(Created page with " ==Abstract== ====Purpose==== We hypothesized that pulmonary vein (PV) orientation influences tissue contact of the contact force (CF) sensing radiofrequency ablation cathe...") |
m (Scipediacontent moved page Draft Content 221299181 to Gal et al 2015a) |
(No difference)
| |
Latest revision as of 11:31, 19 May 2017
Abstract
Purpose
We hypothesized that pulmonary vein (PV) orientation influences tissue contact of the contact force (CF) sensing radiofrequency ablation catheter (CFC) and therefore atrial fibrillation (AF) free survival after pulmonary vein isolation (PVI). The aim of this study was to determine the association between PV orientation, CF and AF free survival in patients undergoing CFC PVI.
Methods
Sixty consecutive patients undergoing CFC PVI were included. ECG-triggered cardiac CT scans were obtained in all patients before PVI, and the PV orientation was measured at the insertion in the LA for all PVs in both the transverse and frontal plane. PVs were assigned to 1 of 4 orientation groups: ventral–caudal, dorsal–caudal, ventral–cranial and dorsal–cranial.
Results
Mean age was 59 years, 88% had paroxysmal AF. AF free survival off anti-arrhythmic drugs after a median follow-up of 12 months was 58% after a single PVI procedure. No association was found between PV orientation and CF. Furthermore, no association was found between PV orientation and AF free survival. In univariate analysis, the number of lesions with a mean CF of 10 g was associated with AF free survival. However, in multivariate analysis, only the AF duration was significantly associated with AF free survival.
Conclusions
This study shows that in patients undergoing PVI with the CFC ablation system, PV orientation does not affect CF and is not associated with AF free survival. PV orientation assessment does not appear to be necessary in patients undergoing CFC PVI.
Keywords
Atrial fibrillation;Pulmonary vein isolation;Radiofrequency catheter ablation;Contact force sensing catheter system;Pulmonary vein orientation
1. Introduction
Pulmonary vein isolation (PVI) is considered the cornerstone in the ablative treatment of atrial fibrillation (AF) [1] ; [2]. Multiple techniques have been developed to perform PVI [3]; [4] ; [5]. Currently, the most widely used technique is point-by-point radiofrequency (RF) ablation guided by a 3D electro-anatomical mapping system. Earlier reports attempting to identify geometrical characteristics of pulmonary veins (PVs) that influence AF free survival in patients undergoing point-by-point PVI yielded conflicting results [6] ; [7]. A recent study showed that PV orientation is associated with AF free survival after laser balloon PVI [8]. We hypothesized that PV orientation influences optimal contact between the ablation catheter and atrial tissue, resulting in less durable lesion sets. The aim of this study was to determine whether PV orientation influences contact force and AF free survival after PVI with a contact force sensing catheter ablation system (CFC).
2. Methods
Sixty consecutive patients with highly symptomatic, drug-refractory AF who underwent a primo PVI using CFC were included. Exclusion criteria were: previous PVI attempt, severe valvular heart disease and contraindications to post-procedural anti-coagulation. A transesophageal echocardiogram to rule out LA thrombus was performed in all patients directly prior to the PVI.
2.1. CT characteristics
All patients underwent CT scanning of the left atrium to guide the procedure. Cardiac multislice CT (MSCT) angiography was performed by a team of very experienced CT technologists using a 64-slice scanner (Lightspeed VCT XT, GE Healthcare). A bolus of 70 ml of nonionic contrast medium of agent (Optiray 350, Mallinckrodt, The Netherlands) was infused through a large antecubital vein at a rate of 5 ml/s, followed by 50-ml saline solution flush. Automatic detection of the contrast bolus in the left atrium was used to time the start of the scan. Delay times varied significantly because of flow rate differences in patients, but were generally in the range of 5–15 s. Craniocaudal scanning was performed during breath-hold and using retrospective ECG gating (to be able to determine volume changes of the LA, but not used in this study). The collimation was 64 × 0.5 mm, rotation time 400 ms, and the tube voltage was 120 kV with mA dose modulation variable between 80 and 200 mA. All images were checked for adequacy before the end of the procedure to guarantee adequate image quality in all patients. After acquisition, the raw MSCT data were exported, post-processed, and analyzed on a dedicated workstation (GE Healthcare). The images were reviewed by an independent investigator who was not involved in the CFC guided PVI ablation procedures and was not informed about the PVI outcome in these patients.
2.2. Pulmonary vein orientation measurement
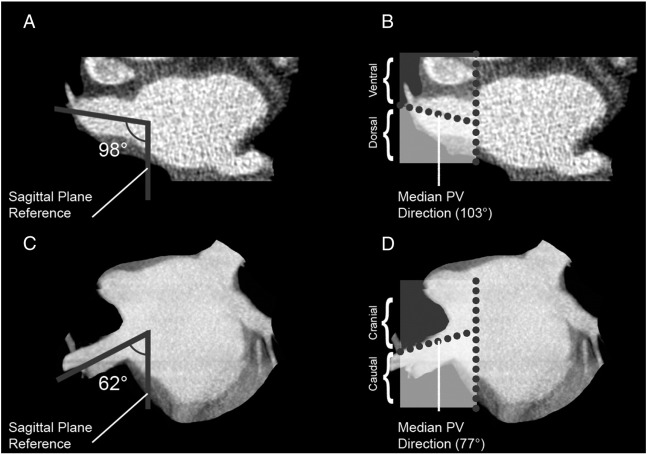
The PV trunk orientation measurement has been described before ([9] ; [10], Association between pulmonary vein orientation and atrial fibrillation free survival in patients undergoing endoscopic laser balloon ablation [8]). The orientation of the PV trunk at the site of insertion into the LA was assessed for all PVs in both the transverse and frontal plane. A line was drawn in the direction of each PV trunk in both the transverse and frontal plane. Thereafter, the angle between the PV trunk direction and the intersection line of the sagittal plane was measured in the transverse and frontal plane (Fig. 1). Median PV trunk angles were calculated in the transverse and frontal plane for all four PV trunks. PVs were assigned to a ventral/dorsal or caudal/cranial orientation depending on the PV trunk angle as compared to the median angle. So, each PV trunk was assigned to one of four orientation groups: ventral–caudal, dorsal–caudal, ventral–cranial and dorsal–cranial.
|
|
|
Fig. 1. Example of right upper pulmonary vein orientation measurement in the transverse and frontal planes. This figure displays the PV orientation measurement in the transverse and frontal plane of the RUPV. In panels A and B, the allocation of the RUPV in this patient in the transverse plane is displayed. The angle between the PV direction and the sagittal plane reference is 98°, as is displayed in panel A. The median RUPV direction in the transverse plane is 103° as can be appreciated from table 2, categorizing this RUPV to the dorsal RUPV orientation group. In panels C and D, the allocation of the RUPV of this patient in the frontal plane is displayed. The angle between the PV direction and the sagittal plane reference is 62°, as is displayed in panel C. The median RUPV direction in the frontal plane is 77°, as can be appreciated from table 2, categorizing this RUPV to the caudal RUPV orientation group. Combining the frontal and transverse plane, the RUPV of this patient is categorized to the dorsal–caudal RUPV orientation group. PV: pulmonary vein; RUPV: right upper pulmonary vein. |
2.3. Electrophysiological procedure
All patients underwent CFC guided PVI under general anesthesia supervised by a cardiovascular anesthesiologist. First, a 6F quadripolar catheter was placed in the coronary sinus to obtain a procedural intracardiac electrogram. Two transseptal punctures were performed using a Brockenbrough needle under fluoroscopy and pressure guidance. 10,000 IU of unfractionated heparin was administered after the first transseptal puncture. A circular mapping catheter (LASSO®, Biosense Webster Inc., Diamond Bar, CA, USA) was inserted into the LA through an 8.5F sheath (SL-1, St. Jude Medical, Minnetonka, MN, USA) and an 8.5F sheath (SL-1, St. Jude Medical, Minnetonka, MN, USA) was used for PV angiography. Both sheaths were flushed continuously with a saline solution containing 2500 UI heparin per 500 ml saline. The targeted activated clotting time was between 300 and 350 s and additional heparin was administered when necessary. The activated clotting time was assessed every 30 min. The CFC catheter was inserted in the LA through an 8.5F sheath and the CFC sensor in the tip electrode was calibrated after positioning the catheter tip in a free floating position in the LA cavity.
2.4. Contact force sensing radiofrequency ablation
The CFC (Smarttouch™, Biosense Webster Inc., Diamond Bar, CA, USA) [3] ; [11] is an externally irrigated catheter with a 3.5 mm tip electrode. The tip electrode is equipped with a contact force sensor which measures both contact pressure and vector of tip deflection. The catheter system is fully integrated within the 3D electro-anatomical mapping system (CARTO 3™, Biosense Webster Inc., Diamond Bar, CA, USA). PVI was performed by delivering RF energy in a point-by-point fashion to the PV antrum creating contiguous circular ablation lesions. RF energy was applied in a temperature-control mode with a temperature setting of 43 °C. RF energy was applied at 30 W with a flow rate of 15 ml/min or at 40 W with a flow rate of 30 ml/min, depending on site of ablation. The endpoint of the ablation procedure was PV isolation, as documented by entrance and exit block or dissociation of PV potentials.
2.5. Follow-up
A blanking period of 3 months was defined after PVI. Patients visited the outpatient clinic at 3, 6, 12, 18 and 24 months after PVI, including Holter ECG, event recorder monitoring and loop recorder monitoring in selected cases. Patients were immediately referred to the emergency room in case of symptoms. 3 months after PVI, an attempt was made in all patients to cease anti-arrhythmic drugs (AADs).
2.6. Study endpoints
The primary endpoint of our study was AF free survival, defined as patients without AF/atrial flutter/atrial tachycardia recurrence after a blanking period of 3 months. AF recurrence was defined as an ECG showing the characteristics of AF, or on a 30 s telemetry strip, in accordance with European Heart Rhythm Association AF ablation guidelines [1]. Ablation points with a contact force < 10 g were assessed separately, based on a study that showed adequate lesions are applied with a mean contact force > 10 g [12].
2.7. Statistical analysis
Continuous variables were expressed as mean with standard deviation in case of normal distribution or median with interquartile range when variables were not normally distributed. Differences in mean contact force among PV orientation configurations was assessed with a Kruskal–Wallis test in case of continuous data and a Chi-square test in case of categorical data. A univariate and multivariate Cox proportional hazard model was used to determine predictors of AF free survival. Statistical analysis was performed using IBM SPSS statistics version 20 (IBM inc., Armonk, NY, USA). A p-value of ≤ 0.05 was considered statistically significant.
3. Results
Our study population consisted of sixty consecutive patients. Baseline characteristics are displayed in Table 1. There were 6 common PVs (2.6%), which were excluded from analysis. No LA thrombi were found during preoperative transesophageal echocardiography or CT scans. Table 2 describes the characteristics of the PV orientation in all patients.
| Total (n = 60) | |
|---|---|
| Gender female (%) | 23% |
| Age (years) | 59.3 (± 9.1) |
| BMI (kg/m2) | 26.9 (± 3.5) |
| AF duration (years) | 5.1 (± 5.4) |
| Paroxysmal AF | 88% |
| Failed AADs (range) | 1.1 (0–3) |
| LA dimension in PSLAX (mm) | 42.2 (± 5.3) |
| LVEF (%) | 58.9 (± 3.8) |
| Hypertension | 38% |
| Previous TIA/stroke | 12% |
| Coronary artery disease | 0% |
| Diabetes | 2% |
Data are presented as percentages or means ± their SD or ranges where appropriate; BMI: body mass index; AF: atrial fibrillation; AAD: anti-arrhythmic drugs; LA: left atrium; PSLAX: parasternal long axis view; LVEF: left ventricular ejection fraction. P-values: comparison between AF free and AF recurrence groups.
| Pulmonary vein | Median angle | Ventral–caudal | Dorsal–caudal | Ventral–cranial | Dorsal–cranial |
|---|---|---|---|---|---|
| Left upper | Transverse: 97.1° (± 32.0) | 28% | 24% | 22% | 26% |
| Frontal: 139.0° (± 19.7) | |||||
| Left lower | Transverse: 62.1° (± 17.6) | 26% | 26% | 24% | 24% |
| Frontal: 86.4° (± 19.4) | |||||
| Right upper | Transverse: 103.0° (± 14.9) | 28% | 23% | 22% | 27% |
| Frontal: 121.8° (± 11.4) | |||||
| Right lower | Transverse: 55.5° (± 16.2) | 20% | 32% | 30% | 18% |
| Frontal: 77.1° (± 14.8) |
Data are presented as absolute median or percentages, ± their SD where appropriate.
3.1. Pulmonary vein isolation results
In 234 out of 234 PVs (100%), acute PV isolation after CFC ablation was confirmed. After a median follow up of 12.3 (interquartile range: 8.3–16.1) months, AF free survival was 57.6% after a single CFC PVI without the use of class I or III AADs.
3.2. Contact force data
Mean contact force was 15.0 g ± 10.9 for the left upper PV (LUPV), 13.5 g ± 9.7 for the left lower PV (LLPV), 17.9 g ± 10.0 for the right upper PV (RUPV) and 15.6 g ± 9.8 for the right lower PV (RLPV). PV orientation was not associated with contact force for the LUPV (p = 0.236), LLPV (p = 0.491), RUPV (p = 0.143) and RLPV (p = 0.718), as is displayed in the supplemental Table 1. Furthermore, no association was found between PV orientation and the number of lesions with a mean contact force < 10 g, as displayed in supplemental Table 2.
3.3. Association with AF free survival
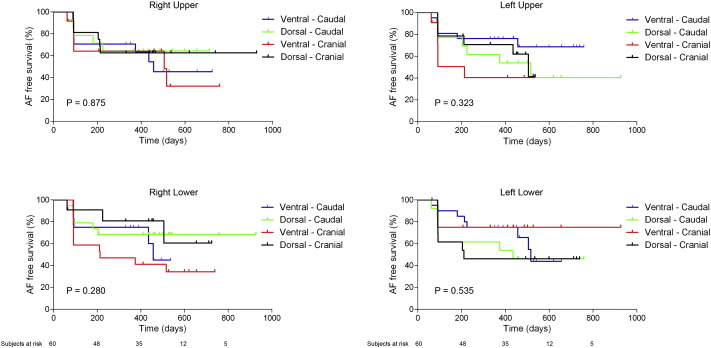
In univariate analysis, none of the baseline characteristics were associated with AF free survival. Moreover, PV orientation was also not associated with AF free survival, as is also displayed in Fig. 2. Table 3 displays the univariate analysis. Of note, mean contact force was not associated with AF free survival, but the number of ablation points with a mean contact force < 10 g was significantly associated with AF free survival. However, in multivariate analysis, only AF duration was associated with AF free survival.
|
|
|
Fig. 2. Association of pulmonary vein orientation and atrial fibrillation free survival. This figure displays the association between PV orientation and AF free survival for all four PVs. There is no significant association between PV orientation and AF free survival. P-value between pulmonary vein orientation groups. AF: atrial fibrillation; PV: pulmonary vein. |
| Univariate analysis | p-value | Hazard ratio | 95% CI | Multivariate analysis | p-value | Hazard ratio | 95% CI |
|---|---|---|---|---|---|---|---|
| Female gender | 0.882 | 1.072 | 0.428–2.687 | Ablation points with mean contact force < 10 g | 0.333 | 1.017 | 0.983–1.053 |
| Age | 0.560 | 0.988 | 0.947–1.030 | AF duration (per year) | 0.017 | 1.128 | 1.021–1.246 |
| BMI | 0.605 | 1.031 | 0.918–1.158 | ||||
| Paroxysmal AF | 0.115 | 0.452 | 0.169–1.212 | ||||
| AF duration (per year) | 0.062 | 1.053 | 0.997–1.112 | ||||
| Failed AADs | 0.907 | 1.032 | 0.607–1.755 | ||||
| LA dimension | 0.468 | 1.028 | 0.954–1.107 | ||||
| LVEF | 0.645 | 1.028 | 0.913–1.159 | ||||
| Hypertension | 0.976 | 0.988 | 0.440–2.216 | ||||
| Mean contact force | 0.305 | 0.890 | 0.711–1.112 | ||||
| Ablation points with mean contact force < 10 g | 0.042 | 1.036 | 1.001–1.072 | ||||
| LUPV orientation | 0.547 | ||||||
| Ventral–caudala | 0.643 | 0.755 | 0.230–2.481 | ||||
| Dorsal–caudala | 0.712 | 1.229 | 0.412–3.665 | ||||
| Ventral–craniala | 0.326 | 1.765 | 0.568–5.488 | ||||
| LLPV orientation | 0.641 | ||||||
| Ventral–caudala | 0.819 | 0.884 | 0.308–2.536 | ||||
| Dorsal–caudala | 0.800 | 1.146 | 0.399–3.287 | ||||
| Ventral–craniala | 0.285 | 0.478 | 0.123–1.852 | ||||
| RUPV orientation | 0.876 | ||||||
| Ventral–caudala | 0.671 | 1.258 | 0.436–3.628 | ||||
| Dorsal–caudala | 0.928 | 0.947 | 0.289–3.103 | ||||
| Ventral–craniala | 0.521 | 1.450 | 0.467–4.505 | ||||
| RLPV orientation | 0.299 | ||||||
| Ventral–caudala | 0.536 | 1.574 | 0.374–6.618 | ||||
| Dorsal–caudala | 0.793 | 1.204 | 0.301–4.820 | ||||
| Ventral–craniala | 0.137 | 2.644 | 0.735–9.513 |
Univariate and multivariate analysis of the association between patient, procedural and PV characteristics and AF free survival after CFC PVI. BMI: body mass index; AF: atrial fibrillation; AAD: anti-arrhythmic drugs; LA: left atrium; PSLAX: parasternal long axis view; LVEF: left ventricular ejection fraction. LUPV: left upper pulmonary vein; LLPV: left lower pulmonary vein; RUPV: right upper pulmonary vein; RLPV: right lower pulmonary vein. CFC: contact force sensing catheter ablation system. P-values between AF free and AF recurrence groups.
a. As compared to the dorsal–cranial orientation group.
4. Discussion
This study shows that in patients undergoing PVI with the CFC ablation system, PV orientation does not affect contact force and is not associated with AF free survival. These results suggest that durable PV lesion sets can be delivered independent of PV orientation with the CFC ablation system. PV orientation assessment does not appear to be necessary in patients undergoing CFC PVI.
4.1. Pulmonary vein anatomy and atrial fibrillation free survival
In a study of 100 patients who underwent RF catheter ablation, a smaller LA size and an atypical right-sided PV anatomy were associated with an increased AF free survival after PVI [7]. In another study of 118 patients who underwent RF catheter ablation, the absence of common PV trunks was associated with an increased AF free survival [13]. However, none of these studies assessed the association between PV orientation and AF free survival, although in a recent study, PV orientation was significantly associated with AF free survival after laser balloon PVI [9].
4.2. Contact force sensing catheter system characteristics
The CFC allows contact force guided RF ablation of AF. Previous studies found an association between pressure–time curves recorded with the CFC and AF free survival, indicating that contact force sensing may assist in the application of durable lesion sets [14] ; [15]. The present study is in line with these results, showing that the number of lesions with a contact force < 10 g was associated with a diminished AF free survival. Hypothetically, pressure-guided ablation should allow the operator to identify inadequate lesions and apply additional ablations when deemed necessary.
4.3. Pulmonary vein orientation and AF free survival
AF recurrences are generally regarded as reconnection between the PV and the LA allowing electrical reconduction [16] ; [17]. Therefore the durability of the applied circumferential lesions are essential in preventing AF recurrences. Hypothetically, the influence of PV orientation may be negated by allowing the operator to identify inadequate catheter-tissue contact due to PV orientation, and increase catheter–tissue contact accordingly, resulting in durable lesion sets.
4.4. Limitations
With regards to interpreting our data, the following limitations should be considered. This is a single center study with a limited number of patients. The patient cohort was limited to those having no or minimal structural heart disease and a normal left ventricular function.
5. Conclusion
This study showed that in patients undergoing PVI with the CFC ablation system, PV orientation does not affect contact force and is not associated with AF free survival. PV orientation assessment does not appear to be necessary in patients undergoing CFC PVI.
Conflict of interest
None.
Funding
None.
Acknowledgments
We thank ms. Vera Derks for the final preparation of this manuscript.
Appendix A. Supplementary data
Supplementary material.
References
- [1] H. Calkins, K.H. Kuck, R. Cappato, J. Brugada, A.J. Camm, S.A. Chen, et al.; 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design; Europace, 14 (2012), pp. 528–606
- [2] M. Haissaguerre, P. Jais, D.C. Shah, A. Takahashi, M. Hocini, G. Quiniou, et al.; Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins; N Engl J Med, 339 (1998), pp. 659–666
- [3] K.H. Kuck, V.Y. Reddy, B. Schmidt, A. Natale, P. Neuzil, N. Saoudi, et al.; A novel radiofrequency ablation catheter using contact force sensing: Toccata study; Heart Rhythm, 9 (2012), pp. 18–23
- [4] V.Y. Reddy, P. Neuzil, S. Themistoclakis, S.B. Danik, A. Bonso, A. Rossillo, et al.; Visually-guided balloon catheter ablation of atrial fibrillation: experimental feasibility and first-in-human multicenter clinical outcome; Circulation, 120 (2009), pp. 12–20
- [5] Y. Van Belle, P. Janse, M.J. Rivero-Ayerza, A.S. Thornton, E.R. Jessurun, D. Theuns, et al.; Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome; Eur Heart J, 28 (2007), pp. 2231–2237
- [6] I. Hof, K. Chilukuri, A. Arbab-Zadeh, D. Scherr, D. Dalal, S. Nazarian, et al.; Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation?; J Cardiovasc Electrophysiol, 20 (2009), pp. 1005–1010
- [7] D.W. den Uijl, L.F. Tops, V. Delgado, J.D. Schuijf, L.J. Kroft, A. de Roos, et al.; Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation; Am J Cardiol, 107 (2011), pp. 243–249
- [8] P.G. Platonov, L.B. Mitrofanova, V. Orshanskaya, S.Y. Ho; Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age; J Am Coll Cardiol, 58 (2011), pp. 2225–2232
- [9] A. Sorgente, G.B. Chierchia, C. de Asmundis, A. Sarkozy, M. Namdar, L. Capulzini, et al.; Pulmonary vein ostium shape and orientation as possible predictors of occlusion in patients with drug-refractory paroxysmal atrial fibrillation undergoing cryoballoon ablation; Europace, 13 (2011), pp. 205–212
- [10] P.H. van der Voort, H. van den Bosch, J.C. Post, A. Meijer; Determination of the spatial orientation and shape of pulmonary vein ostia by contrast-enhanced magnetic resonance angiography; Europace, 8 (2006), pp. 1–6
- [11] M. Martinek, C. Lemes, E. Sigmund, M. Derndorfer, J. Aichinger, S. Winter, et al.; Clinical impact of an open-irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation; Pacing Clin Electrophysiol, 35 (2012), pp. 1312–1318
- [12] C.I. Park, H. Lehrmann, C. Keyl, R. Weber, J. Schiebeling, J. Allgeier, et al.; Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance; J Cardiovasc Electrophysiol, 25 (2014), pp. 701–708
- [13] M. Kubala, J.S. Hermida, G. Nadji, S. Quenum, S. Traulle, G. Jarry; Normal pulmonary veins anatomy is associated with better AF-free survival after cryoablation as compared to atypical anatomy with common left pulmonary vein; Pacing Clin Electrophysiol, 34 (2011), pp. 837–843
- [14] P. Neuzil, V.Y. Reddy, J. Kautzner, J. Petru, D. Wichterle, D. Shah, et al.; Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study; Circ Arrhythm Electrophysiol, 6 (2013), pp. 327–333
- [15] V.Y. Reddy, D. Shah, J. Kautzner, B. Schmidt, N. Saoudi, C. Herrera, et al.; The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study; Heart Rhythm, 9 (2012), pp. 1789–1795
- [16] R. Cappato, S. Negroni, D. Pecora, S. Bentivegna, P.P. Lupo, A. Carolei, et al.; Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation; Circulation, 108 (2003), pp. 1599–1604
- [17] K. Lemola, B. Hall, P. Cheung, E. Good, J. Han, K. Tamirisa, et al.; Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation; Heart Rhythm, 1 (2004), pp. 197–202
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?