Summary
The suitable size of a graft is a key element in the success of liver transplantation. A small-for-size liver graft is very likely to sustain a significant degree of injury as a result of ischemia, preservation, reperfusion, and rejection. Usually, small-for-size grafts are a concern in living-donor liver transplantation rather than in deceased-donor liver transplantation. Here, we describe the successful transplantation of a liver from a 2-year-old deceased donor to a 61-year-old male recipient who suffered from liver failure related to hepatitis B. No report of successful deceased-donor liver transplantation with discrepancies between donor and recipient age and size to such an extent has been found in the literature. Despite unusually large discrepancies, with effort in minimizing the ischemic time, revised surgical techniques, and strong regenerative power of the “young” graft, the old patients liver function gradually returned to normal. This again proves that the definition of a “suitable graft” evolves with time and experience.
Keywords
deceased-donor liver transplantation;graft-weight-to-recipient-weight ratio;pediatric donor
1. Introduction
The disparity between demand and supply of liver grafts has led the liver transplant community to look beyond the conventional donor pool toward more marginal donor candidates. Strategies to increase the number of liver grafts include liver splitting, use of steatotic livers and livers with familial amyloidotic polyneuropathy disease, and accepting donors over 70 years old1 and donations after cardiac death.2 These strategies are particularly important in Asian regions, including Hong Kong, where there is a serious shortage of livers from brain-dead donors.3 Here, we describe a case of liver transplantation where a liver donated by a 28-month-old brain-dead boy was transplanted to a 61-year-old man.
2. Case report
2.1. Donor
The donor was a 28-month-old Chinese–Japanese boy with a body weight of 12 kg and a body height of 90 cm. He sustained severe head injury, resulting in cerebral hemorrhage. He received craniectomy but finally succumbed to brain death 4 days afterward. Throughout his 4-day stay in the pediatric intensive care unit, he was hemodynamically stable. Right at the time of his death, there was no pediatric liver transplant candidate in the city who was compatible with the donor.
2.2. Recipient
The recipient was a 61-year-old Chinese man, a hepatitis B virus (HBV) carrier with a known history of diabetes mellitus. He was admitted to the hospital because of acute-on-chronic liver failure related to an acute flare of hepatitis B. His serum total bilirubin level on admission was 373 μmol/L and the international normalized ratio was 2.5. His HBV DNA was 1.5 × 105 copies/mL. His condition deteriorated rapidly, with the model for end-stage liver disease score rising to 40 despite commencing antiviral therapy. He developed hepatic encephalopathy and required intubation 12 days after admission. Six days later, he received liver transplantation. His body weight was 64.5 kg and body height was 1.53 m at the time of transplantation, giving an estimated standard liver volume of 1264 mL.4
2.3. Operation
In the donor, the celiac trunk and portal vein were isolated at the beginning of the liver-recovering procedure so as to reduce the cold ischemic time. The liver was then retrieved with the standard technique with kocherization of the duodenum, isolation of the inferior vena cava (IVC), and cannulation of the aorta and inferior mesenteric vein. Cross-clamping and perfusion with Viaspan were not performed until the recipient was almost ready for implantation. The liver weighed 360 g. The graft-weight-to-recipient-weight ratio was 0.56% and the graft-weight-to-standard-liver-volume ratio was 28.4%. The graft was trimmed at the back table and the infrahepatic IVC was closed with a Tyco Autosuture TA30 linear stapler.
In the recipient, the native liver was cirrhotic with moderate splenomegaly. Total hepatectomy was performed with preservation of the IVC. Venovenous bypass was not used. The right hepatic vein and the common trunk of the middle and left hepatic veins were closed with an Ethicon Endopath ATW35 articulating linear cutter. A separate opening was made in the IVC below the hepatic vein orifice to allow caudal shifting of the graft so as to facilitate portal vein and bile duct anastomosis. The piggyback technique was adopted because of the discrepancy between the size of the donor IVC and that of the recipient IVC. Implantation of graft was started with an end-to-side anastomosis of the graft suprahepatic IVC to the recipient IVC with a 5/0 Prolene suture. After portal vein reconstruction, hepatic artery anastomosis was performed under an operation microscope with a 9/0 nylon interrupted suture, and duct-to-duct anastomosis was performed with a 6/0 polydioxanone continuous suture for the posterior layer and an interrupted suture for the anterior layer. At the end of the operation, Doppler ultrasound study confirmed that all vessels were patent. Portal pressure measured after implantation was 16 mmHg and portal flow 333 mL/100 g/min. The cold ischemic time was 214 minutes and warm ischemic time 43 minutes.
2.4. Postoperative course
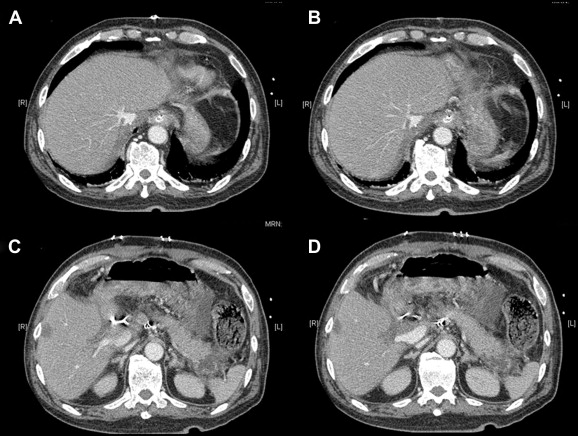
A computed tomography scan 1 week after the operation showed hypertrophy of the liver graft (Fig. 1). Pathology of his excised liver confirmed chronic hepatitis B with cirrhosis. His serum total bilirubin level went down from 582 to 392 μmol/L after the operation and reached a peak level of 550 μmol/L 9 days after the operation. The level gradually returned to normal in 2 months' time. His international normalized ratio returned to normal 11 days after the operation. Daily abdominal drain output was around 500–1000 mL in the early postoperative period, which gradually reduced to an insignificant amount 50 days after the operation. He underwent re-laparotomy for intra-abdominal collection, with peritoneal fluid growing Citrobacter freundii, and gradually recovered. At the time of writing of this article, he has remained alive for 16 months after transplantation.
|
|
|
Figure 1. Computed tomography scan 1 week after the operation showed hypertrophy of the liver graft: (A) small graft hepatic vein, (B) inferior vena cava, (C) small graft portal vein, and (D) normal recipient portal vein. |
3. Discussion
The expanding request for liver transplantation, coupled with the serious shortage of liver grafts, has increased the use of extended-criteria donors worldwide. There are reports on cases of deceased-donor liver transplantation (DDLT) using previously forbidden livers such as those with large cavernous hemangioma,5 ; 6 polycystic livers,7 and livers from donors positive of hepatitis B surface antigen.8
Livers from pediatric donors are ideally used for pediatric recipients. However, there are occasions where such a liver is allocated to an adult recipient, e.g., if the pediatric donor and an adult patient are within the same body weight range. However, no report of successful DDLT with discrepancies between donor and recipient age and size to an extent as described herein has been found in the literature. At the time of this transplantation, no pediatric liver transplant candidate in the city was compatible with the donor. On the other hand, for this patient with a disease of UNOS status 1A, liver transplantation was the only curative option. We therefore decided to go ahead with the transplantation, and all details regarding the donor and the situation of the patient were discussed with the patients family before the transplantation could proceed.
Emre et al9 reviewed their experience of using pediatric donor livers in adult liver transplantations and found that the incidence of hepatic artery thrombosis was significantly higher in the pediatric donor group (12.9%) when compared with the adult donor group (3.8%; p = 0.0003). Adam et al 10 reviewed their use of small donor livers for adult recipients and found that risk factors for complications were graft weight below 600 g, graft-weight-to-recipient-weight ratio below 0.5, and preservation time exceeding 12 hours.
The suitable size of a graft is a key element in the success of adult-to-adult living-donor liver transplantation (LDLT). A small-for-size graft is very likely to sustain a significant degree of injury as a result of ischemia, preservation, reperfusion, and rejection. Early graft dysfunction predisposes the patient to complications such as sepsis and intracranial hemorrhage. In our early experience, a graft-weight-to-standard-liver-volume ratio of >40% correlated with a patient survival rate of 95%, and a <40% ratio correlated with a 40% survival rate only.11 On reaching the first 100 cases of right-liver LDLT, we reckoned that a graft larger than 35% of the standard liver volume may suffice.12 Through accumulation of experience, small-for-size grafts have become less important as a factor in hospital mortality at our center.13 Similarly, Nishizaki et al14 have suggested that a small graft with a graft-weight-to-standard-liver volume ratio of 26–29% can be used for LDLT.
In the literature, most of the studies on small-for-size liver graft have their focus on LDLT, as the minimum graft size for DDLT is expected to be higher because of various adverse events on brain-dead donors as well as the longer ischemic time. The DDLT described herein is quite unusual, with a graft-weight-to-recipient-weight ratio of only 0.56% and a graft-weight-to-standard-liver-volume ratio of only 28.4%. Nonetheless, with revised techniques such as caudal shifting of graft, piggyback technique for implantation, effort in minimizing the ischemic time, and the stronger regenerative power of the “young” graft,15 the old patients liver function gradually returned to normal. This again proves that the definition of a “suitable graft” evolves with time and experience.
References
- 1 D.Y. Kim, S.P. Cauduro, H.E. Bohorquez, M.B. Ishitani, S.L. Nyberg, C.B. Rosen; Routine use of livers from deceased donors older than 70: is it justified?; Transplant Int, 18 (2005), pp. 73–77
- 2 Bernat JL, D'Alessandro AM, Port FK, et al. Report of a national conference on donation after cardiac death. Am J Transplant. 2006;6:281–91.
- 3 C.M. Lo; Liver transplantation in Asia—challenges and opportunities; Asian J Surg, 25 (2002), p. 270
- 4 S.C. Chan, C.L. Liu, C.M. Lo, et al.; Estimating liver weight of adults by body weight and gender; World J Gastroenterol, 12 (2006), pp. 2217–2222
- 5 F.N. Aucejo, W.A. Ortiz, D. Kelly, et al.; Expanding the donor pool: safe transplantation of a cadaveric liver allograft with a 10 cm cavernous hemangioma—a case report; Liver Transpl, 12 (2006), pp. 687–689
- 6 S. Nikeghbalian, K. Kazemi, H. Salahi, et al.; Transplantation of a cadaveric liver allograft with right lobe cavernous hemangioma, without back-table resection: a case report; Transplant Proc, 39 (2007), pp. 1691–1692
- 7 Z.A. Stewart, T. Kozlowski, D.L. Segev, R.A. Montgomery, A.S. Klein; Successful transplantation of cadaveric polycystic liver: case report and review of the literature; Transplantation, 81 (2006), pp. 284–286
- 8 J.K. Ho, P.R. Harrigan, C.H. Sherlock, et al.; Utilization of a liver allograft from a hepatitis B surface antigen positive donor; Transplantation, 81 (2006), pp. 129–131
- 9 S. Emre, Y. Soejima, G. Altaca, et al.; Safety and risk of using pediatric donor livers in adult liver transplantation; Liver Transpl, 7 (2001), pp. 41–47
- 10 R. Adam, D. Castaing, H. Bismuth; Transplantation of small donor livers in adult recipients; Transplant Proc, 25 (1993), pp. 1105–1106
- 11 C.M. Lo, S.T. Fan, C.L. Liu, et al.; Minimum graft size for successful living donor liver transplantation; Transplantation, 68 (1999), pp. 1112–1116
- 12 S.T. Fan, C.M. Lo, C.L. Liu, B.H. Yong, J. Wong; Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation; Ann Surg, 238 (2003), pp. 864–869
- 13 S.C. Chan, C.M. Lo, K.K.C. Ng, S.T. Fan; Alleviating the burden of small-for-size graft in right liver living donor liver transplantation through accumulation of experience; Am J Transplant, 10 (2010), pp. 859–867
- 14 T. Nishizaki, T. Ikegami, S. Hiroshige, et al.; Small graft for living donor liver transplantation; Ann Surg, 233 (2001), pp. 575–580
- 15 H. Yokoi, S. Isaji, K. Yamagiwa, et al.; Donor outcome and liver regeneration after right-lobe graft donation; Transplant Int, 18 (2005), pp. 915–922
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?