the follow-up. The occurrence of AF was defined as documentation during ECG or ECG Holter monitoring of at least 30 s of AF. The strategy for AAD therapy after CTI ablation was to maintain the treatment in patients with prior AF and withdraw the drug in patients with isolated AFl. Long-term maintenance of AAD therapy was at discretion of the responsible clinician.
2.5. Statistical analysis
Continuous data are presented as the mean ± standard deviation. Categorical variables are expressed as counts and percentages. We used a t-test if the continuous variables were normally distributed and a Wilcoxon two-sample test if the continuous variables were not normally distributed. The chi-squared test was used to compare categorical variables. Survival data were described using a Kaplan–Meier analysis.
Survival time was defined as the time from the date of AFl ablation to the date of AF, as verified during the follow-up. Differences between pairs of survival curves were assessed using the log-rank test.
All statistical analyses were performed in R using the survival package (for fitting parametric Cox models). These packages are freely available at http://cran.r-project.org.
This study was performed in accordance with the Declaration of Helsinki (1975) and approved by the Ethics Committee of Clinical Investigation in Galicia. All enrolled patients gave their written informed consent [23].
3. Results
Four hundred and eight patients with typical AFl who underwent CTI ablation were followed for a mean time of 5.9 ± 3.1 years (range, 1.6–13.5 years). There were 13 patients (3.2%) with AF during follow-up who underwent pulmonary vein isolation. These patients were included in the series. Acute bidirectional CTI block was achieved in all of the patients.
They were classified into four groups based on the occurrence of AF (Table 1). There was no difference in age among the groups. There was a trend to a higher proportion of diabetes in groups with AF and the prevalence of COPD was higher in the new-onset AF group compared with the others. There was also a trend toward a lower rate of stroke in the group with no AF (1.6%). There were no differences in the HATCH score among the groups.
All patients were discharged with at least four weeks on OAC therapy or enoxaparin. Patients with old AF (73%), prior and post AF (71%) and new AF (62%) had a rate of OAC at the end of follow-up that was significantly higher than patients with no AF (50%).
Before CTI ablation, the number of patients taking AAD treatment was significantly higher between groups with prior AF than in groups without prior AF. Patients without prior AF were off AAD after CTI ablation. At the end of follow-up, the number of patients taking AAD who kept taking the therapy was significantly higher between groups with prior AF than in groups without prior AF, with a rate of 14.4% in patients with new-onset AF (Table 1).
There were no differences in left ventricular ejection fraction between the groups. However, we found statistically significant differences in the left atrium dimensions (LAD) between the groups with a rate of normal LAD that more than doubled in the No AF patients group (48%) compared with the new-onset AF group (22%; p = 0.001; Table 2).
| LA size | New AF (N = 97) | Old AF (N = 49) | Prior and post AF (N = 75) | No AF (N = 187) | Total (N = 408) | p |
|---|---|---|---|---|---|---|
| Normal | 21 (21.6) | 21 (42.8) | 23 (30.7) | 89 (47.6) | 154 (37.7) | p = 0.001 |
| Mild dilatation | 47 (48.5) | 19 (38.7) | 25 (33.3) | 71 (38.0) | 162 (39.7) | |
| Moderate dilatation | 15 (15.5) | 6 (12.3) | 19 (25.3) | 19 (10.1) | 59 (14.5) | |
| Severe dilatation | 14 (14.4) | 3 (6.1) | 8 (10.7) | 8 (4.3) | 33 (8.1) |
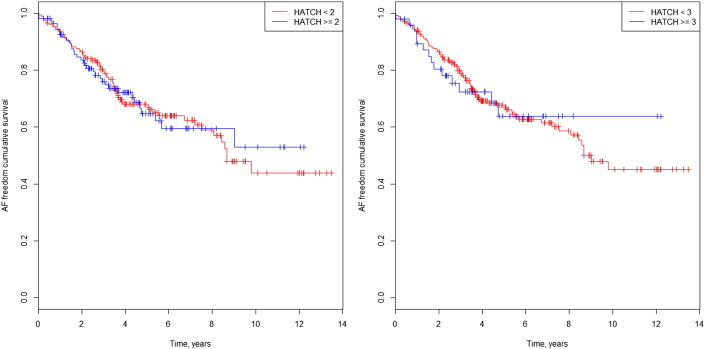
The Kaplan–Meier curves in Fig. 1a and b illustrate survival freedom from AF during follow-up according to risk stratification by HATCH score and prior AF. In patients without prior AF and HATCH ≥ 2, the hazard ratio (HR) for developing AF during follow-up was 0.98 (CI 95%: 0.65–1.50; p = 0.95). In patients without prior AF and HATCH ≥ 3, the HR for developing AF was 1.00 (CI 95%: 0.57–1.77; p = 0.98). In patients with prior AF and HATCH ≥ 2, the HR for developing AF during follow-up was 1.41 (CI 95%: 0.87–2.28; p = 0.17). In patients with prior AF and HATCH ≥ 3, the HR for developing AF was 1.79 (CI 95%: 0.96–3.35; p = 0.06) (Fig. 2a and b).
|
|
|
Fig. 1. a. AF freedom cumulative survival curve in isolated AFl patients and HATCH 2. b. AF freedom cumulative survival curve in isolated AFl patients and HATCH 3. |
|
|
|
Fig. 2. a. AF freedom cumulative survival curve in AF prior patients and HATCH 2. b. AF freedom cumulative survival curve in AF prior patients and HATCH 3. |
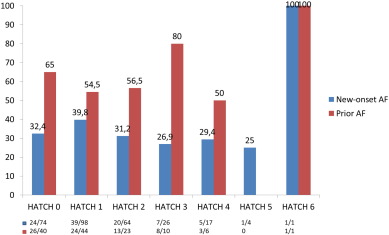
The HATCH score did not predict new-onset AF in the follow up period, and the rate of AF after 3 years of follow up was 25% in the HATCH ≥ 2 group and 20% in the HATCH < 2 group (Fig. 1). The results for new-onset AF were similar using a cut-off value of 3 for the HATCH score. In patients with prior AF who developed AF during follow-up, the rate of AF was higher in the HATCH ≥ 2 group (64%) than in the HATCH < 2 group (44%) at the 3-year follow-up visit. In addition, the difference was higher using a cut-off value of 3 for the HATCH score, with an AF rate of 75% in patients with HATCH ≥ 3, which was higher than the 46% rate of AF in patients with HATCH < 3 at the 3 year follow-up visit. However, these differences were not statistically significant. Most of the AF occurrence was noted near the beginning of the follow-up, with an AF rate of 53% during the first year (Fig. 2b). Fig. 3 shows the AF occurrence in each HATCH category among the groups.
|
|
|
Fig. 3. AF during follow-up based on HATCH category. |
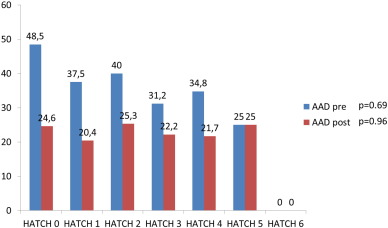
Patients on AAD treatment prior CTI ablation and at the end of the study, among the different HATCH categories, are depicted in Fig. 4. There were no significant differences among the HATCH categories in the percentage of patients on AAD not only in the whole population but in the isolated typical AFl group.
|
|
|
Fig. 4. Patients on AAD prior CTI ablation and at the end of the study (%). |
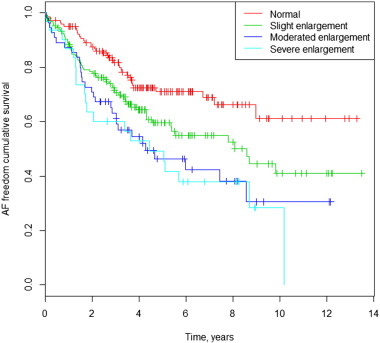
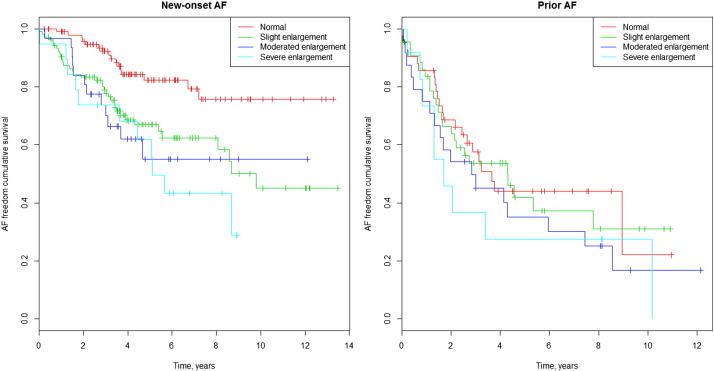
There was a positive correlation between LAD and AF occurrence during follow-up. Only 22% of patients with new AF and 31% of patients with prior and post AF had a normal basal LAD. On the other hand, 52% of patients with no AF during follow-up had a LA enlargement (Table 2). The Kaplan–Meier survival curve for the occurrence of AF during follow-up among different LAD values is shown in Fig. 5. Multivariate Cox analysis shows an HR of 1.64 (CI 95%: 1.09–2.47; p = 0.02); 2.31 (CI 95%: 1.43–3.74; p = 0.01); and 2.65 (CI 95%: 1.54–4.55; p = 0.00), for slight, moderately and severely dilated LAD, respectively, compared with the normal LAD. The Kaplan–Meier curves in Fig. 6a and b illustrate survival freedom from AF during follow-up based on risk stratification for LAD and prior AF. In patients without prior AF, the HR for developing AF during follow-up was 2.44 (CI 95%: 1.35–4.40; p = 0.003); 2.88 (CI 95%: 1.36–6.10; p = 0.006) and 3.68 (CI 95%: 1.71–7.94; p = 0.001) for slight, moderate and severely dilated LAD, respectively, compared with normal LAD. In patients with prior AF, the HR for developing AF during follow-up was 1.06 (CI 95%: 0.59–1.89; p = 0.84); 1.43 (CI 95%: 0.77–2.68; p = 0.26) and 1.65 (CI 95%: 0.75–3.54; p = 0.21) for slight, moderate and severely dilated LAD, respectively, compared with normal LAD.
|
|
|
Fig. 5. AF freedom cumulative survival curves and left atrial dimension. |
|
|
|
Fig. 6. a. AF freedom cumulative survival curves and left atrial dimension in patients without prior AF. b. AF freedom cumulative survival curves and left atrial dimension in patients with prior AF. |
4. Discussion
In our cohort of patients with typical AFl who underwent CTI ablation, the HATCH score was not effective to predict new-onset AF. The HATCH score performance was slightly better when considering only the group of patients with prior AF.
In a recent report [9], the HATCH score was a good predictor of new-onset AF after typical isolated AFl ablation, with an area under the curve of 0.74. However, we did not find it to be a reliable predictor for detecting new-onset AF at any of the HATCH score cut-off values. There may be several reasons for such a difference.
First, in Chens report [9], the age of patients who developed AF was 65 years, which was significantly higher than the age of patients who did not develop AF (61 years). Other studies [5]; [10] ; [11] did not find a statistically significant difference in the occurrence of AF related to age, and in two reports [12] ; [13], patients who developed AF during follow-up were significantly younger than patients who did not develop AF (HR of 1.46 for those < 65 years of age). In our series, patients who developed AF during follow-up were also younger than patients who did not. These data suggest that fibrosis related to age is not a determining factor in the occurrence of AF after typical AFl ablation in most studies.
Second, in Chens report, the frequency of heart failure among patients with HATCH ≥ 2 was high (70%). We had a much lower frequency of heart failure (50%) and in one-third of patients, the systolic dysfunction was reversible [2].
Third, in this report [9], the frequency of prior cerebrovascular disease was significantly higher in HATCH ≥ 2 patients than in HATCH < 2 patients (19% vs. 2%). However, the frequency of hypertension was significantly lower in HATCH ≥ 2 patients than in HATCH < 2 patients (58% vs. 70%). AF-related strokes are responsible for about one-third of all ischemic strokes [21], but this population consisted of isolated AFl cases and was, therefore, free from AF. On the other hand, hypertension is the main risk factor for having an ischemic stroke that is not related to AF. What, then, was the mechanism of prior stroke in the HATCH ≥ 2 population? Taking into account the difficulty in using clinical manifestations to accurately identify AF and AFl prior to ablation, it is possible that the prior AF patients were under-recognized in this series. In our series, the frequency of prior cerebrovascular disease was 2% in HATCH ≥ 2 patients including patients with prior AF. Taken together, this population [9] seems to be sicker than those in previously reported studies [5]; [10]; [11]; [12] ; [13].
Fourth, AAD treatment during the follow-up was also different between our series (AAD allowed and under clinician criteria after CTI ablation) and Chens report (AAD off after CTI ablation). However, in our series, the rate of withdrawal during follow-up was high with only 40% of patients with prior AF on AAD treatment at the end of the study and only 13% of patients among the new-onset AF group. The main reason for AAD withdrawal was the lack of efficacy. In a previous short-term follow-up study (16 months), including patients with AAD-related AFl who underwent CTI ablation, the rate of cessation of AAD was 25% and the change of AAD due to lack of efficacy was 33% [24]. In another study Anastasio et al., reported a rate of AF recurrence as high as 90% in a 5 year follow-up study with all patients on AAD at the end of the study. [25] Therefore the effect of AAD in preventing AF after CTI ablation for typical AFl is modest. Anyhow, we did not find any differences in AAD treated patients at the end of study among the HATCH categories either in the whole population or in isolated typical AFl group.
AF is a progressive arrhythmia and its progression is driven by underlying heart disease, which is described well by the HATCH score. An increasing HATCH score indicates a significantly higher proportion of patients in whom AF progressed to a long-lasting form [6]. This fact could explain why the HATCH score had a slightly better prediction ability between patients who had prior AF.
Enlargement of LA was correlated with occurrence of AF during follow-up and it reflects the structural remodeling of LA [14] ; [15]. It is a consistent factor of AF occurrence after CTI in most of the series [3]; [9]; [11] ; [12] although a lack of correlation has also been reported [10]. However, more than half of the patients with no AF during follow-up had a dilated LA, and 43% of patients with prior AF that did not develop AF during follow-up had normal LA dimensions. In addition, LAD correlation was especially observed in patients without prior AF and when the patients had prior AF, this correlation disappeared. The occurrence of prior AF is likely the strongest factor for predicting AF after CTI ablation because it gathers electrical and structural remodeling factors and attenuates the predictive ability for structural factors such as LAD or HATCH factors alone [7].
Thus, it may seem necessary an electrical remodeling to occur along with structural remodeling for AF to develop in typical AFl patients who undergo CTI [16]. This interaction between AF and AFl has long been studied [17] ; [18]. Recently a clinical study has proposed the AF ablation strategy in patients with isolated AFl on top to CTI ablation with a significant reduction of new-onset AF assessed by a continuous implantable cardiac monitor [19]. The HATCH score likely failed to identify patients with isolated AFl who develop new-onset AF because it takes into account only structural factors of underlying heart disease progression and COPD but it does not consider the arrhythmic substrate [20]. When the HATCH score is applied to prior AF patients (considering electrical remodeling), the ability of the score to predict AF improves. Recently, it was shown that AF inducibility after CTI ablation for typical AFl was a predictive factor of AF occurrence in patients with isolated AFl, but not in prior AF patients [22]. It is necessary to identify structural factors that should include LAD and electrical factors that could express atrial electrical vulnerability (such as AF inducibility), to improve the ability to predict AF in patients with isolated atrial flutter who are undergoing CTI. This will help with the difficult issue of long-term anticoagulation therapy in this population.
4.1. Limitations
Our study represents a single centers experience. The exact incidence of arrhythmia episodes, especially those that were asymptomatic, is not known and it is difficult to estimate. The retrospective nature of the study constitutes another limitation and data should be analyzed with caution.
5. Conclusions
The HATCH score failed to predict new onset AF in patients with isolated typical AFl who are undergoing CTI ablation. Basal LAD could help predict new-onset AF after CTI ablation.
Conflicts of interest
The authors have no conflicts of interest.
Author contribution
Garcia Seara J, Martinez Sande JL and Fernández López XA designed the study; García Seara, Alvarez Alvarez and Iglesias Alvarez performed the study; Gude Sampedro analyzed the data; García Seara wrote the paper and Gonzalez Melchor and Gonzalez Juanatey critically revised the manuscript.
The authors declared not to have conflict of interest for this publication.
References
- [1] F.J. Pérez, C.M. Schubert, B. Parvez, V. Pathak, K.A. Ellenbogen, M.A. Wood; Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis; Circ. Arrhythm. Electrophysiol., 2 (2009), pp. 393–401
- [2] B. Brembilla-Perrot, N. Girerd, J.M. Sellal, A. Olivier, V. Manenti, T. Villemin, D. Beurrier, C. de Chillou, P. Louis, O. Selton, A.T. de la Chaise; Risk of atrial fibrillation after atrial flutter ablation: impact of AF history, gender, and antiarrhythmic drug medication; J. Cardiovasc. Electrophysiol., 25 (2014), pp. 813–820
- [3] J. Voight, M. Akkaya, P. Somasundaram, R. Karim, S. Valliani, Y. Kwon, S. Adabag; Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter; Heart Rhythm., 11 (2014), pp. 1884–1889
- [4] S. Mittal, E. Pokushalov, A. Romanov, M. Ferrara, A. Arshad, D. Musat, M. Preminger, T. Sichrovsky, J.S. Steinberg; Long-term ECG monitoring using an implant-able loop recorder for the detection of atrial fibrillation after cavotricuspid isthmus ablation in patients with atrial flutter; Heart Rhythm., 10 (2013), pp. 1598–1604
- [5] J. García Seara, S. Raposeiras Roubin, F. Gude Sampedro, V. Balboa Barreiro, J.L. Martínez Sande, M. Rodríguez Mañero, J.R. González Juanatey; Failure of hybrid therapy for the prevention of long term recurrence of atrial fibrillation; Int. J. Cardiol., 176 (2014), pp. 74–79
- [6] C.B. De Vos, R. Pisters, R. Nieuwlaat, M.H. Prins, R.G. Tieleman, R.J. Coelen, A.C. van den Heijkant, M.A. Allessie, H.J. Crijns; Progression from paroxysmal to persistent atrial fibrillation; J. Am. Coll. Cardiol., 55 (2010), pp. 725–731
- [7] J.G. Seara, S.R. Roubin, F. Gude Sampedro, V.B. Barreiro, J.M. Sande, M.R. Mañero, P.C. Grandio, B. Alvarez, J.G. Juanatey; Risk of atrial fibrillation, stroke, and death after radiofrequency catheter ablation of typical atrial flutter; Clin. Res. Cardiol., 103 (2014), pp. 543–552
- [8] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, M.H. Picard, M.J. Roman, J. Seward, J.S. Shanewise, S.D. Solomon, K.T. Spencer, M.S. Sutton, W.J. Stewart; American Society of Echocardiographys Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification*; Eur. J. Echocardiogr., 7 (2006), pp. 79–108
- [9] K. Chen, R. Bai, W. Deng, C. Gao, J. Zhang, X. Wang, S. Wang, H. Fu, Y. Zhao, J. Zhang, J. Dong, C. Ma; HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter; Heart Rhythm., 12 (2015), pp. 1483–1489
- [10] J.S. Chinitz, E.P. Grestenfeld, F.E. Marchlinski, D.J. Callans; Atrial fibrillation is common after ablation of isolated atrial flutter during long-term follow up; Heart Rhythm., 4 (2007), pp. 1029–1033
- [11] K. Ellis, O. Wazni, N. Marrouche, D. Martin, M. Gillinov, P. McCarthy, E.B. Saad, M. Bhargava, R. Schweikert, W. Saliba, D. Bash, A. Rossillo, D. Erciyes, P. Tchou, A. Natale; Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter; J. Cardiovasc. Electrophysiol., 18 (2007) (799–02)
- [12] E. Bertaglia, F. Zoppo, A. Bonso, E. Bertaglia, F. Zoppo, A. Bonso, A. Proclemer, R. Verlato, L. Corò, R. Mantovan, D. D'Este, F. Zerbo, P. Pascotto, Northeastern Italian Study on Atrial Flutter Ablation Investigators; Long term follow up of radiofrequency catheter ablation of atrial flutter: clinical course and predictors of atrial fibrillation occurrence; Heart, 90 (2004), pp. 59–63
- [13] A. Da Costa, C. Romeyer, S. Mourot, M. Messier, A. Cerisier, E. Faure, K. Isaaz; Factors associated with early atrial fibrillation after ablation of common atrial flutter; Eur. Heart J., 23 (2002) (498–06)
- [14] W.P. Abhayaratna, J.B. Seward, C.P. Appleton, P.S. Douglas, J.K. Oh, A.J. Tajik, T.S. Tsang; Left atrial size: physiologic determinants and clinical applications; J. Am. Coll. Cardiol., 47 (2006), pp. 2357–2363
- [15] J.B. Morton, M.J. Byrne, J.M. Power, J. Raman, J.M. Kalman; Electrical remodeling of the atrium in an anatomic model of atrial flutter: relationship between substrate and triggers for conversion to atrial fibrillation; Circulation, 105 (2002), pp. 258–264
- [16] A.L. Waldo, G.K. Feld; Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications; J. Am. Coll. Cardiol., 51 (2008), pp. 779–786
- [17] W. Moreira, C. Timmermans, H.J. Wellens, Y. Mizusawa, S. Philippens, D. Perez, L.M. Rodriguez; Can common-type atrial flutter be a sign of an arrhythmogenic substrate in paroxysmal atrial fibrillation? Clinical and ablative consequences in patients with coexistent paroxysmal atrial fibrillation/atrial flutter; Circulation, 116 (2007), pp. 2786–2792
- [18] S. Mohanty, P. Mohanty, L. Di Biase, R. Bai, P. Santangeli, M. Casella, A. Dello Russo, C. Tondo, S. Themistoclakis, A. Raviele, A. Rossillo, A. Corrado, G. Pelargonio, G. Forleo, A. Natale; Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL); Circulation, 127 (2013), pp. 1853–1860
- [19] J.S. Steinberg, A. Romanov, D. Musat, M. Preminger, S. Bayramova, S. Artyomenko, V. Shabanov, D. Losik, A. Karaskov, R.E. Shaw, E. Pokushalov; Prophylactic pulmonary vein isolation during isthmus ablation for atrial flutter: the PReVENT AF Study I; Heart Rhythm., 11 (2014), pp. 1567–1572
- [20] V. Bazan, N. Grau, E. Valles, M. Felez, C. Sanjuas, M. Cainzos-Achirica, B. Benito, M. Jauregui-Abularach, J. Gea, J. Bruguera-Cortada, J. Marti-Almor; Obstructive sleep apnea in patients with typical atrial flutter: prevalence and impact on arrhythmia control outcome; Chest, 143 (2013), pp. 1277–1283
- [21] Y. Winter, C. Wolfram, M. Schaeg, J. Reese, W. Oertel, R. Dodel, T. Back; Evaluation of costs and outcome in cardioembolic stroke or TIA; J. Neurol., 256 (2009), pp. 954–963
- [22] J. Joza, K.B. Filion, M. Eberg, R. Proietti, T. Nascimento, M. Bernier, T. Hadjis, V. Essebag; Prognostic value of atrial fibrillation inducibility after right atrial flutter ablation; Heart Rhythm., 11 (2014), pp. 1870–1876
- [23] L.G. Shewan, G.M.C. Rosano, M.Y. Henein, A.J.S. Coats; A statement on ethical standards in publishing scientific articles in the International Journal of Cardiology family of journals; Int. J. Cardiol., 170 (2014), pp. 253–254
- [24] C. Reithmann, U. Dorwarth, M. Dugas, et al.; Risk factors for recurrence of atrial fibrillation in patients undergoing hybrid therapy for antiarrhythmic drug-induced atrial flutter; Eur. Heart J., 24 (2003), pp. 1264–1272
- [25] N. Anastasio, D. Frankel, M. Deyell, et al.; Nearly uniform failure of atrial flutter ablationm and continuation of antiarrhythmic agents (hybrid therapy) for the long-term control of atrial fibrillation; J. Interv. Card. Electrophysiol., 35 (2012), pp. 57–61
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?