Summary
Introduction
Compared with intravenous chemotherapy, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been shown to improve survival in patients with recurrent colorectal disease confined to the peritoneum. We report our experience with CRS and HIPEC for colorectal cancer patients with peritoneal carcinomatosis, evaluating prognostic factors for disease-free survival (DFS), overall survival (OS), and perioperative morbidity and mortality.
Methods
All patients who underwent CRS and HIPEC were included in our study. Clinical characteristics, operative data, and 30-day morbidity and mortality were collected and evaluated.
Results
Between January 2001 and December 2012, there were 35 consecutive patients who underwent CRS and HIPEC at our institution. Thirty-three patients (94%) had optimal cytoreduction. No 30-day mortality was reported, but 14 patients had postoperative complications. The median DFS was 9.4 months (95% confidence interval 5.5–18.7 months), and DFS at 1 year, 3 years, and 5 years were 43.8%, 22.3%, and 22.3%, respectively. The median OS was calculated to be 27.1 months (95% confidence interval 15.3–39.1), and the OS at 1 year, 3 years, and 5 years were 83.7%, 38.2%, and 19.1%, respectively.
Conclusion
CRS and HIPEC can provide survival benefit, with reasonable morbidity and mortality for Asian patients with peritoneal carcinomatosis from colorectal cancer. Patient selection and perioperative management of the patients are key to the success of the procedure.
Keywords
colorectal cancer;cytoreductive surgery;hyperthermic intraperitoneal chemotherapy;peritoneal carcinomatosis
1. Introduction
Colorectal peritoneal metastases (CPM) occur in up to 20% of colorectal cancers, and 40–70% of all recurrent diseases. In 10–30% of these recurrences, the disease is confined to the peritoneum, conferring a median survival of 7 months.1 In these patients, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been shown to improve survival when compared to intravenous chemotherapy alone.2 The success of CRS and HIPEC is based on the underlying principle of peritoneal disease being a locoregional disease, and not a “true” metastasis.3 Hence, surgical resection of the entire macroscopic tumor by CRS, and HIPEC targeting the microscopic disease, renders the patient potentially tumor-free. Prognostic factors include ability to achieve complete cytoreduction,4 ; 5 tumor burden,6 and primary tumor histology7; therefore, patient selection is of utmost importance. We report our (tertiary) institutions experience with CRS and HIPEC for colorectal cancer patients with peritoneal carcinomatosis (PC), evaluating the prognostic factors for disease-free survival (DFS) and overall survival (OS) and the perioperative morbidity and mortality.
2. Methods
The study was conducted with the approval of the Centralized Institutional Review Board of the Singapore Health Services. Data were prospectively collected for consecutive cases of colorectal cancer patients with PC treated by CRS and HIPEC at the National Cancer Centre Singapore between January 2001 and December 2012. Our primary end points were OS and DFS. Clinical characteristics, operative data, and 30-day morbidity and mortality were also evaluated.8 All of the cases had surgery performed by either one of two surgeons (K.C. S. and M. T.).
2.1. Patient selection
All patients who were selected to undergo CRS and HIPEC in our institution were of Eastern Cooperative Group (ECOG) performance status 0 or 1, with no distant metastases evaluated with computed tomography (CT) or positron emission tomography-CT scans. In addition, the primary tumor histology and stage, the disease-free interval (DFI), response to chemotherapy, tumor burden, and the possibility of complete cytroreduction are discussed at multidisciplinary tumor board meetings, with radiologists, pathologists, as well as radiation, medical, and surgical oncologists present. The patients were also evaluated with colonoscopy to exclude synchronous and metachronous colonic lesions. Prior to the surgery, all patients were subjected to a physical examination and routine blood tests, including tumor markers.
2.2. Surgical procedure
CRS was performed as described by Sugarbaker.9 The procedure aims to remove all macroscopic peritoneal disease, and resection of involved visceral organs is typically performed first followed by the removal of sections of involved peritoneum. Bowel anastomoses are typically performed after HIPEC. Mitomycin C was the drug of choice for our patients with colorectal PC.
HIPEC targets the microscopic diseases, working on lesions less than 3 mm. Owing to the peritoneal–plasma barrier, a higher dose of chemotherapy can be delivered with less systemic toxicity. The high temperature increases the drug penetration and provides a synergistic effect with the intraperitoneal chemotherapy. At our institution, a closed technique for HIPEC, with the chemotherapy agent (Mitomycin C) diluted in 2–3 L of peritoneal dialysis solution at 42°C, is used to distend the abdomen and ensure the greatest exposure to the chemotherapy agent. The temperature is measured via temperature probes attached to the inflow and outflow catheters. Currently, we are using the Belmont hyperthermia pump (Belmont Instrument Corporation, Billerica, MA, USA) to deliver HIPEC via a single inflow catheter, and drainage is via four intra-abdominal drains. In our initial experience, typically only two intra-abdominal drains were placed for drainage. HIPEC is administered for 60 minutes. A dedicated anesthetist monitors the patients parameters, including the core temperature via an esophageal temperature probe and keeps the patient adequately volume-filled.
2.3. Peritoneal Cancer Index and completeness of cytoreduction score
The Peritoneal Cancer Index (PCI) score was used to describe the extent of peritoneal disease.10 The completeness of resection was measured prospectively in all patients using the completeness of cytoreduction (CC) score. This score, which measures the amount of disease left behind,11 has been shown in several studies to be the strongest prognostic indicator in patients with PC undergoing CRS and HIPEC.4 ; 5 Patients with a CC score of 0 and 1 are considered to have achieved optimal cytoreduction because chemotherapy can penetrate these small nodules. In patients with a CC score of 2 and 3, surgery does not provide additional survival benefit when compared to conservative management.
2.4. Postoperative care
Following CRS and HIPEC, four intra-abdominal drains were left in place, and early postoperative intraperitoneal chemotherapy (EPIC) was initiated for 5 days. EPIC is used as the resection site, and stripped peritoneal surfaces are at high risk for tumor cell implantation in the postoperative period.10 5-Fluorouracil was the chemotherapy agent used for EPIC in this group of patients.
The patients were transferred to the surgical intensive care unit or high dependency unit postoperatively. All intraoperative and 30-day postoperative complications were recorded. Morbidity was evaluated using the common terminology criteria for adverse events version 3.0 of the National Institute of Health criteria.8
The patients were followed up at the surgical oncology outpatient unit at the National Cancer Centre Singapore at approximately 1 week after discharge, and at least every 3–6 months thereafter. At each follow-up visit and when clinically indicated, CT scans of the thorax, abdomen, and pelvis were performed, along with tumor markers (as appropriate). Most of the patients were also followed up with medical oncologists and received adjuvant systemic chemotherapy at the discretion of the oncologist. Events of recurrent disease and their sites were recorded.
2.5. Statistical analysis
The Kaplan–Meier method was used to derive the survival functions for OS and DFS, from which median OS and DFS were derived. 95% confidence intervals for the medians were calculated using the log–log method. Median follow-up duration was derived using the reverse Kaplan–Meier method. The effect of individual variables on the occurrence of postoperative complications was tested with the Mann–Whitney U test, Pearson χ2 test, or Fishers exact test, where appropriate. Logistic regression models were used to evaluate the effect of multiple variables on the occurrence of postoperative complications. A two-sided p value of <0.05 was taken as significant. All analyses were performed in STATA 11.2 (StataCorp LP. http://www.stata.com/).
3. Results
A total of 35 consecutive patients underwent CRS and HIPEC between January 2001 and December 2012. Preoperative data are summarized in Table 1. DFI was defined as the time between primary surgery and recurrence. T and N staging was based on the American Joint Committee on Cancer Staging Manual, 7th edition.12
| Variable | Frequency | |
|---|---|---|
| Age (y) | 51 (14–71) | |
| Race | Chinese | 31 (89) |
| Indian | 1 (3) | |
| Others | 3 (9) | |
| Sex | Female | 24 (69) |
| Male | 11 (31) | |
| Histological diagnosis | Intestinal | 23 (66) |
| Mucinous | 12 (34) | |
| Site of primary tumor | Right colon | 15 (43) |
| Left colon | 20 (57) | |
| T stage of primary tumor (n = 32) | 1 | 1 |
| 2 | 0 | |
| 3 | 9 | |
| 4 | 22 | |
| N stage of primary tumor (n = 30) | N0 | 10 |
| N1 (1–3 LN) | 10 | |
| N2 (4 or more LN) | 10 | |
| Grade of primary tumor (n = 33) | 1 | 9 |
| 2 | 20 | |
| 3 | 4 | |
| Disease-free interval (mo) | 15 (1.7–95.9) |
Data are presented as n, n (%), or median (range).
LN = lymph node.
The median PCI was 12, and 33 patients (94%) had a complete cytoreduction (CC-0). One patient achieved optimal cytoreduction with CC-1, and one patient did not achieve optimal cytoreduction with a CC-3 score. Median operating time and intraoperative blood loss were 505 minutes and 1000 mL, respectively. Median intensive care unit and hospital stays were 1 day and 14 days, respectively. The procedures and operative factors are summarized in Table 2.
| Variable | No. of patients | Median (range) |
|---|---|---|
| PCI | 25 | 12 (1–27) |
| Operation duration (min) | 35 | 505 (195–960) |
| Average blood loss (mL) | 34 | 1000 (200–4500) |
| Hospital stay (d) | 35 | 14 (9–36) |
| ICU stay (d) | 35 | 1 (0–5) |
| Time to feeds (d) | 35 | 5 (2–11) |
| Disease-free interval1 (mo) | 35 | 15.0 (1.7–95.9) |
| CC score | 35 | 0 (0–3) |
| Total no. of procedures | 35 | 2 (1–5) |
| Colectomy | 15 | |
| Small bowel resection | 15 | |
| Splenectomy | 7 | |
| Gastrectomy | 1 | |
| Total hysterectomy and bilateral salpingoopherctomy (THBSO) | 12 | |
| Cholecystectomy | 5 | |
| Bladder resection (wedge) | 1 | |
| Diaphragmatic peritonectomy | 25 |
CC = completeness of cytoreduction; ICU = intensive care unit; PCI = Peritoneal Cancer Index.
3.1. Morbidity and mortality
Postoperative complications occurred in 14 patients. For patients who experienced more than one complication, the worse grade was used. We observed four low-grade (grades 1 and 2) and 10 high-grade complications (grades 3–5; Table 3). Pleural effusions and intra-abdominal collections requiring percutaneous drainage accounted for the majority of the latter. There was one postoperative hemorrhage necessitating reexploration. There was no 30-day mortality.
| Postoperative complication | No. of patients |
|---|---|
| Respiratory (pleural effusion) | 6 |
| Intra-abdominal collection | 5 |
| Enterocutaneous fistula | 1 |
| Bleeding | 2 |
On univariate analysis, patients who underwent a colectomy as part of the CRS and HIPEC were significantly more likely to experience a postoperative complication. In addition, patients who had four or more procedures performed during the CRS and those who received less blood transfusion were more likely to experience a high-grade complication. Multivariate analyses were not performed because of the small number of events.
3.2. OS and DFS
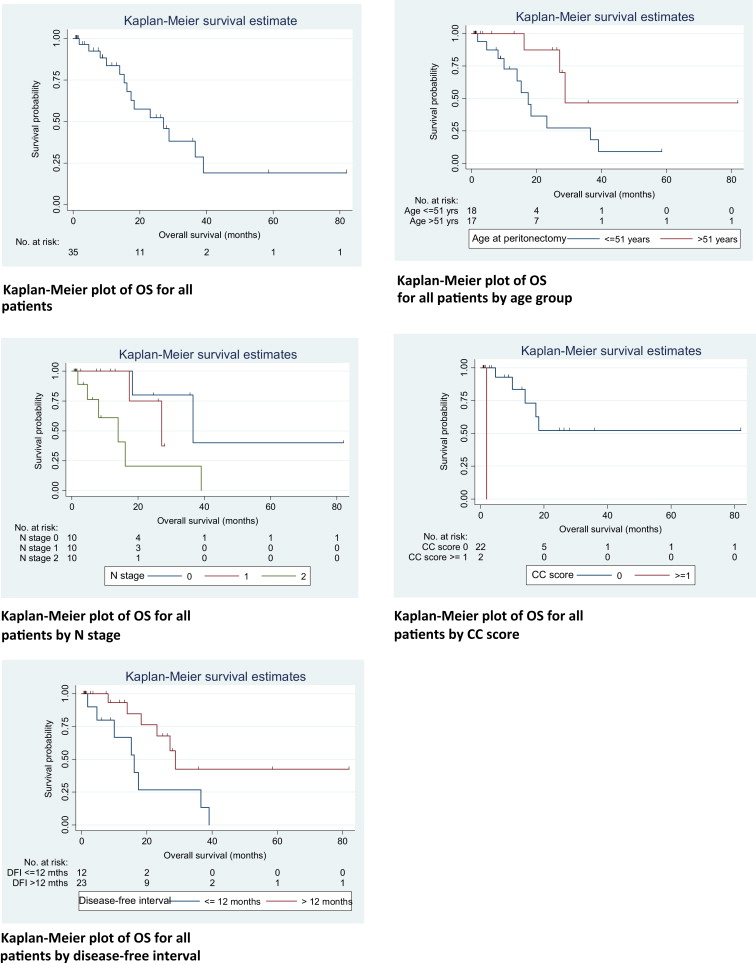
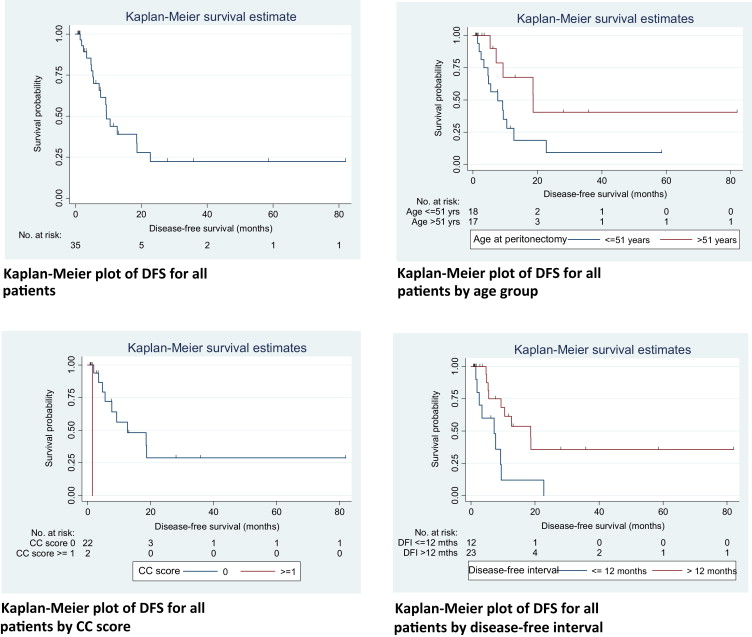
After a median follow-up of 24.7 months (95% CI 0.6–81.8 months), 18 (51.4%) patients recurred and 13 passed away. Four patients (11.4%) had isolated peritoneal recurrence, five patients (14.3%) had isolated distant metastasis, and eight (22.8%) had both peritoneal and distant relapse at first recurrence. The median time to recurrence for the patients with isolated peritoneal recurrence, isolated distant metastases, and both peritoneal and distant relapse was 10 months, 5 months, and 15 months, respectively. The median DFS for the 35 patients was 9.4 months (95% CI 5.5–18.7), with 1 year, 3 year, and 5 year DFS at 43.8%, 22.3%, and 22.3%, respectively. The patients age at surgery, CC score, and DFI were significant on log-rank test. The N stage showed a trend toward significance, with a p = 0.094.
The median OS was calculated to be 27.1 months (95% CI 15.3–39.1). The 1 year, 3 year, and 5 year OS rates were 83.7%, 38.2%, and 19.1%, respectively. Factors influencing OS were age at surgery, N stage, CC score, and DFI. The Kaplan–Meier curves and univariate analysis of prognostic factors for OS and DFS are depicted in Figure 1 ; Figure 2, and Table 4 ; Table 5, respectively. Multivariate analyses were not performed owing to the small number of events.
|
|
|
Figure 1. Kaplan–Meier curves of overall survival (OS). |
|
|
|
Figure 2. Kaplan–Meier curves for disease-free survival (DFS). |
| No. of deaths/no. of patients | Median OS, mo (95% CI) | p (log-rank test) | Hazard ratio (95% CI) | p (Cox model) | |
|---|---|---|---|---|---|
| All patients | 14/35 | 27.1 (15.3–39.1) | |||
| Age at peritonectomy | |||||
| ≤51 y | 11/18 | 17.4 (10.0–36.6) | 1 | ||
| >51 y | 3/17 | 28.8 (16.2–UD) | 0.038 | 0.28 (0.08–1.01) | 0.033 |
| Histology | |||||
| Intestinal | 8/23 | 28.8 (14.0–UD) | 1 | ||
| Mucinous | 6/12 | 27.1 (4.7–UD) | 0.325 | 1.73 (0.57–5.23) | 0.337 |
| T stage | |||||
| 1 | 0/1 | UD (UD) | Omitted | ||
| 3 | 1/9 | UD (1.9–UD) | 1 | ||
| 4 | 11/22 | 23.2 (14.0–39.1) | 0.676 | 1.29 (0.16–10.39) | 0.805 |
| N stage | |||||
| 0 | 2/10 | 36.6 (18.3–UD) | 1 | ||
| 1 | 2/10 | 27.1 (17.4–UD) | 1.67 (0.23–12.19) | ||
| 2 | 6/10 | 14.0 (1.9–UD) | 0.045 | 5.89 (1.14–30.54) | 0.061 |
| PCI score | |||||
| ≤15 | 5/19 | 18.3 (10.0–UD) | 1 | ||
| >15 | 2/6 | UD (1.9–UD) | 0.470 | 1.83 (0.35–9.73) | 0.496 |
| CC score | |||||
| 0 | 13/33 | 27.1 (16.2–39.1) | Model did not converge | ||
| ≥1 | 1/2 | 1.9 (UD) | <0.001 | ||
| No. of procedures | |||||
| <4 | 10/28 | 27.1 (16.2–39.1) | 1 | ||
| ≥4 | 4/7 | 36.6 (1.9–UD) | 0.986 | 0.99 (0.31–3.21) | 0.986 |
| Gastrectomy | |||||
| No | 14/33 | 23.2 (15.3–39.1) | Model did not converge | ||
| Yes | 0/2 | UD (UD) | 0.356 | ||
| Colectomy | |||||
| No | 6/20 | 27.1 (14.0–UD) | 1 | ||
| Yes | 8/15 | 18.3 (4.7–36.6) | 0.342 | 1.67 (0.58–4.82) | 0.344 |
| SB resection | |||||
| No | 10/20 | 23.2 (10.0–36.6) | 1 | ||
| Yes | 4/15 | 27.1 (15.3–UD) | 0.327 | 0.56 (0.17–1.81) | 0.317 |
| Splenectomy | |||||
| No | 10/28 | 23.2 (15.3–UD) | 1 | ||
| Yes | 4/7 | 27.1 (1.9–UD) | 0.458 | 1.56 (0.48–5.08) | 0.476 |
| Diaphragm | |||||
| No | 1/10 | 18.3 (18.3–UD) | 1 | ||
| Yes | 13/25 | 27.1 (14.0–39.1) | 0.492 | 2.02 (0.26–15.76) | 0.458 |
| Disease-free interval | |||||
| ≤12 mo | 8/12 | 16.2 (1.9–36.6) | 1 | ||
| >12 mo | 6/23 | 28.8 (18.3–UD) | 0.028 | 0.32 (0.11–0.93) | 0.037 |
CC = completeness of cytoreduction; CI = confidence interval; SB = small bowel; UD = undefined.
| No. of relapses/no. of patients | Median DFS, mo (95% CI) | p (log-rank) | Hazard ratio (95% CI) | p (Cox model) | |
|---|---|---|---|---|---|
| All patients | 18/35 | 9.4 (5.5–18.7) | |||
| Age at peritonectomy | |||||
| ≤51 y | 13/18 | 7.7 (3.4–12.7) | 1 | ||
| >51 y | 5/17 | 18.7 (5.3–UD) | 0.038 | 0.35 (0.12–0.98) | 0.035 |
| Histology | |||||
| Intestinal | 10/23 | 10.5 (4.8–UD) | 1 | ||
| Mucinous | 8/12 | 9.4 (2.5–UD) | 0.325 | 1.62 (0.62–4.23) | 0.333 |
| T stage | |||||
| 1 | 1/1 | UD (UD) | 1 | ||
| 3 | 2/9 | UD (1.5–UD) | 1.02 (0.09–11.73) | ||
| 4 | 13/22 | 9.4 (7.2–18.7) | 0.995 | 1.08 (0.14–8.45) | 0.995 |
| N stage | |||||
| 0 | 3/10 | 18.7 (4.7–UD) | 1 | ||
| 1 | 4/10 | 9.4 (1.9–UD) | 2.18 (0.48–9.83) | ||
| 2 | 7/10 | 7.2 (1.5–UD) | 0.094 | 4.14 (1.05–16.42) | 0.102 |
| PCI score | |||||
| ≤15 | 8/19 | 9.2 (4.7–UD) | 1 | ||
| >15 | 3/6 | 18.7 (1.5–UD) | 0.867 | 1.12 (0.30–4.23) | 0.869 |
| CC score | |||||
| 0 | 17/33 | 10.5 (7.2–18.7) | Model did not converge | ||
| ≥1 | 1/2 | 1.5 (UD) | <0.001 | ||
| No. of procedures | |||||
| <4 | 14/28 | 9.4 (5.5–18.7) | 1 | ||
| ≥4 | 4/7 | 12.7 (1.5–UD) | 0.699 | 0.80 (0.26–2.46) | 0.694 |
| Gastrectomy | |||||
| No | 18/33 | 9.4 (5.5–18.6) | Model did not converge | ||
| Yes | 0/2 | UD (UD) | 0.191 | ||
| Colectomy | |||||
| No | 9/20 | 12.7 (7.2–UD) | 1 | ||
| Yes | 9/15 | 5.3 (2.5–18.6) | 0.168 | 1.90 (0.75–4.83) | 0.178 |
| SB resection | |||||
| No | 12/20 | 9.4 (5.3–18.7) | 1 | ||
| Yes | 6/15 | 18.6 (2.5–UD) | 0.364 | 0.63 (0.24–1.71) | 0.358 |
| Splenectomy | |||||
| No | 13/28 | 9.2 (5.3–UD) | 1 | ||
| Yes | 5/7 | 9.4 (1.5–UD) | 0.526 | 1.40 (0.49–4.01) | 0.537 |
| Diaphragm | |||||
| No | 2/10 | UD (4.7–UD) | 1 | ||
| Yes | 16/25 | 9.4 (5.3–18.7) | 0.398 | 1.87 (0.43–8.15) | 0.368 |
| Disease-free interval | |||||
| ≤12 mo | 9/12 | 7.2 (1.5–9.4) | 1 | ||
| >12 mo | 9/23 | 18.6 (5.5–UD) | 0.008 | 0.29 (0.11–0.76) | 0.014 |
CC = completeness of cytoreduction; CI = confidence interval; SB = small bowel; UD = undefined.
4. Discussion
The role of CRS and HIPEC for CPM was established in the first randomized prospective trial in 2003.2 In the trial, 105 patients with CPM were assigned to either systemic chemotherapy (5-fluorouracil/lecovorin) with or without palliative surgery, or CRS and HIPEC with mitomycin C, followed by systemic chemotherapy. The preliminary results showed a median survival of 12.6 months and 22.3 months in the standard treatment and CRS and HIPEC arms, respectively (p = 0.032), but with a mortality of 8% with the CRS arm. The study was updated in 2008 and reported disease-specific survivals of 12.6 months and 22.2 months in the control and CRS and HIPEC arms, 13 respectively. The trial was criticized for its high mortality rate, and the chemotherapy regime used in the standard arm is now outdated. Glehen et al14 conducted the largest study, involving 506 patients treated at 28 institutions, and reported outcomes of CRS and HIPEC for CPM. Morbidity and mortality rates of 22.9% and 4%, respectively and OS of 19.2 months were attained.
CRS and HIPEC are gradually becoming accepted as the standard of treatment for patients with colorectal PC. However, data supporting the use of CRS and HIPEC for colorectal PC in an Asian population is lacking. In our cohort, the majority of patients were Chinese, with a smaller percentage of patients from other communities. Our Japanese counterparts have reported morbidity and mortality rates of CRS and HIPEC performed for pseudomyxoma peritonei and PC from colorectal and gastric cancers15 at 49% and 3.5%, respectively, whereas other Japanese reports have been on CRS and HIPEC for gastric cancer, with a reported median OS of 11.5 months, and a 5-year survival rate of 6.7%.16 To our knowledge, this is the first study to report on the outcomes for CRS and HIPEC for colorectal PC in Asian patients. Our reported median OS of 27.1 months (95% CI 15.3–39.1) and of 83.7%, 38.2%, and 19.1% at 1 year, 3 years, and 5 years, respectively, is comparable to that reported in other Western centers.17 ; 18
As with most surgical interventions, the selection of patients for treatment is crucial for success. The CC score remains the most important prognostic indication for survival in patients undergoing CRS and HIPEC.4 ; 19 The PCI score has also been shown to be a useful prognostic measure for patients with colorectal or appendiceal PC.7 ; 10 In our study, the age of the patient, CC score, nodal status, and the DFI significantly affected OS. The PCI score did not affect OS, as optimal cytoreduction was achieved in all but one of our patient. In addition, only 25 of the 35 patients had records of their PCI score, which may also limit the analysis of this factor on OS and DFS.
Our younger patients (<52 years) did worse than the older patients. There are small studies that show that young colorectal cancer patients have a more aggressive disease, with poorer survival.20 ; 21 Larger studies depict young colorectal cancer patients as having later-stage and higher-grade tumors, but equivalent 5-year cancer-specific survival compared to older patients.22 However, our study found that our Asian patients who were aged <51 years at surgery had a median OS that was 11 months less than that of older patients (Table 4), but there were fewer patients (12.5%) with N0 disease in the younger, as compared to the older group of patients (42%). This may account for the poorer survival as nodal status affected OS. Patients with N1 or more fared at least two times worse than patients with N0 disease (Table 4). We tended to be more aggressive with younger patients with good ECOG status, willing to perform CRS and HIPEC even if their DFI was less than 12 months and they had N2 disease. Patients, in whom CC-0 was not achieved during surgery, also had significantly inferior OS. The analysis of the CC score was, however, limited by the small number of patients who had a CC score of more than 1 (n = 1). Nevertheless, the CC score remains an important prognostic indicator and is well established in the literature. 22 Lastly, a DFI of more than 1 year also provided good OS, as this likely reflected a better tumor biology and disease profile.
The DFS was similarly affected by age, CC score, and DFI. Again, our younger patients tended to recur earlier. Patients in whom CC-0 was not achieved also had significantly inferior DFS, as did patients with DFI of less than 1 year (Table 5). As shown in the Kaplan–Meier curve (Fig. 1), the majority of patients who were disease-free after 2 years, remained disease-free, whereas patients who had recurrence of disease tended to recur early, i.e., within the first 2 years. This can be explained by the fact that PC behaves like a locoregional disease, and when recurrences occur, they tend to recur early. The absence of disease at 2 years after CRS portends good survival results. Consequently, it is vital to select patients with a low risk of systemic metastases that can be predicted by nodal status and DFI. This is further supported by the analysis showing that nodal status significantly affected OS in our patients. In this group of patients with “high-risk” features, we propose a plan of watchful waiting with or without systemic chemotherapy, and repeat imaging in 3 months. In the event that they remain systemically free of metastases, CRS and HIPEC can then be planned. This facilitates better selection of patients, allowing us to perform CRS and HIPEC for patients who will derive the greatest benefits.
In our study, among the 34 patients who underwent CRS and HIPEC, 10 patients (28%) suffered major complications, the majority of which were pleural and intra-abdominal collections requiring percutaneous drainage. Since 2010, have we placed pleural and subdiaphragmatic drainage catheters intraoperatively, if the diaphragmatic peritoneum is stripped, to reduce intra-abdominal collections.23 This is likely to improve our morbidity rates with the next analysis. Patients who underwent a colectomy as part of the CRS or had more than four procedures performed, were more likely to experience a postoperative complication.
Patients undergoing CRS and HIPEC experience significant pathophysiological alterations during surgery, i.e., massive blood loss and raised intra-abdominal pressure.24 Perioperative anesthetic care is critical in the CRS and HIPEC. Postoperative complications are reduced when patients are kept well resuscitated intraoperatively. This is also suggested by our analysis showing that patients with fewer intraoperative blood transfusions were more prone to major complications, indicating that patients who were under-resuscitated during the surgery suffered from higher grade complications, whereas those who received adequate blood products had fewer high-grade complications.
5. Conclusion
Our data show that CRS and HIPEC can provide survival benefits, with reasonable morbidly and mortality for Asian patients with PC from colorectal cancer. The key to the success of the procedure lies in patient selection and perioperative management of the patients.
References
- 1 D.G. Jayne, S. Fook, C. Loi, F. Seow-Choen; Peritoneal carcinomatosis from colorectal cancer; Br J Surg, 89 (2002), pp. 1545–1550
- 2 V.J. Verwaal, S. van Ruth, E. de Bree, et al.; Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer; J Clin Oncol, 21 (2003), pp. 3737–3743
- 3 P.H. Sugarbaker; Review Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology; Cancer Treat Res, 82 (1996), pp. 79–100
- 4 P.H. Sugarbaker, D. Chang, P. Koslowe; Prognostic features for peritoneal carcinomatosis in colorectal and appendiceal cancer patients when treated by cytoreductive surgery and intraperitoneal chemotherapy; Cancer Treat Res, 81 (1996), pp. 89–104
- 5 P.H. Sugarbaker, B.M. Ronnett, A. Archer, et al.; Pseudomyxoma peritonei syndrome; Adv Surg, 30 (1997), pp. 233–280
- 6 P. Jacquet, P.H. Sugarbaker; Current methodologies for clinical assessment of patients with peritoneal carcinomatosis; J Exp Clin Cancer Res, 15 (1996), pp. 49–58
- 7 J. Esquivel, R. Sticca, P.H. Sugarbaker, et al.; Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancy of colonic origin: a consensus statement. Society of Surgical Oncology; Ann Surg Oncol, 14 (2007), pp. 128–133
- 8 R. Younan, S. Kusamura, D. Baratti, A.S. Cloutier, M. Deraco; Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy; J Surg Oncol, 98 (2008), pp. 253–257
- 9 P.H. Sugarbaker; Peritonectomy procedures; Ann Surg, 221 (1995), pp. 29–42
- 10 P.L. Wagner, D. Jones, A. Aronova, et al.; Early postoperative intraperitoneal chemotherapy following cytoreductive surgery for appendiceal mucinous neoplasms with isolated peritoneal metastasis; Dis Colon Rectum, 55 (2012), pp. 407–415
- 11 P.H. Sugarbaker, K.A. Jablonsky; Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy; Ann Surg, 221 (1995), pp. 124–132
- 12 Colon and rectum; S.B. Edge, D.R. Byrd, C.C. Compton (Eds.), et al., AJCC Cancer Staging Manual (7th ed.), Springer, New York, NY (2010), pp. 143–402
- 13 V.J. Verwaal, S. Bruin, H. Boot, et al.; 8-year follow-up of randomized trials: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer; Ann Surg Oncol, 15 (2008), pp. 2426–2432
- 14 O. Glehen, F. Kwiatkowski, P.H. Sugarbaker, et al.; Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study; J Clin Oncol, 22 (2004), pp. 3284–3292
- 15 A. Mizumoto, E. Canbay, M. Hirano, et al.; Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan; Gastroenterol Res Pract, 2012 (2012), p. 836425 Epub 2012 Jun 18
- 16 Y. Yonemura, Y. Endou, M. Shinbo, et al.; Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: selection for cytoreductive surgery; J Surg Oncol, 100 (2009), pp. 311–316
- 17 V.J. Verwaal, S. Van Ruth, A. Witkamp, et al.; Long-term survival of peritoneal carcinomatosis of colorectal origin; Ann Surg Oncol, 12 (2005), pp. 65–71
- 18 F. Cavaliere, M. Valle, M. De Simone, et al.; 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra-abdominal hemohyperthermia: a S.I.T.I.L.O. multicentric study; In Vivo, 20 (2006), pp. 747–750
- 19 D. Elias, F. Gilly, F. Boutitie, et al.; Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study; J Clin Oncol, 28 (2010), pp. 63–68
- 20 R.B. Adkins, J.B. DeLozier, W.G. McKnight, et al.; Carcinoma of the colon in patients 35 years of age and younger; Am Surg, 53 (1987), pp. 141–145
- 21 F.E. Rosato, T.G. Frazier, E.M. Copeland, et al.; Carcinoma of the colon in young people; Surg Gynecol Obstet, 129 (1969), pp. 29–32
- 22 J.B. O'Connell, M.A. Maggard, J.H. Liu, D.A. Etzioni, E.H. Livingston, C.Y. Ko; Do young colon cancer patients have worse outcomes?; World J Surg, 28 (2004), pp. 558–562
- 23 M. Teo, K.F. Foo, W.H. Koo, L.T. Wong, K.C. Soo; Lessons learned from initial experience with peritonectomy and intra-peritoneal chemotherapy infusion; World J Surg, 30 (2006), pp. 2132–2135
- 24 C. Schmidt, S. Moritz, S. Rath, et al.; Perioperative management of patients with cytoreductive surgery for peritoneal carcinomatosis; J Surg Oncol, 100 (2009), pp. 297–301
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?